Introduction
Novel effective treatments are required for patients
with recurrent metastatic breast cancer, particularly for those who
fail to respond to third-line and subsequent lines of chemotherapy
(1). Overexpression of the oncogene
human epidermal growth factor receptor 2 (HER-2) is an independent
indicator of poor prognosis (2).
HER-2 overexpression has been associated with decreased survival
time and resistance to certain chemo- and endocrine therapies in
patients with breast cancer (3,4). Although
the high efficacy of trastuzumab, a drug that targets the HER-2
oncogene, has been widely recognized, its efficiency remains at
~30% (5,6). Patients who fail to response to
third-line or subsequent lines of chemotherapy are usually of poor
physical condition and have difficulty tolerating additional
chemotherapy (7). Therefore, methods
for optimizing effective biological therapies to improve patient
quality of life and survival times are urgently required in
clinical practice.
Tumor initiation and development are a result of
multiple genetic abnormalities; therefore, blocking the expression
of cancer-promoting genes at multiple points and along numerous
signaling pathways may theoretically limit tumor development
(8). Studies have demonstrated that
the expression of the urokinase-type plasminogen activator (uPA)
and its receptor (uPAR) system is significantly increased in
various human tumor tissues, including breast, lung and colorectal
cancer (9–11). Furthermore, uPA-uPAR expression has
been significantly associated with tumor growth and patient
prognosis (12–14). As uPA-uPAR has key roles in tumor
metastasis and growth, it has become an ideal candidate for
targeted cancer therapy. Interference with uPA-uPAR interactions
not only inhibits tumor infiltrations but also is able to block
tumor angiogenesis, thereby effectively controlling tumor growth
(15,16). In a previous study by the authors, an
antibody-like molecule comprised of the amino-terminal fragment
(ATF) of uPA and is conjugated to the Fc fragment of immunoglobulin
(Ig) G1 (ATF-Fc), was engineered. The specific inhibitory effect of
the antibody-like molecule on tumors was evaluated (17).
In the present study, the authors proposed using the
ATF-Fc fusion protein to target uPA-uPAR and trastuzumab signaling
pathways, and to inhibit HER-2 oncogene function in breast cancer.
In addition, the effect of ATF-Fc in combination with trastuzumab
on the tumor apoptosis and metastasis of HER-2-overexpressing
breast cancer was evaluated in vivo.
Materials and methods
Preparation of an engineered
antibody-like molecule ATF-Fc
ATF-Fc-2E9, a recombinant Chinese hamster ovary cell
line which was stably transfected with ATF-Fc (17), was gifted by Professor Haifeng Duan
(Beijing Institute of Radiation Medicine, Beijing, China). The
ATF-Fc expression level of the ATF-Fc-2E9 cell line was ~20
µg/106 cells/day. A fusion protein with a molecular
weight of ~96 kDa under non-reducing conditions and ~45 kDa under
reducing conditions was obtained, according to the protocols
previously published (17,18). The fusion protein consists of the ATF
of human uPA and is conjugated with the Fc fragment of human
IgG1.
Experimental drug trastuzumab
The monoclonal antibody trastuzumab (catalog no.,
10172501; 440 mg/vial) was provided by (Roche Diagnostics, Basel,
Switzerland), and was freshly prepared prior to each experiment.
Briefly, each 440 mg vial of trastuzumab was reconstituted with 20
ml of bacteriostatic water for injection, containing 1.1% benzyl
alcohol as a preservative to yield a multi-dose solution containing
21 mg/ml trastuzumab. The drug was then diluted to the required
concentration (0.12 mg/ml) using sterile saline.
Cell lines and experimental
animals
The EC-109 human esophageal cancer cell line and the
MCF-7 and SK-BR-3 human breast cancer cell lines were purchased
from the Cell Culture Center of the Chinese Academy of Science
(Beijing, China). The EC-109 and MCF-7 cells were maintained in
Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA), while the SK-BR-3 cells were maintained
in RPMI-1640 (HyClone; GE Healthcare Life Sciences), supplemented
with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), 100 U/ml streptomycin and 100 U/ml penicillin
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). All the cells were incubated in a humidified incubator
containing 5% CO2 at 37°C. The experimental models used
were 35 4-6-week-old female BALB/C nude mice, which were purchased
from the Animal Center of the Peking Union Medical College Hospital
(Beijing, China). The experimental protocol was approved by the
Experimental Animal Center of China. All experiments were performed
according to the standards of animal care as outlined in the Guide
for the Care and Use of Experimental Animals of Peking Union
Medical College. Briefly, the mice were maintained in an
air-conditioned specific-pathogen-free laboratory with 12/12-h
light-dark cycle and 24±1°C and 5±5% relative humidity, and had
free access to food and water during the study. Following one week
of acclimatization, the animals were subcutaneously injected into
the right flank with SK-BR-3 cells suspended in serum-free medium
in the exponential growth stage at a dosage of 1×107
cells/nude mouse. The period of tumor formation was 7–10 days. When
the tumors reached a volume of ~3 cm3 calculated as
previously described (19,20), the mice were sacrificed and the tumor
tissues were dissected, minced, ground, homogenized and re-injected
into another four groups of the same type of mice (7 mice per
group). Following 1–2 weeks, tumors developed, and the animals were
randomly grouped for subsequent experiments.
Detection of uPA and uPAR expression
in tumor cell lines
The serum-free supernatant from the EC-109, MCF-7
and SK-BR-3 cell cultures was collected and centrifuged at 850 × g
for 10 min at 4°C (ThermoHeraeus Multifuge X1R; Thermo Fisher
Scientific, Inc.) for detection of uPA (three samples from each
cell line). Triple wells were used in each cell line. An ELISA was
performed to determine the level of uPA secreted from the cells
using an uPA ELISA kit (catalog no., ab108917; Abcam, Cambridge,
MA, USA) The assay was performed according to the manufacturer's
instructions. The extracellular level of uPAR was determined using
a FACscan flow cytometer (BD Biosciences, San Jose, CA, USA) (3
samples in each cell line). The procedure is briefly described, as
follows: 1×106 EC-109, MCF-7 and SK-BR-3 cells were
incubated with an anti-uPAR antibody (10 µg per 1×106
cells) (catalog no., sc-13522; Santa Cruz Biotechnologies, Dallas,
USA) for 2 h at 4°C. The cells were washed three times with PBS
containing 0.05% Tween-20 to remove the unbound antibody and then
incubated with a fluorescein isothiocyanate-conjugated secondary
antibody (dilution, 1:500; catalog no., ab6785; Abcam) for 30 min
at room temperature. The normal mouse IgG (cat. no., sc-2025; Santa
Cruz) were used as the controls. The flow cytometry results were
analyzed using a FACScan instrument (CellQuest Pro Software 5.1; BD
Biosciences).
