Introduction
Colorectal cancer (CRC) is one of the most common
causes of cancer-associated mortality worldwide (1,2). CRC has a
poor prognosis due to the insidious symptomatology, rapid
progression and late clinical presentation, causing a poor 5-year
overall survival rate (3,4), which is <10% in advanced stages of
CRC (5). Although a number of novel
therapeutic strategies targeting epidermal growth factor receptor
(EGFR) and vascular endothelial growth have been identified, the
most frequently utilized frontline regimen for patients with
metastatic CRC is a combination of oxaliplatin (OXA) and
fluoro-pyrimidines (6,7). The cytotoxic effects of OXA on cancer
cells mainly depend on the formation of platinum-DNA adducts, which
may result in replication blockade, DNA damage and the activation
of programmed cell death of cancer cells (8). In the clinic, not all patients with CRC
are sensitive to OXA therapy due to developing drug resistance,
which is the main obstacle for therapeutic effectiveness (5). However, the mechanisms for the
OXA-induced drug resistance in CRC cancer cells are elusive.
The lysosome-associated protein transmembrane
(LAPTM) protein family includes LAPTM4α, LAPTM4β and LAPTM5
(9). Among these LAPTMs, LAPTM4α and
LAPTM4β are ubiquitously expressed, and LAPTM5 is expressed in
immune cells (10,11). Previous studies have reported that
LAPTM4β mediates multidrug resistance (MDR) in cancer cells via
interacting with multidrug resistance protein (12,13). A
previous study reported that LAPTM4β is overexpressed in numerous
cancer cells and is involved in tumorigenic processes (14). Therefore, it was speculated that
LAPTM4β may enhance the proliferation and/or detoxification
potential of cancer cells. Recently, Xia et al (15) demonstrated that LAPTM4β-35 was
significantly over-expressed in various cancers including
hepatocellular carcinoma, breast cancer, cervical carcinoma,
gallbladder carcinoma and ovarian carcinoma. Kang et al
(16) reported that LAPTM4β-35
overexpression may be an independent factor in CRC prognosis, which
may be a critical potential biomarker for CRC.
The present study attempted to establish OXA
drug-resistant CRC cell lines, and detect the expression of LAPTM4β
and LAPTM4β-35. Therefore, the present study aimed to investigate
the drug resistance mechanism of OXA in CRC cell lines, and
identify a specific and sensitive biomarker for CRC.
Materials and methods
Cell culture
The CRC cell line, including 47 strains of
Oxaliplatin resistant HT-29 (HT-29/L-OHP) cells and 31 strains of
HT-29 cells were obtained from the Shanghai Institute for
Biological Sciences, Chinese Academy of Science (Shanghai, China).
HT-29 cells were maintained and cultured in RPMI-1640 growth medium
(Gibco BRL; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Gibco BRL; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml streptomycin
at 37°C in an atmosphere containing 5% CO2.
Establishment of drug-resistant cell
lines
The OXA-resistant cell line was established in
General Surgery laboratory of the Second Affiliated Hospital of
Harbin Medical University (Harbin, China) over a period of 12
months by continuous exposure of the HT-29 cell line to gradually
increasing concentrations of OXA (4–15 µmol/l) according to a
previous study (17). The established
OXA-resistant cell line was termed HT-29/L-OHP. The HT-29 cell line
was passaged three times at each drug concentration and the cell
vials were frozen at each increase in drug concentration. Prior to
the following experiments, the HT-29 cells were maintained in
no-drug RPMI-1640 medium for at least 7 days. The established
HT-29/L-OHP cells were cultured in RPMI-1640 medium with 4
µmol/lOXA solution (final concentration) for subsequent
experiments.
Cell morphology observation
The cells were stained using the 10% Giemsa's
staining solution at room temperate for 15 min. The cell morphology
of the HT-29/L-OHP and HT-29 cells in the logarithmic growth phase
were observed and captured under an inverted fluorescence
microscope, as previously described (17).
Flow cytometry
The HT-29 cells were harvested by scraping the cells
and centrifuging at the speed of 500 × g for 5 min at room
temperature. Subsequently, the cells were seeded on 6-well plates
at a density of 1×106 cells/well. Cell apoptosis was
evaluated by flow cytometry, which monitors annexin V-fluorescein
isothiocyanate (FITC) binding (Trevigen, Inc., Gaithersburg, MD,
USA) and propidium iodide (PI; Trevigen, Inc.) uptake
simultaneously. Subsequent to culturing for 24 h at 37°C, the HT-29
cells were harvested by scraping the cells and centrifuging at the
speed of 500 × g for 5 min at room temperature. Subsequently, the
cells were resuspended in annexin V-FITC (at a concentration of 1X)
and PI (at a concentration of 5 µg/ml) in the dark at room
temperature for 15 min. Subsequently, the cell samples were
examined by FACScan flow cytometry (BD Biosciences, Franklin Lakes,
NJ, USA). The produced annexin V-FITC fluorescence was monitored
via the 530/30-nm band filter (FL-1), while the produced PI
fluorescence was monitored via the 585/42-nm band filter
(FL-2).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In order to examine the mRNA expression of LAPTM4β,
a RT-qPCR assay was performed. Primers sequences are presented in
Table I. β-actin was used as the
internal control. Total RNA was extracted with the RNA simple Total
RNA kit (Tiangen Biotech Co., Ltd., Beijing, China) according to
the manufacturer's protocol. The integrity of RNA was checked by 2%
agarose gel electrophoresis and visualized using the ethidium
bromide. The concentration of the obtained RNA was examined with an
ultraviolet spectrophotometer (DU800; Beckman Coulter, Inc., Brea,
CA, USA) according to the manufacturer's protocol. RNA (~2 µg) was
reverse transcribed following the protocol of the PrimeScript™ II
1ststrand cDNA Synthesis kit (catalog no., 6210A; Takara Bio, Inc.,
Otsu, Japan). The obtained complementary DNAs were amplified by
using the Sybgreen qPCR kit (Tiangen Biotech Co. Ltd.) in a volume
of 20 µl under the following amplification conditions: 95°C for 3
min, 95°C for 10 sec and 60°C for 30 sec, for 40 cycles. The
temperature was then successively increased between 70 and 90°C
(intervals of 0.5°Cevery5 sec). The melting curve assay was
employed to demonstrate the purity of the PCR products, as
described previously study (17). The
experiments were performed in at least three wells and repeated at
least three times. Subsequent to electrophoresis on 1.4% agarose
gels and visualized using the ethidium bromide, the images were
digitally captured with acharge coupled device camera. The captured
images were analyzed by NIH Imager beta (version 2.0; Matrix
Science, Inc., Boston, MA, USA). The relative levels of target
genes were calculated using the 2−ΔΔCq method (18).
 | Table I.Primers for the LAPTM4β and β-actin
genes. |