HER-2 oncogene immunohistochemistry
(IHC) and fluorescence in situ hybridization (FISH)
Anti-HER-2 monoclonal antibody (cat. no., ZM0065;
dilution, 1:200) and PV6000 detection kit (both Zhongshan Golden
Bridge Biotechnology Co., Ltd., Beijing, China) were used for IHC
analysis. The IHC detection of HER-2 protein was performed
according to the manufacturer's instructions. Plasmalemma
exhibiting brown particles was considered positively stained. Based
on the intensity and continuity of the color, the staining results
were classified as ‘negative’ for no color (0), ‘positive’ for
light-yellow (1+), ‘strong positive’ for brownish-yellow (2+) and
‘extreme positive’ for brown (3+).
The HER-2 gene FISH detection kit was provided by
Daan Gene Co., Ltd. FISH detection of HER-2 was performed according
to the manufacturer's instructions. In a clear tumor area, the
total number of HER-2 (red) and CEP-17 (green) signals in 60
cell nuclei were counted, and the ratio of the number of
HER-2 to CEP-17 signals was calculated. The following
assessment criteria were used: If the ratio was ≥2.2, the result
was considered positive (with amplification); if the ratio was
<1.8, the result was considered negative (without
amplification); if the ratio was close to borderline values
(1.8–2.2), the signals in an additional 20 nuclei were counted
(21).
In vitro analysis of apoptosis status
in a breast cancer cell line
SK-BR-3 cells in the exponential growth stage were
seeded in 96-well tissue culture plates (Costar; Corning
Incorporated, Corning, NY, USA) at a density of 5×104
cells/well. When the cells reached 65–70% confluency, ATF-Fc (final
concentration of 50 µg/ml), trastuzumab (final concentration of 10
mg/ml) or ATF-Fc (50 µg/ml) plus trastuzumab (10 mg/ml) was added.
Saline was added to the control group. The cells were continuously
cultured for an additional 72 h at 37°C in a humidified incubator
containing 5% CO2. The culture medium was subsequently
removed and the cells were collected. The cells were then stained
with Annexin-V and 7-amino-actinomycin D (7-AAD), according to the
manufacturer's instructions (BD Biosciences). Briefly, subsequent
to washing with ice-cold PBS, SK-BR-3 cells were resuspended in 200
µl of 1X annexin-V binding buffer. Next, 5 µl of annexin-V-APC and
5 µl of 7-AAD were added to the tubes and incubated for 10 min at
4°C in the dark. The samples were placed on ice and the cellular
apoptotic rate was determined using flow cytometry. Triplicate
wells were used in the experimental and in the control groups.
In vivo analysis of tumor growth and
metastasis in breast cancer xenograft mouse model
As aforementioned, female BALB/C nude mice
inoculated with SK-BR-3 cells were randomly grouped for
experimentation when the size of the tumors reached 100
mm3. Each group consisted of seven mice. The following
four experimental groups were included: ATF-Fc alone; trastuzumab
alone; ATF-Fc plus trastuzumab; a blank control group. ATF-Fc (10
mg/kg) was administered once every two days via tail vein injection
for three continuous weeks, and trastuzumab (6 mg/kg) was
administered once a week for two weeks. The medication regimen of
ATF-Fc plus trastuzumab group was ATF-Fc (10 mg/kg) administered
once every 2 days via tail vein injection for 3 continuous weeks,
combined with trastuzumab (6 mg/kg) administered once a week for 2
weeks. An equal volume of saline (1 ml/time) was used once every
two days for the control group. Following drug administration, the
mice were observed for 3 weeks. The longitudinal and transverse
diameters of the tumors were measured every other day, and the
tumor volume was calculated as previously described (19,20). After
3 weeks, the animals were sacrificed. The tumor tissues were
isolated and weighed to calculate the tumor inhibition rate. The
mice were dissected to examine the liver metastasis status. The
number of mice with liver metastasis and the number of metastatic
foci in each liver were recorded. Briefly, specimens for
histological examination were fixed in 10% formalin for 24 h. To
ensure systematic uniform and random sampling, the entire liver of
each animal was cut transversally to the portal vein into 2 mm
thick parallel sections, the cut surfaces were examined and the
specimens were embedded in paraffin. Sections (5 µm) were cut and
stained with hematoxylin and eosin and Periodic Acid-Schiff (G1281;
Beijing Solarbio Science & Technology Co., Ltd.) to confirm the
presence of cancerous cells by light microscopy. The equation for
calculating tumor volume is as follows (19,20):
V=LxD2/2
(L, maximum diameter of the tumor; D, minimum
diameter of the tumor). The equation for calculating the tumor
inhibition rate of drug effect based on the tumor weight is as
follows:
(a–b)/ax100%
(a, mean tumor weight in the control group; b, mean
tumor weight in the treatment group).
Statistical analysis
The results are presented as the mean ± standard
deviation. Group differences were analyzed with the non-parametric
Kruskal-Wallis test. Comparisons of the tumor weight and the liver
metastasis rate in the nude mice with tumors were analyzed using
the Pearson χ2 test. In experiments involving histology
or flow cytometry, the figures presented are representative of ≥3
experiments performed on different days on the tissue sections or
tumor cells. All statistical assessments were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference. All analyses were performed using SPSS software
(version 15.0; SPSS Inc., Chicago, IL, USA).
Results
Determination of uPA, uPAR and HER-2
expression in tumors cell lines
The expression levels of uPA, uPAR, and HER-2 in
EC-109, MCF-7 and SK-BR-3 cells were determined as aforementioned.