Table I.
Primers for the LAPTM4β and β-actin
genes.
| Gene | Primers |
|---|
| LAPTM4β |
|
|
Forward |
5′-GGAAGCAGGACAGCCAACTT-3′ |
|
Reserve |
5′-TTATTCTCGATCTCACAACCAAAC-3′ |
| β-actin |
|
|
Forward |
5′-CCTGTGGCATCCACGAAACT-3′ |
|
Reserve |
5′-GAAGCATTTGCGGTGGACGAT-3′ |
Western blot analysis
The HT-29 cellular lysates were harvested by 0.25%
trypsin/EDTA in PBS solution, pelleted by short centrifugation at
speed of 500 × g for 5 min at room temperature, and suspended in
lysis buffer (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) to
extract the total proteins. The concentration of the extracted
proteins was examined with a bicinchoninic acid protein
quantification kit (Beyotime Institute of Biotechnology, Haimen,
China). Cell lysates were separated by 15% SDS-PAGE (loading, 50
µg/well) and electrotransferred to polyvinylidene fluoride
membranes. Subsequently, membranes were blocked with 5% defatted
milk for 1 h at room temperature in PBS-Tween-20 solution (PBST;
PBS adjusted to pH 7.6, containing 0.05% Tween-20). The membranes
were incubated with rabbit anti-human LAPTM4β polyclonal antibody
(catalog no., ab82810; dilution, 1:2,000; Abcam, Cambridge, UK) and
mouse anti-human β-actin monoclonal antibody (catalog no.,
sc-130300; dilution, 1:3,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) in PBST at 4°C overnight. The membranes were
washed with PBST solution for 10 min three times. The membranes
were then incubated with horseradish peroxidase-conjugated goat
anti-rabbit polyclonal antibody (catalog no., ab6721; dilution,
1:2,000; Abcam) and goat anti-mouse polyclonal antibody (catalog
no., ab6789; dilution, 1:2,000; Abcam) in PBST at 37°C for 1 h. The
membranes were continuously washed with PBST three times, for 10
min each time. The reactive signals were visualized using an
enhanced chemiluminescence luminescence kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
immunoblot was scanned with GE Typhoon TM FLA 7000 (GE Healthcare
Life Sciences, Uppsala, Sweden) and images were captured. The
quantitative analysis for the immunoblot images was performed using
Image J software (version 2.0; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed with SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). The differences between
the groups were analyzed by a paired Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Cell morphology of HT-29/L-OHP
drug-resistant cell line
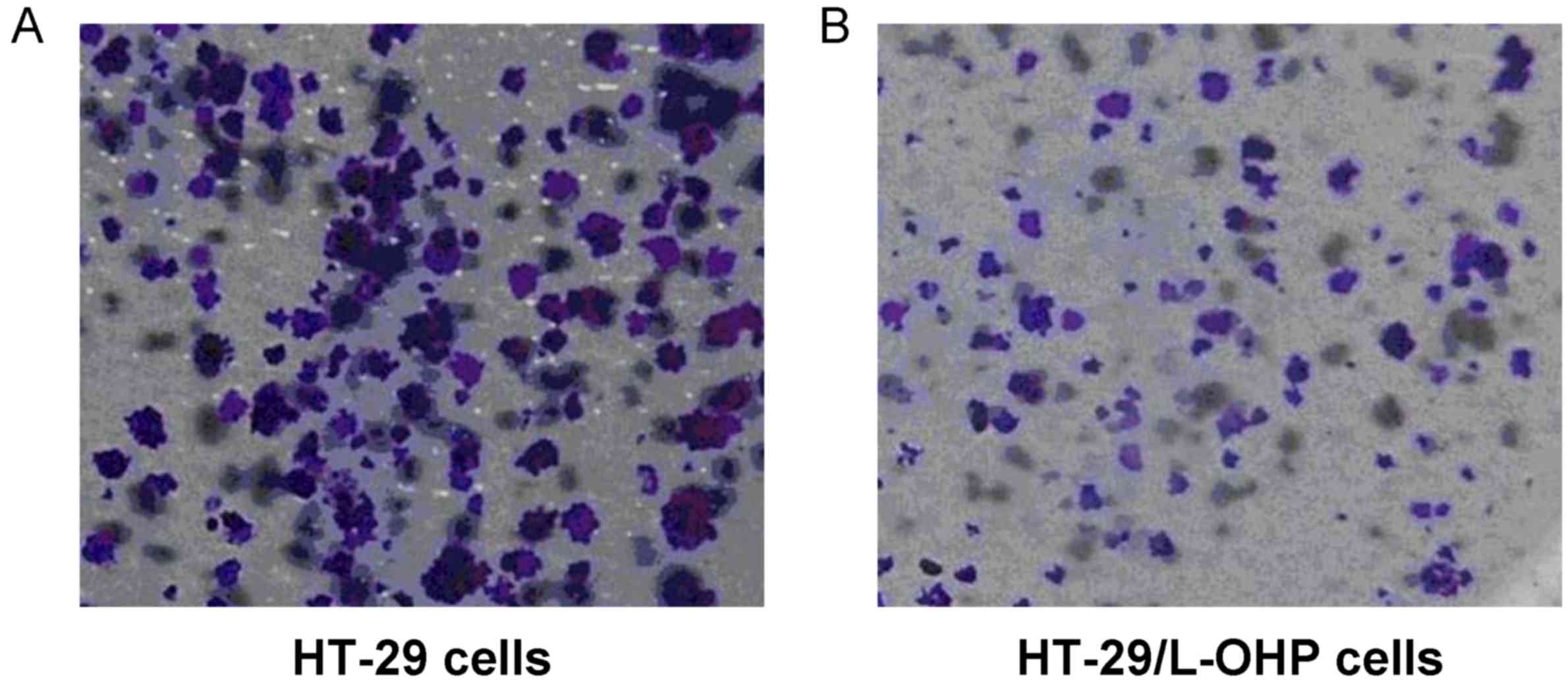
The cell morphology of the HT-29/L-OHP and HT-29
cells in the logarithmic growth phase was observed and captured
under an inverted fluorescence microscope. The results indicated
that the intercellular space among the HT-29 cells was small, with
aggregative growth (Fig. 1A).
However, the intercellular space among the HT-29/L-OHP cells was
large, with scattered growth (Fig.
1B).
Apoptotic rate is inhibited in
HT-29/L-OHP cells
The HT-29/L-OHP cell apoptotic rate was observed by
the annexin V-FITC/PI double staining method. The apoptotic rate
was calculated as the early apoptosis [quadrant (Q)4-upper left]
plus the late apoptosis (Q4-upper right). The results demonstrated
that the apoptotic rate in the HT-29/L-OHP cells (11.7%) was
significantly lower compared with that in the HT-29 cells (17.7%)
(P<0.05; Fig. 2). This result
suggests that the HT-29/L-OHP cells were resistant to OXA
application.
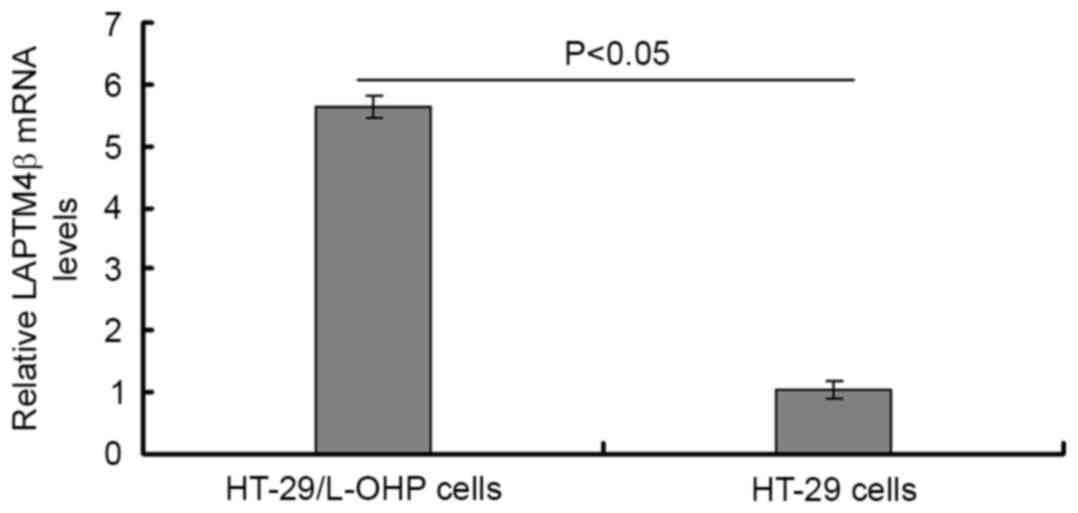
LAPTM4β mRNA expression is enhanced in
HT-29/L-OHP cells
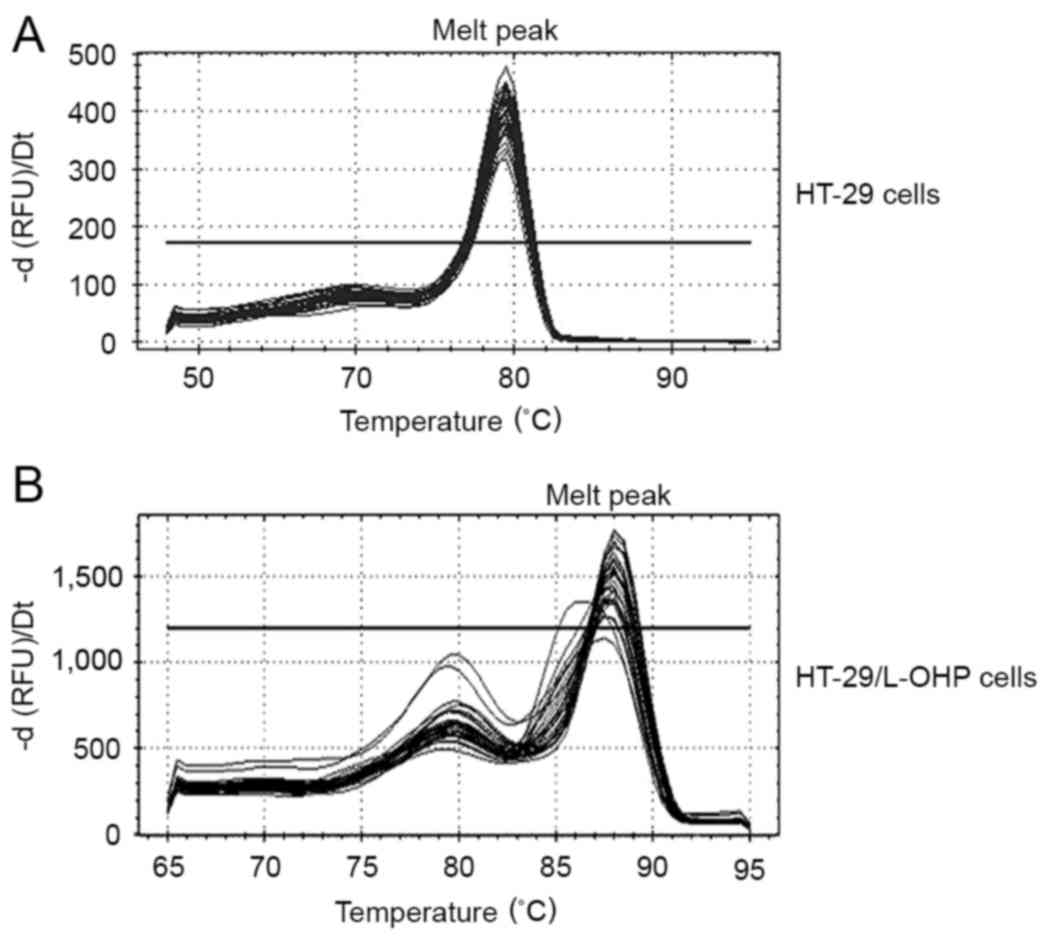
LAPTM4β mRNA was evaluated in 47 strains of
HT-29/L-OHP cells and 31 strains of HT-29 cells by qPCR assay. The
melting curve demonstrated that the melt peak is homogeneous;
therefore, the PCR product was purified (Fig. 3). The results indicated that the
LAPTM4β mRNA expression in HT-29/L-OHP cells was significantly
increased compared with that in the HT-29 cells (P<0.05;
Table II; Fig. 4).
 | Table II.Gene changes in the HT-29/L-OHP and
HT-29 cells analyzed by the 2−ΔΔCq method. |
Table II.
Gene changes in the HT-29/L-OHP and
HT-29 cells analyzed by the 2−ΔΔCq method.
| Cell line | Mean fold change in
gene expression | SD |
|---|
| HT-29/L-OHP | 5.61a | 0.22 |
| HT-29 | 1.00 | 0.15 |
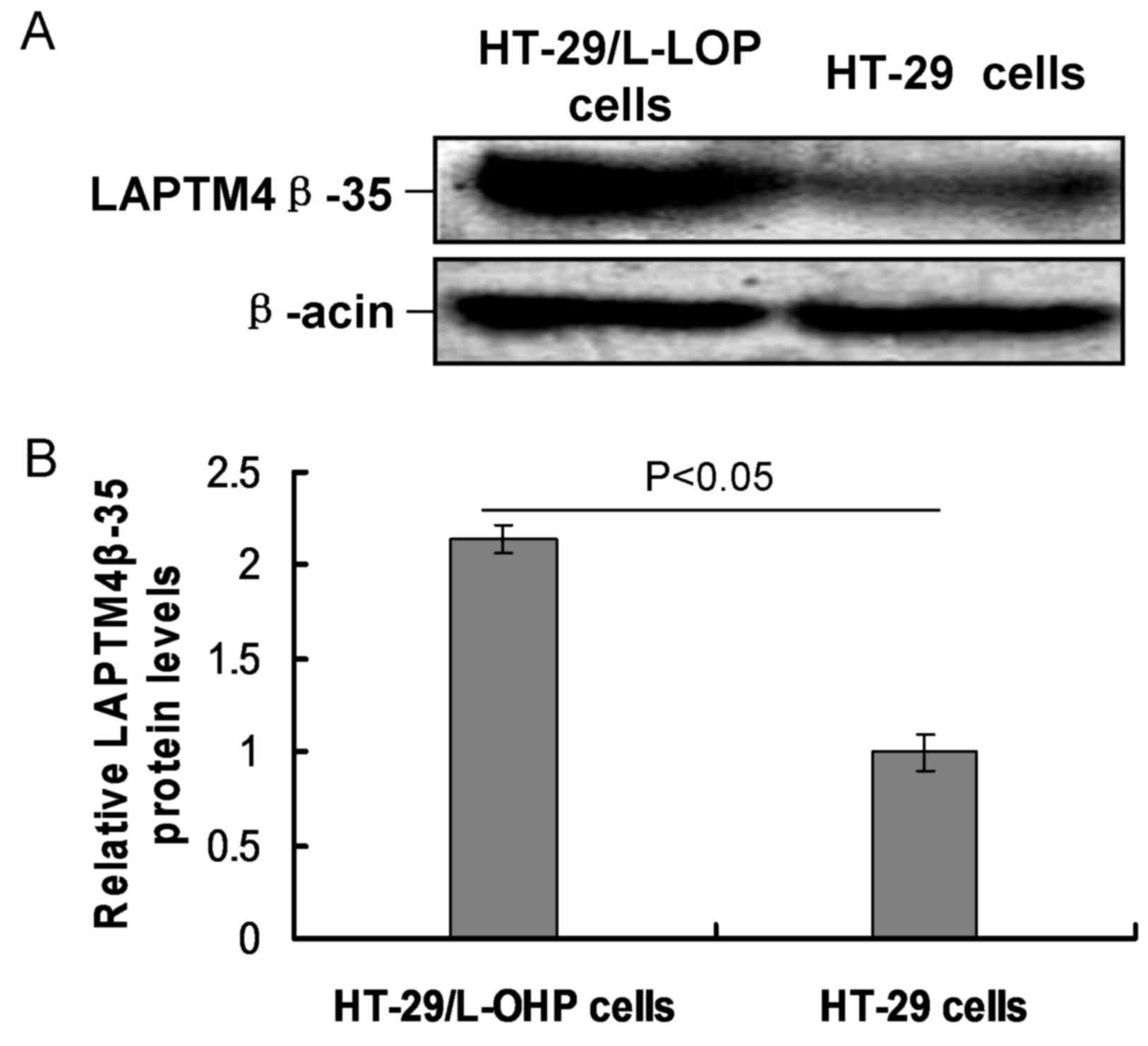