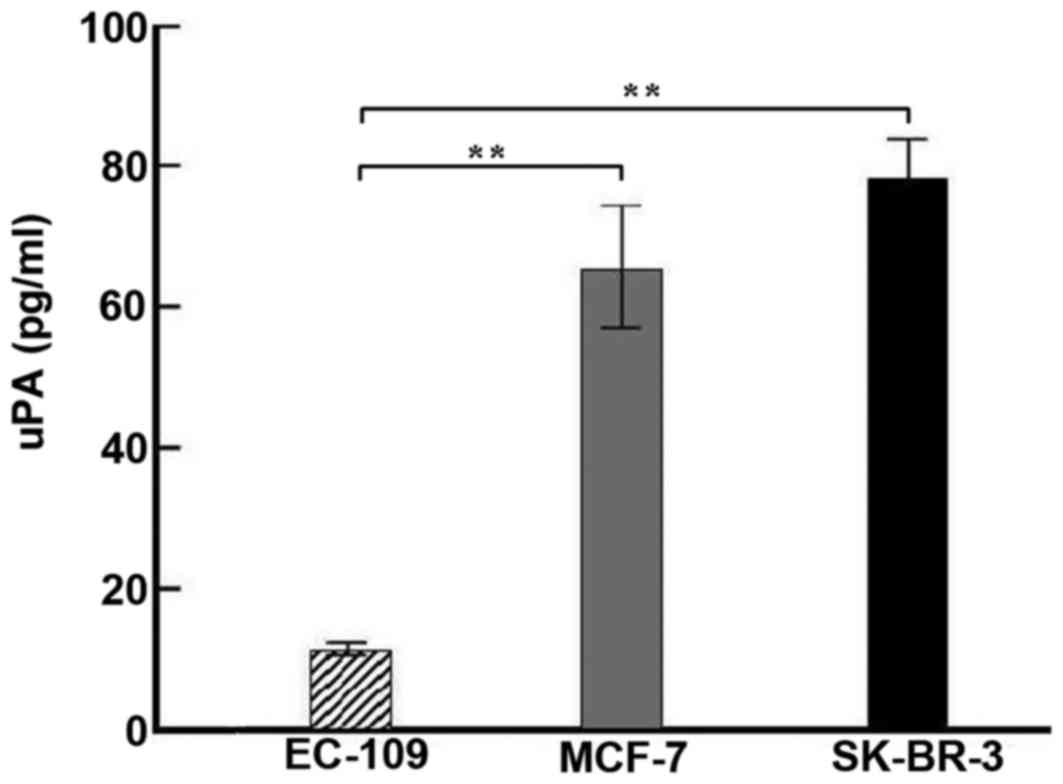
As presented in Fig. 1, the
expression level of uPA in EC-109, MCF-7 and SK-BR-3 cells was
12.32±1.18, 63.77±9.26, and 78.62±6.09 pg/ml, respectively. The
uPAR expression rate was 6.5, 56.32 and 69.87%, respectively
(Fig. 2A and B). The SK-BR-3 cells
expressed significantly increased levels of uPA and uPAR compared
with EC-109 cells, (**P-value<0.01). The HER-2 expression, as
determined by IHC, revealed that EC-109 and MCF-7 cells were
negative for HER-2 expression and that the nuclei were blue. By
contrast, the SK-BR-3 cells exhibited strong positive expression,
and the nuclear membrane appeared dark-brown (Fig. 2C). FISH analysis verified that the
HER-2 gene was amplified in the SK-BR-3 cells (Fig. 2D). These results suggested that
SK-BR-3 cells exhibit elevated levels of uPA and uPAR expression,
and high levels of HER-2 expression. Therefore, SK-BR-3 cells were
selected for further experiments to determine the inhibitory effect
of ATF-Fc in combination with trastuzumab on cells.
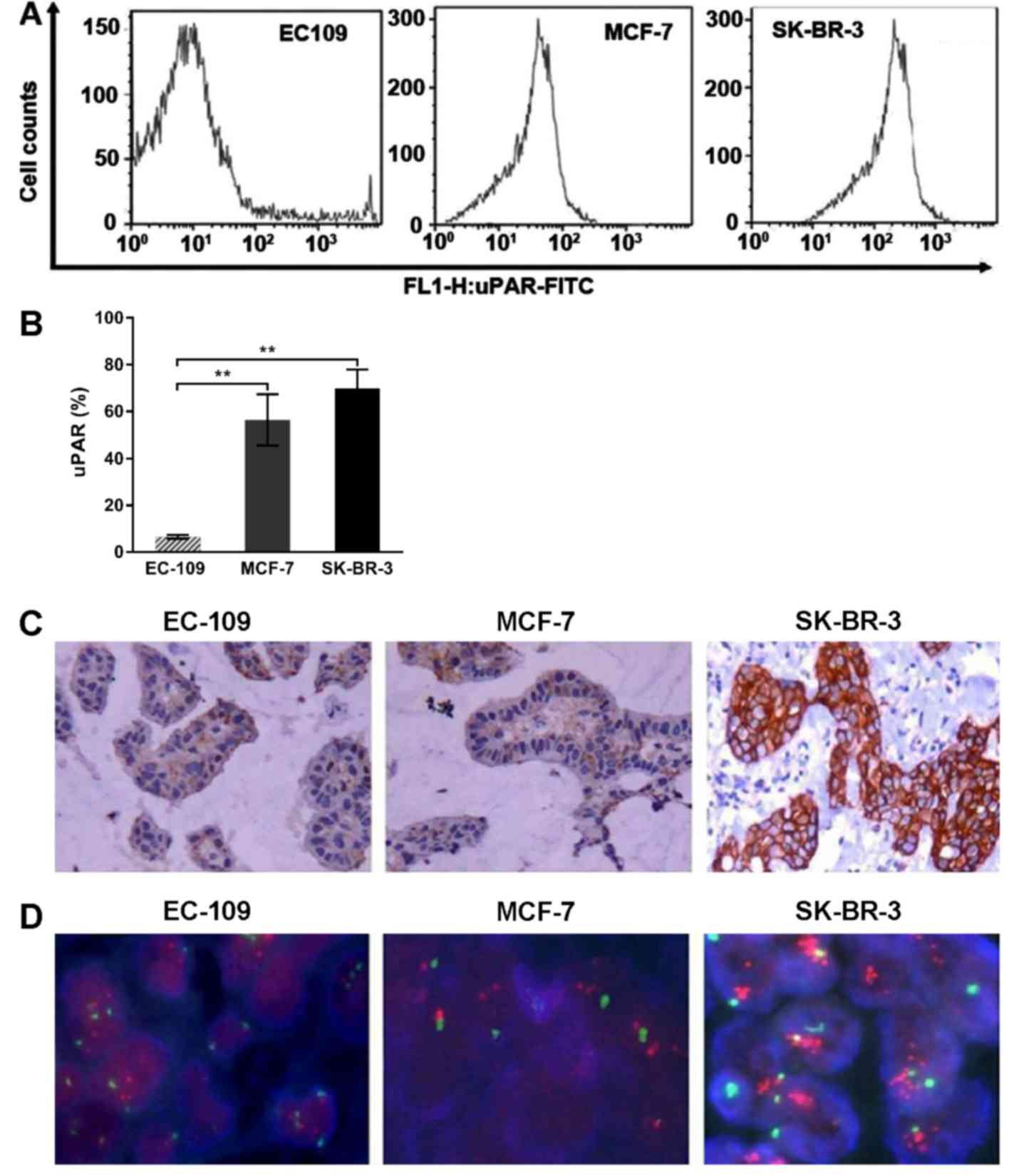 | Figure 2.Expression of uPAR and HER-2 in
various cell lines. (A) The uPAR expression levels in EC-109, MCF-7
and SK-BR-3 cells were determined by flow cytometry. The uPAR