LAPTM4β-35 expression is increased in
HT-29/L-OHP cells
In the present study, LAPTM4β-35 expression was
examined by western blot analysis. The results demonstrated that
the relative expression of LAPTM4β-35 protein in HT-29/L-OHP cells
was significantly higher compared with that in the HT-29 cells
(P<0.05; Fig. 5).
Discussion
CRC is one of the most prevalent malignant tumors,
the reoccurrence and metastasis of which is usually treated by
chemotherapy (19). However, the
outcomes are usually poor for patients with CRC. MDR is one of the
most important factors leading to the reduction in chemotherapeutic
effects in the clinic (20–22). The classical mechanism of the MDR
protein mainly results in the overexpression of the adenosine
triphosphate-binding cassette family protein, which also interacts
with the drug to decrease the drug concentration to sub-lethal
levels (23,24).
LAPTM4β is a novel carcinoma-associated gene, which
has been mapped to chromosome 8q22.1, spanning at least 50 kb, is
composed of 7 exons and 6 introns (25). The LAPTM4β gene codes a 35-kDa
membrane glycoprotein (25). The
LAPTM4β gene-coded LAPTM4β-35 protein has been shown to be
upregulated in numerous cancers, including gastric cancer (26), prostate cancer (27), cervical carcinoma (28) and hepatocellular carcinoma (29), and performs an important role. Lee
et al (30) reported that
LAPTM4β upregulation is associated with activation of the
phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling
transduction pathway. Other studies also demonstrated that the
PI3K/Akt signaling transduction pathway could regulate and
strengthen the MDR (31,32). Therefore, it was speculated that
LAPTM4β may also be associated with CRC.
The establishment of drug-resistant cell lines may
provide a strategy for cancer therapy and neoplasm metastasis
mechanism (33). In the present
study, the drug-resistant CRC cell line HT-29/L-OHP was
established, which could stably grow in OXA solution at a
concentration of 15 µmol/l. LAPTM4β mRNA levels and LAPTM4β-35
protein levels were detected by qPCR and western blot analysis,
respectively. The results indicated that the mRNA and protein
expression levels in HT-29/L-OHP cells were significantly higher
compared with those in the HT-29 cells. These results indicate that
long-term OXA treatment could enhance LAPTM4β expression, which may
be an important drug-resistant mechanism for CRC therapy.
A previous study revealed that the upregulated
LAPTM4β in drug-resistant HT-29/L-OHP cells could increase the
efflux of OXA in tumor cells, which becomes a critical reason for
drug resistance in CRC cells (12).
Li et al (12) reported that
LAPTM4β induces MDR of cancer cells by promoting drug efflux via
the co-localization and interaction with P-glycoprotein (P-gp), and
anti-apoptosis by triggering the signaling pathway of PI3K/Akt.