expression rate was 6.5, 56.32 and 69.87%. (B) The SK-BR-3 cells
exhibited significantly increased levels of expression compared
with EC-109 cells (**P<0.01). (C) The HER-2 protein expression
was determined via immunohistochemistry in EC-109, MCF-7 and
SK-BR-3 cells. HER-2 was not present in EC-109 and MCF-7 cells. By
contrast, the SK-BR-3 cells exhibited brown staining, indicating
positive expression. (D) The HER-2 gene expression was detected by
fluorescence in situ hybridization. The SK-BR-3 cells
exhibited positive amplification with a HER-2/CEP-17 ratio of 4.38,
whereas the EC-109 and MCF-7 cells were negative for amplification
and had HER-2/CEP-17 ratios of 1.41 and 1.26, respectively. FITC,
fluorescein isothiocyanate; HER-2, human epidermal growth factor
receptor; uPAR, urokinase plasminogen activator receptor; uPA,
urokinase-type plasminogen activator. |
Apoptosis status of breast cancer
cells following treatment with ATF-Fc or trastuzumab alone and in
combination
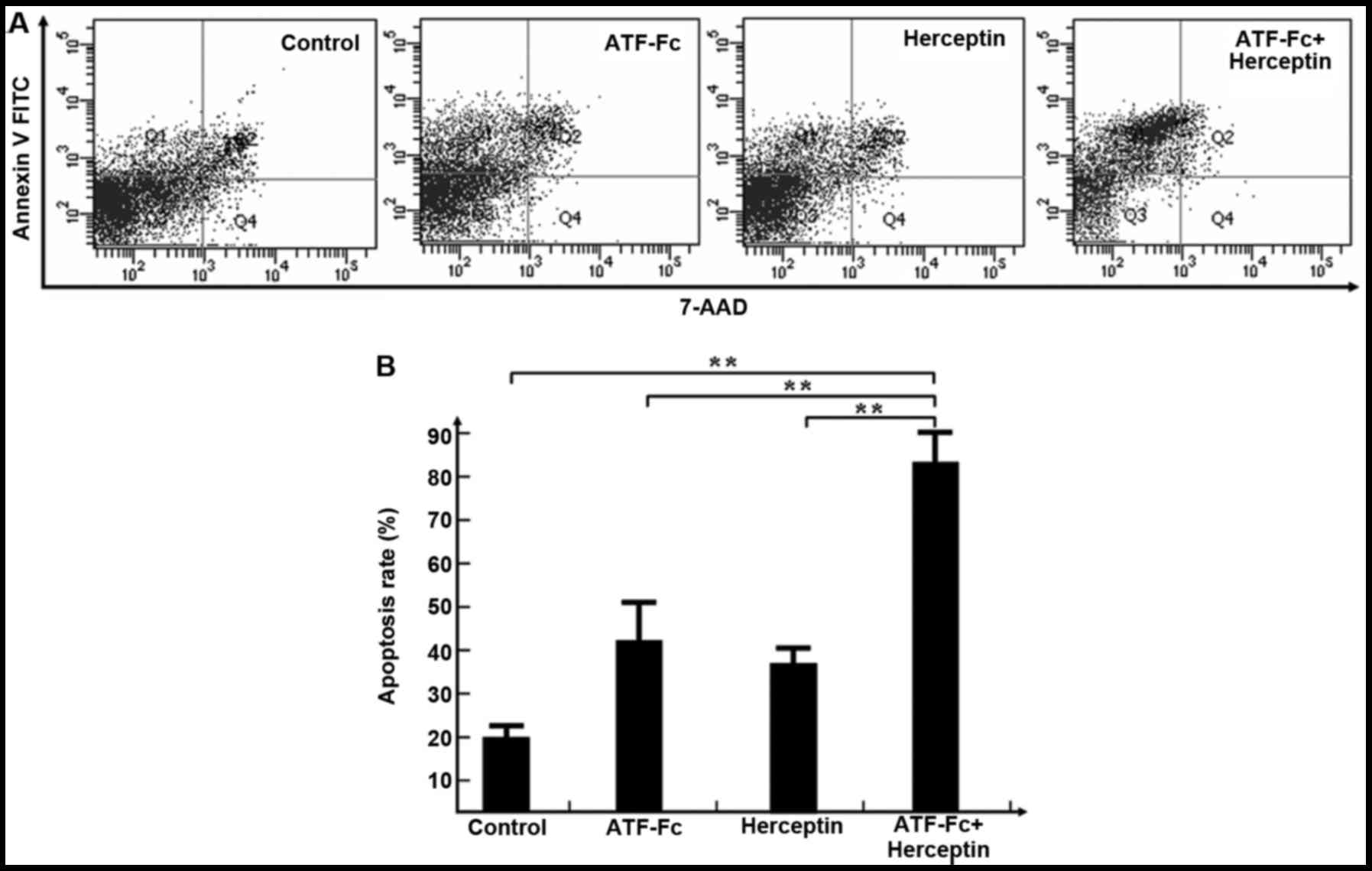
Following the treatment of SK-BR-3 cells (in the
exponential growth stage) with ATF-Fc (50 µg/ml) or trastuzumab (10
mg/ml) alone and in combination for 72 h, the cells were stained
with Annexin-V and 7-AAD, and then analyzed by flow cytometry.