Another study (34) also reported
that the PI3K/Akt pathway was involved in the modulation of
P-gp-mediated MDR in the mouse leukemic L1210/VCR cell line.
Furthermore, MDR may reversely affect the effects of LY294002 on
vincristine-induced apoptosis in HeLa cells. Li et al
(35) also revealed that MDR could
increase drug efflux and decrease the drug concentration entering
into nucleus by P-gp, and reduce drug-induced DNA injury and
drug-caused apoptosis. Tan et al (36) reported that LAPTM4β may promote EGFR
association with the autophagy inhibitor Rubicon, which in turn
disassociates Beclin 1 from Rubicon to initiate autophagy. Li et
al (37) reported that LAPTM4β
renders the tumor cells resistant to anthracycline by triggering
lysosome-mediated cell death. However, the drug-resistant mechanism
for OXA remains unknown.
In conclusion, the present study established a
stable OXA-resistant CRC cell line. The LAPTM4β gene and the
LAPTM4β-35 protein expression levels in this drug-resistant cell
line were significantly increased, compared with those in the
normal CRC cell line, which suggests that LAPTM4β is involved in
the MDR processes of CRC. Therefore, LAPTM4β may be become a novel
biomarker for drug resistance of CRC.
Acknowledgements
The present study was funded by the Science and
Technology Project of the Education Department of Heilongjiang
Province (grant no., 12541303) and a Project from the Natural
Foundation of Heilongjiang Province General Program (grant no.,
H2015104).
References
|
1
|
Takahashi K, Hosokawa M, Kasajima H,
Hatanaka K, Kudo K, Shimoyama N and Miyashita K: Anticancer effects
of fucoxanthin and fucoxanthinol on colorectal cancer cell lines
and colorectal cancer tissues. Oncol Lett. 10:1463–1467.
2015.PubMed/NCBI
|
|
2
|
Geryk E, Horváth T and Konecný M: The
expected worldwide burden of oesophagus, stomach and colorectal
cancers. Vnitr Lek. 57:1006–1011. 2011.(In Czech). PubMed/NCBI
|
|
3
|
Bahl R, Arora S, Nath N, Mathur M, Shukla
NK and Ralhan R: Novel polyumorphism in p21(waf1/cip1) cyclin
dependent kinase inhibitor gene: Association with human esophageal
cancer. Oncogene. 19:323–328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Klintrup K, Mäkinen JM, Kauppila S, Väre
PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ and
Mäkinen MJ: Inflammation and prognosis in colorectal cancer. Eur J
Cancer. 41:2645–2654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ginés A, Bystrup S, de Porras Ruiz V,
Guardia C, Musulén E, Martínez-Cardús A, Manzano JL, Layos L, Abad
A and Martínez-Balibrea E: PKM2 subcellular localiczation is
involved in oxaliplatin resistance acquisition in HT29 human
colorectal cancer cell lines. PLoS One. 10:e01238302015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weitz J, Koch M, Debus J, Höhler T, Galle
PR and Büchler MV: Colorectal cancer. Lancet. 365:153–165. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kelland L: The resurgence of
platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shao GZ, Zhou RL, Zhang QY, Zhang Y, Liu
JJ, Rui JA, Wei X and Ye DX: Molecular cloning and characterization
of LAPTM4B, a novel gene upregulated in hepatocellular carcinoma.
Oncogene. 22:5060–5069. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kasper G, Vogel A, Klaman I, Gröne J,
Petersen I, Weber B, Castaños-Vélez E, Staub E and Mennerich D: The
human LAPTM4b transcript is upregulated in various types of solid
tumours and seems to play a dual functional role during tumour
progression. Cancer Lett. 224:93–103. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hogue DL, Kerby L and Ling V: A mammalian
lysosomal membrane protein confers multidrug resistance upon
expression in Saccharomyces cerevisiae. J Biol Chem.