Representative dot plots indicating the presence of early and late
apoptotic tumor cells are depicted in Fig. 3A. As illustrated in Fig. 3B, treatment with ATF-Fc or trastuzumab
alone resulted in a cellular apoptotic rate of 42±7.79 and
38±4.55%, respectively. By contrast, treatment with a combination
of ATF-Fc and trastuzumab resulted in an apoptotic rate of
83±6.58%. The apoptotic rates of tumor cells were significantly
higher in the ATF-Fc and trastuzumab combination treatment group,
as compared with in the ATF-Fc (P<0.01) or trastuzumab alone
groups (P<0.01). These results indicate that specifically
blocking the uPA-uPAR and HER-2 pathways may effectively promote
the apoptosis of breast cancer cells in vitro.
In vivo analysis of inhibition of
tumor cell proliferation
Tumor-bearing nude mice were administered ATF-Fc
alone, trastuzumab alone or a combination of ATF-Fc and trastuzumab
according to the protocols previously described. The control group
received saline via tail vein injection. In the present study, the
mice did not exhibit symptoms of fever, vomiting, diarrhea or skin
rash following administration of ATF-Fc or trastuzumab alone or
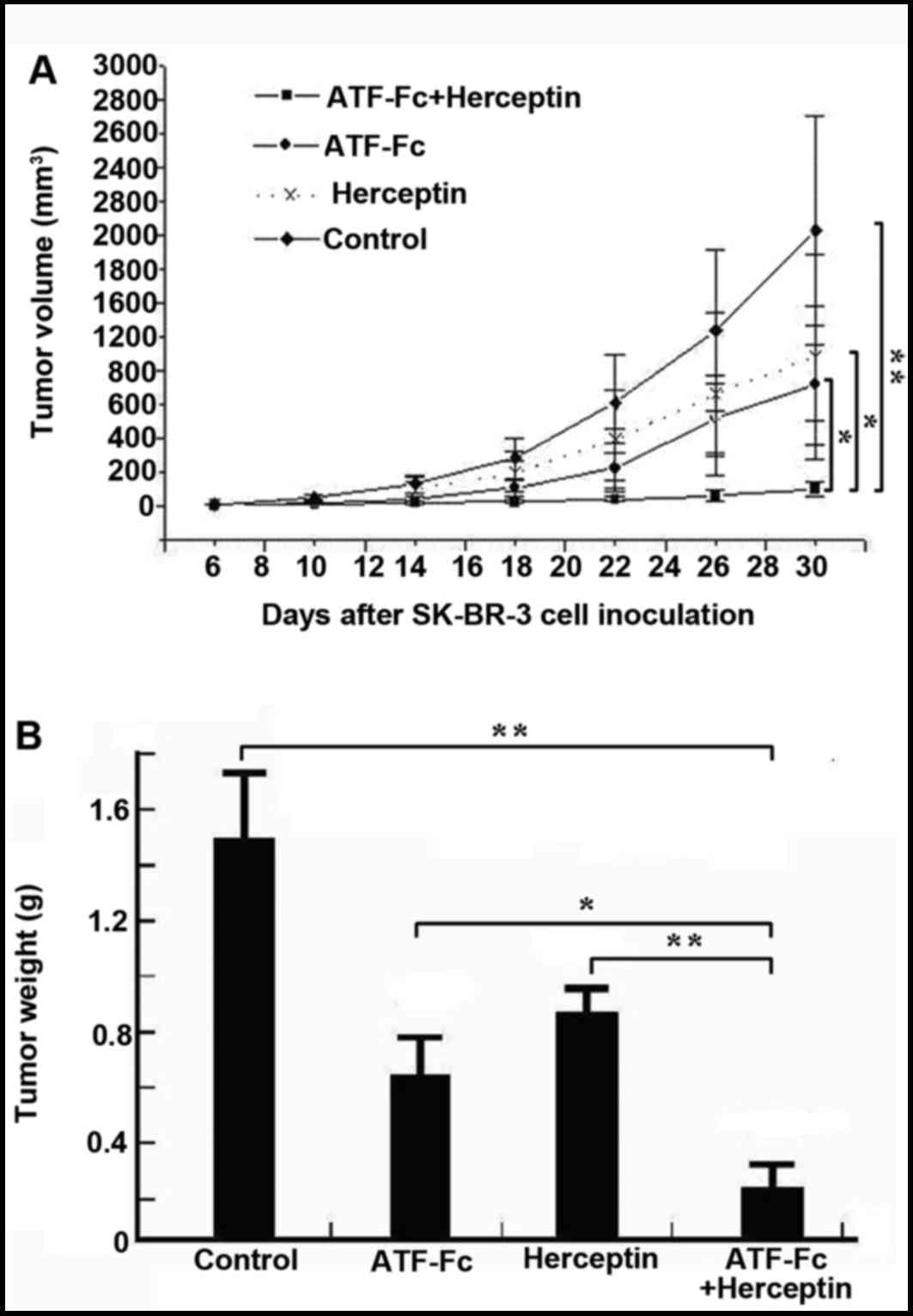
combination with ATF-Fc and trastuzumab. As illustrated in Fig. 4A, compared with the control group,
treatment with ATF-Fc in combination with trastuzumab significantly
reduced SK-BR-3 tumor volume (P<0.01). The inhibitory effect of
the combination treatment was significantly higher, as compared
with treatment with either ATF-Fc alone (P<0.05) or trastuzumab
alone (P<0.05). Fig. 4B indicates
that the treatment of ATF-Fc in combination with trastuzumab
significantly reduced the SK-BR-3 tumor volume compared with the
control group, ATF-Fc alone and trastuzumab alone (P<0.05 and
P<0.01, respectively). The tumor inhibition rates were 60, 74,
and 84% for ATF-Fc alone, trastuzumab alone and combination
treatments, respectively.
Inhibitory effect on liver metastasis
in tumor-bearing nude mice
Following the administration of the drugs to the
mice for three weeks, the mice were sacrificed and dissected. The
tissues were subsequently evaluated for liver metastasis status,
and the number of metastatic foci was determined. Compared with the
control group, treatment with a combination of ATF-Fc and
trastuzumab had a significant inhibitory effect on liver metastasis
in the tumor-bearing nude mice. The differences in the rate of
liver metastasis and the number of metastatic foci between the
group treated with trastuzumab or ATF-Fc or ATF-Fc plus trastuzumab
and the control group were statistically significant. In addition,
the administration of ATF-Fc in combination with trastuzumab
significantly reduced the rate of liver metastasis and the number
of metastatic foci, compared with ATF-Fc or trastuzumab alone
(Table I). These results suggest that
treatment with ATF-Fc in combination with trastuzumab may increase
the inhibitory effect on breast cancer metastasis.
 | Table I.Inhibitory effect of trastuzumab,
ATF-Fc or ATF-Fc plus trastuzumab on liver metastasis. |
Table I.
Inhibitory effect of trastuzumab,
ATF-Fc or ATF-Fc plus trastuzumab on liver metastasis.
| Group | Metastasis rate
(%) | Number of foci |
|---|
| Control | 71.4 (5/7) | 69.8±11.9 |
| aTrastuzumab (6 mg/kg) | 42.9 (3/7) | 52.5±9.6 |
| aATF-Fc (10 mg/kg) | 28.6 (2/7) | 44.8±5.1 |
| a–cATF-Fc plus trastuzumab | 14.3 (1/7) | 13.7±4.8 |
Discussion
Chemotherapy is an effective method for treating
malignant tumors. However, due to the presence of primary and
secondary drug resistance, tumors in numerous patients ultimately
progress and their health deteriorates (22). Therefore, studies in oncology are
constantly investigating and searching for more effective
therapeutics.
Targeted (biological) therapy has an increasingly
critical role in treating malignant tumors. For example, rituximab
specifically targets cluster of differentiation (CD)20 (23). Trastuzumab specifically targets HER-2
(24), and bevacizumab specifically
targets vascular endothelial growth factor (25,26).
Rituximab, trastuzumab and bevacizumab have already exhibited
notable efficacy in the treatment of multiple human malignant
tumors (27).
The uPA-uPAR system has an essential role in tumor
growth and metastasis, and overexpression of these molecules is
strongly associated with poor prognosis in a variety of malignant
tumors (12–13,28). The
monoclonal antibody against uPA or uPAR has been demonstrated to be
effective in inhibiting the proliferation, migration and
invasiveness of cancer in vitro (29,30).
Another known antagonist of uPA-uPAR is the ATF of
higher-molecular-weight uPA, which contains an epidermal growth
factor-like domain and a kringle domain (31). ATF is a fragment of uPA without enzyme
activity that exhibits a strong affinity for uPAR (32). In 1993, Crowley et al (33) constructed an antibody-like molecule
that is comprised of 1–137 amino acids (AA) of uPA and is
conjugated with the Fc fragment of an IgG molecule. The binding
target of this molecule was uPAR, and the molecule exhibited an
inhibitory effect on tumor cell dissemination (33). However, the anti-tumor effect of this
molecule is not ideal. Further study revealed that the uPA fragment
in Crowley's construct contained the enzymatic site for plasmin
(uPA, AA135 and 136). This defect meant that the molecule is
susceptible to enzymatic digestion in tumor tissues that are rich
in plasmin, thus preventing it from targeting uPAR (17).
In order to overcome this defect in the present
study, a novel ATF-Fc fragment that did not contain the enzymatic
site for plasmin was constructed. In vitro and in
vivo studies demonstrated that ATF-Fc exhibits an inhibitory
effect on tumor growth, metastasis and angiogenesis. These findings
indicate that ATF-Fc is a promising antibody drug with the
potential to serve a crucial role in anti-tumor therapy in the
future (17).
Overexpression of HER-2 or uPAR in breast cancer has
been associated with an increased aggressive primary tumor
phenotype and a poor prognosis (4,34).
Although the role of the HER-2 oncogene has been recognized,
≤70% of HER-2-positive breast cancer patients exhibit primary
resistance to trastuzumab (35).
Additionally, 70% of the patients who effectively respond to the
treatment eventually develop drug resistance-associated relapse
within one year (36,37). A number of studies have demonstrated
that multiple genetic abnormalities are involved in tumor
initiation, development and metastasis (38,39).
Therefore, ideal anti-tumors effects are unlikely to be achieved by
blocking or inhibiting the expression of a single oncogene. A
previous study suggested that the overexpression and gene
amplification of HER-2 and uPAR occurred most frequently in the
same individual tumor cells in primary breast carcinoma, and in the
circulating tumor cells of patients with advanced breast carcinoma
(40). Pierga et al (41) reported comparable results in
disseminated tumor cells from the bone marrow of patients with
breast cancer. These findings indicate that targeting HER-2 and
uPAR together may provide a more efficient therapy for patients
with breast cancer.
A previous study by the authors (17) demonstrated that treatment with ATF-Fc
resulted in significant suppression of the growth and metastasis of
xenograft human tumors (MCF-7 breast cancer and BGC-823 gastric
cancer) in athymic nude mice. ATF-Fc could specifically inhibit the
uPA-uPAR interaction, but trastuzumab did not have this effect. The
present study indicates that when combined with trastuzumab, a drug
used to target HER-2, ATF-Fc can synergistically inhibit
HER-2-positive breast cancer cell proliferation and metastasis by
interfering with the uPA-uPAR system. To identify an effective
biological therapeutic method, the SK-BR-3 breast cancer cell line
was selected, which overexpresses uPA/uPAR and HER-2, to establish
a tumor-bearing animal model. Subsequently, the effect of ATF-Fc in
combination with trastuzumab on the growth and metastasis of the
tumors was determined by interfering with two pathways.
In the present study, significant inhibition of
breast cancer cell proliferation has been demonstrated in
vitro, and significant inhibition of tumor metastasis has been
observed in vivo. The inhibitory effect of the combination
treatment with trastuzumab and ATF-Fc was significantly increased
compared with treatment with trastuzumab or ATF-Fc alone. However,
the mechanisms of action of the combination treatment remain
unknown.
Several studies (42–44) have
suggested that epidermal growth factor receptor (EGFR) may function
as a transducer of the signaling between uPAR to ERK, which
indicates the existence of crosstalk between EGFR and uPAR
signaling pathways. By contrast, it has been demonstrated that
depletion of HER-2 and uPAR by small interfering RNA is able to
suppress mitogen-activated protein kinase signaling pathways,
resulting in a decrease of extracellular-signal-regulated kinase
(ERK) activity and a high p38/ERK activity ratio, which is involved
in the synergistic suppression of breast cancer cell growth
(45). Therefore, crosstalk between
HER-2 and uPAR signaling pathways may exist, and may be a potential
underlying mechanism of the synergistic anti-tumor activity
profiles of combined ATF-Fc and trastuzumab treatments in breast
cancer cells. Together, these findings suggest that ATF-Fc has high
therapeutic value for use in combination with trastuzumab in
patients with HER-2-positive breast cancer, where primary
resistance to trastuzumab is also present.
One limitation of the current study was that only
one cell line, which overexpresses HER-2, was used to investigate
the synergistic inhibitory effects of ATF-Fc and trastuzumab on
tumor growth and invasion. Additionally, the potential underlying
mechanism of the synergistic inhibitory effects has yet to be
determined. These critical issues must be addressed in future
investigations.
In summary, the present study demonstrates that a
combination of ATF-Fc (an antibody-like molecule that targets uPAR)
and trastuzumab, (a monoclonal antibody that targets HER-2)
exhibits synergistic inhibitory effects on tumor growth and
metastasis, and may serve a key role in the treatment of cancer in
the future.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81071839 and
30971297).
Glossary
Abbreviations
Abbreviations:
|
HER-2
|
human epidermal growth factor receptor
2
|
|
uPA
|
urokinase-type plasminogen
activator
|
|
CHO
|
Chinese hamster ovary
|
|
SPF
|
specific-pathogen-free
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
IHC
|
immunohistochemistry
|
|
FISH
|
fluorescence in situ
hybridization
|
References
|
1
|
Saji S: Evolving approaches to metastatic
breast cancer patients pre-treated with anthracycline and taxane.
BioDrugs. 27:469–478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Riou G, Mathieu MC, Barrois M, Le Bihan
ML, Ahomadegbe JC, Bénard J and Lê MG: c-erbB-2 (HER-2/neu) gene
amplification is a better indicator of poor prognosis than protein
over-expression in operable breast-cancer patients. Int J Cancer.
95:266–270. 2001.PubMed/NCBI
|
|
3
|
Pegram MD, Finn RS, Arzoo K, Beryt M,
Pietras RJ and Slamon DJ: The effect of HER-2/neu overexpression on
chemotherapeutic drug sensitivity in human breast and ovarian
cancer cells. Oncogene. 15:537–547. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang GS, Zhu H and Bi SJ: Pathological
features and prognosis of different molecular subtypes of breast
cancer. Mol Med Report. 6:779–782. 2012. View Article : Google Scholar
|
|
5
|
Heyerdahl H, Abbas N, Brevik EM, Mollatt C
and Dahle J: Fractionated therapy of HER2-expressing breast and
ovarian cancer xenografts in mice with targeted alpha emitting
227Th-DOTA-p-benzyl-trastuzumab. PLoS One. 7:e423452012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vu T and Claret FX: Trastuzumab: Updated
mechanisms of action and resistance in breast cancer. Front Oncol.
2:622012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maeda S, Saimura M, Minami S, Kurashita K,
Nishimura R, Kai Y, Yano H, Mashino K, Mitsuyama S, Shimokawa M, et
al: Efficacy and safety of eribulin as first-to third-line
treatment in patients with advanced or metastatic breast cancer
previously treated with anthracyclines and taxanes. Brest.
32:66–72. 2017. View Article : Google Scholar
|
|
8
|
Berger C, Madshus IH and Stang E:
Cetuximab in combination with anti-human IgG antibodies efficiently
down-regulates the EGF receptor by macropinocytosis. Exp Cell Res.
318:2578–2591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng S, Tripathy D, Shete S, Ashfaq R,
Saboorian H, Haley B, Frenkel E, Euhus D, Leitch M, Osborne C, et
al: uPAR and HER-2 gene status in individual breast cancer cells
from blood and tissues. Proc Natl Acad Sci USA. 103:17361–17365.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su CY, Liu YP, Yang CJ, Lin YF, Chiou J,
Chi LH, Lee JJ, Wu AT, Lu PJ, Huang MS and Hsiao M: Plasminogen
activator inhibitor-2 plays a leading prognostic role among
protease families in non-small cell lung cancer. PLoS One.
10:e01334112015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Märkl B, Renk I, Oruzio DV, Jähnig H,
Schenkirsch G, Schöler C, Ehret W, Arnholdt HM, Anthuber M and
Spatz H: Tumour budding, uPA and PAI-1 are associated with
aggressive behaviour in colon cancer. J Surg Oncol. 102:235–241.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim TD, Song KS, Li G, Choi H, Park HD,
Lim K, Hwang BD and Yoon WH: Activity and expression of
urokinase-type plasminogen activator and matrix metalloproteinases
in human colorectal cancer. BMC Cancer. 6:2112006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Foekens JA, Peters HA, Look MP, Portengen
H, Schmitt M, Kramer MD, Brünner N, Jänicke F, Meijer-van Gelder
ME, Henzen-Logmans SC, et al: The urokinase system of plasminogen
activation and prognosis in 2780 breast cancer patients. Cancer
Res. 60:636–643. 2000.PubMed/NCBI
|
|
14
|
Look MP, van Putten WL, Duffy MJ, Harbeck
N, Christensen IJ, Thomssen C, Kates R, Spyratos F, Fernö M,
Eppenberger-Castori S, et al: Pool analysis of prognostic impact of
urokinase-type plasminogen activator and its inhibitor PAI-1 in
8377 breast cancer patients. J Natl Cancer Inst. 94:116–128. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laufs S, Schumacher J and Allgayer H:
Urokinase-receptor (u-PAR): An essential player in multiple games
of cancer: A review on its role in tumors progression, invasion,
metastasis, proliferation/dormancy, clinical outcome and minimal
residual disease. Cell Cycle. 5:1760–1771. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sidenius N and Blasi F: The urokinase
plasminogen activator system in cancer: Recent advances and
implication for prognosis and therapy. Cancer Metastasis Rev.
22:205–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu XW, Duan HF, Gao LH, Pan SY, Li YM, Xi
Y, Zhao SR, Yin L, Li JF, Chen HP and Wu CT: Inhibition of tumors
growth and metastasis by ATF-Fc, an engineered antibody targeting
urokinase receptor. Cancer Biol Ther. 7:651–659. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu X, Xiao C, Huang Z, Guo Z, Zhang Z and
Li Z: Pilot production of u-PA with porous microcarrier cell
culture. Cytotechnology. 33:13–19. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanaka M, Obata T and Sasaki T: Evaluation
of antitumor effects of docetaxel (Taxotere) on human gastric
cancer in vitro and in vivo. Eur J Cancer. 32A:226–230. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Evans BD, Smith IE, Shorthouse AJ and
Millar JL: A comparison of the response of human lung carcinoma
xenografts to vindesine and vincristine. Br J Cancer. 45:466–468.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wolff AC, Hammond ME, Schwartz JN, Hagerty
KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer
A, et al: American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 test in breast cancer. J Clin Oncol. 25:118–145.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glasgow MD and Chougule MB: Recent
developments in active tumor targeted multifunctional nanoparticles
for combination chemotherapy in cancer treatment and imaging. J
Biomed Nanotechnol. 11:1859–1898. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Griffiths R, Mikhael J, Gleeson M, Danese
M and Dreyling M: Addition of rituximab to chemotherapy alone as
first-line therapy improves overall survival in elderly patients
with mantle cell lymphoma. Blood. 118:4808–4816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Seal MD, Speers CH, O'Reilly S, Gelmon KA,
Ellard SL and Chia SK: Outcomes of woman with early-stage breast
cancer receiving adjuvant trastuzumab. Curr Oncol. 19:197–201.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Altomare I, Bendell JC, Bullock KE, Uronis
HE, Morse MA, Hsu SD, Zafar SY, Blobe GC, Pang H, Honeycutt W, et
al: A phase II trial of bevacizumab plus everolimus for patients
with refractory metastatic colorectal cancer. Oncologist.
16:1131–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fleitas T, Martínez-Sales V, Vila V,
Reganon E, Mesado D, Martín M, Gómez-Codina J, Montalar J and
Reynés G: Circulating endothelial cells and microparticles as
prognostic markers in advanced non-small cell lung cancer. PLoS
One. 7:e473652012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vacchelli E, Pol J, Bloy N, Eggermont A,
Cremer I, Fridman WH, Galon J, Marabelle A, Kohrt H, Zitvogel L, et
al: Trial watch: Tumor-targeting monoclonal antibodies for
oncological indications. Oncoimmunology. 4:e9859402015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kargiotis O, Chetty C, Gogineni V, Gondi
CS, Pulukuri SM, Kyritsis AP, Gujrati M, Klopfenstein JD, Dinh DH
and Rao JS: uPA/uPAR downregulation inhibits radiation-induced
migration, invasion and angiogenesis in IOMM-Lee menigioma cells
and decreases tumors growth in vivo. Int J Oncol. 33:937–947.
2008.PubMed/NCBI
|
|
29
|
Rabbani SA and Gladu J: Urokinase receptor
antibody can reduce tumors volume and detect the presence of occult
tumors metastases in vivo. Cancer Res. 62:2390–2397.
2002.PubMed/NCBI
|
|
30
|
Kobayashi H, Gotoh J, Shinohara H, Moniwa
N and Terao T: Inhibition of the metastasis of Lewis lung carcinoma
by antibody against urokinase-type plasminogen activator in the
experimental and spontaneous metastasis model. Thromb Haemost.
71:474–480. 1994.PubMed/NCBI
|
|
31
|
Beloglazova IB, Beabealashvilli RSh,
Gursky YG, Bocharov EV, Mineev KS, Parfenova EV and Tkachuk VA:
Structural investigations of recombinant urokinase growth
factor-like domain. Biochemistry (Mosc). 78:517–530. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bifulco K, Longanesi-Cattani I, Franco P,
Pavone V, Mugione P, Di Carluccio G, Masucci MT, Arra C, Pirozzi G,
Stoppelli MP and Carriero MV: Single amino acid substitutions in
the chemotactic sequence of urokinase receptor modulate cell
migration and invasion. PLoS One. 7:e448062012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Crowley CW, Cohen RL, Lucas BK, Liu G,
Shuman MA and Levinson AD: Prevention of metastasis by inhibition
of the urokinase receptor. Proc Natl Acad Sci USA. 90:5021–5025.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chandran Indira V, Eppenberger-Castori S,
Venkatesh T, Vine KL and Ranson M: HER2 and uPAR cooperativity
contribute to metastatic phenotype of HER2-positive breast cance.
Oncoscience. 2:207–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oliveras-Ferraros C, Corominas-Faja B,
Cufí S, Vazquez-Martin A, Martin-Castillo B, Iglesias JM,
López-Bonet E, Martin AG and Menendez JA: Epithelial-to-mesenchymal
transition (EMT) confers primary resistance to trastuzumab
(Herceptin). Cell Cycle. 11:4020–4032. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Capelan M, Pugliano L, De Azambuja E,
Bozovic I, Saini KS, Sotiriou C, Loi S and Piccart-Gebhart MJ:
Pertuzumab: New hope for patients with HER2-positive breast cancer.
Ann Oncol. 24:273–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gajria D and Chandarlapaty S:
HER2-amplified breast cancer: Mechanisms of trastuzumab resistance
and novel targeted therapies. Expert Rev Anticancer Ther.
11:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baylin SB and Jones PA: A decade of
exploring the cancer epigenome-biological and translational
implications. Nat Rev Cancer. 11:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Esteller M: Cancer epigenomics: DNA
methylomes and histone-modification maps. Nat Rev Genet. 8:286–298.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Meng S, Tripathy D, Shete S, Ashfaq R,
Saboorian H, Haley B, Frenkel E, Euhus D, Leitch M, Osborne C, et
al: uPA and HER-2 gene status in individual breast cancer cells
from blood and tissues. Proc Natl Acad Sci USA. 103:17361–17365.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pierga JY, Bonneton C, Magdelénat H,
Vincent-Salomon A, Nos C, Boudou E, Pouillart P, Thiery JP and de
Cremoux P: Real-time quantitative PCR determination of
urokinase-type plasminogen activator receptor (uPAR) expression of
isolated micrometastatic cells from bone marrow of breast cancer
patients. Int J Cancer. 114:291–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu D, Ghiso Aguirre J, Estrada Y and
Ossowski L: EGFR is a transducer of the urokinase receptor
initiated signal that is required for in vivo growth of a human
carcinoma. Cancer Cell. 1:445–457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jo M, Thomas KS, O'Donnell DM and Gonias
SL: Epidermal growth factor receptor-dependent and -independent
cell-signaling pathways originating from the urokinase receptor. J
Biol Chem. 278:1642–1646. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guerrero J, Santibañez JF, González A and
Martínez J: EGF receptor transactivation by urokinase receptor
stimulus through a mechanism involving Src and matrix
metalloproteinases. Exp Cell Res. 292:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li C, Cao S, Liu Z, Ye X, Chen L and Meng
S: RNAi-mediated downregulation of uPAR synergizes with targeting
of HER2 through the ERK pathways in breast cancer cells. Int J
Cancer. 127:1507–1516. 2010. View Article : Google Scholar : PubMed/NCBI
|