274:12877–12882. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Wei XH, Pan YP, Li HC, Yang H, He
QH, Pang Y, Shan Y, Xiong FX, Shao GZ and Zhou RL: LAPTM4B: A novel
cancer-associated gene motivates multidrug resistance through
efflux and activating PI3K/AKT signaling. Oncogene. 29:5785–5795.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, Zou L, Li Q, Haibe-Kains B, Tian R,
Li Y, Desmedt C, Sotiriou C, Szallasi Z, Iglehart JD, et al:
Amplification of LAPTM4B and YWHAZ contributes to chemotherapy
resistance and recurrence of breast cancer. Nat Med. 16:214–218.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Milkereit R and Rotin D: A role for the
ubiquitin ligase Nedd4 in membrane sorting of LAPTM4 proteins. PLoS
One. 6:e274782011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia LZ, Yin ZH, Ren YW, Shen L, Wu W, Li
XL, Guan P and Zhou BS: The relationship between LAPTM4B
polymorphisms and cancer risk in Chinese Han population: A
meta-analysis. Springerplus. 4:1792015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang Y, Yin M, Jiang W, Zhang H, Xia B,
Xue Y and Huang Y: Overexpression of LAPTM4B-35 is associated with
poor prognosis in colorectal carcinoma. Am J Surg. 204:677–683.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jensen NF, Stenvang J, Beck MK, Hanáková
B, Belling KC, Do KN, Viuff B, Nygård SB, Gupta R, Rasmussen MH, et
al: Establishment and characterization of models of chemotherapy
resistance in colorectal cancer: Towards a predictive signature of
chemoresistance. Mol Oncol. 9:1169–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crea F, Nobili S, Paolicchi E, Perrone G,
Napoli C, Landini I, Danesi R and Mini E: Epigenetics and
chemoresistance in colorectal cancer: An opportunity for treatment
tailoring and novel therapeutic strategies. Drug Resist Updat.
14:280–296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pasquier E, Kavallaris M and André N:
Metronomic chemotherapy: New rational for new directions. Nat Rev
Clin Oncol. 7:455–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Joyce H, McCann A, Clynes M and Larkin A:
Influence of multidrug resistance and drug transport proteins on
chemotherapy drug metabolism. Expert Opin Drug Metab Toxicol.
11:795–809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhirde AA, Chikkaveeraiah BV, Srivatsan A,
Niu G, Jin AJ, Kapoor A, Wang Z, Patel S, Patel V, Gorbach AM, et
al: Targeted therapeutic nanotubes influence the viscoelasticity of
cancer cells to overcome drug resistance. ACS Nano. 8:4177–4189.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Larsen AK, Escargueil AE and Skladanowski
A: Resistance mechanisms associated with altered intracellular
distribution of anticancer agents. Pharmacol Ther. 85:217–229.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gottesman MM: Mechanisms of cancer drug
resistance. Ann Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng XJ, Xu W, Zhang QY and Zhou RL:
Relationship between LAPTM4B gene polymorphism and susceptibility
of colorectal and esophageal cancers. Ann Oncol. 19:527–532. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng X, Zheng Z, Bu Z, Wu X, Zhang L,
Xing X, Wang X, Hu Y, Du H, Li L, et al: LAPTM4B-35, a cancer
related gene, is associated with poor prognosis in TNM stages I–III
gastric cancer patients. PLoS One. 10:e01215592015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Wei Q, Liu R, Qi S, Liang P, Qi
C, Wang A, Sheng B, Li L and Xu Y: Overexpression of LAPTM4B-35: A
novel marker of poor prognosis of prostate cancer. PLoS One.
9:e910692014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Meng F, Luo C, Hu Y, Yin M, Lin M, Lou G
and Zhou R: Overexpression of LAPTM4B-35 in cervical carcinoma: A
clinicopathologic study. Int J Gynecol Pathol. 29:587–593. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang H, Xiong F, Qi R, Liu Z, Lin M, Rui
J, Su J and Zhou R: LAPTM4B-35 is a novel prognostic factor of
hepatocellular carcinoma. J Surg Oncol. 101:363–369. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee JT Jr, Steelman LS and McCubrey JA:
Phosphatidylinositol 3′-kinase activation leads to multidrug
resistance protein 1 expression and subsequent chemoresistance in
advanced prostate cancer cells. Cancer Res. 64:8397–8404. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Knuefermann C, Lu Y, Liu B, Jin W, Liang
K, Wu L, Schmict M, Mills GB, Mendelsohn J and Fan Z:
HER2/PI-3K/Akt activation leads to a multidrug resistance in human
breast adenocarcinoma cells. Oncogene. 22:3205–3212. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdul-Ghani R, Serra V, Györffy B,
Jürchott K, Solf A, Dietel M and Schäfer R: The PI3K inhibitor
LY294002 blocks drug export from resistant colon carcinoma cells
overexpressing MRP1. Oncogene. 25:1743–1752. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rad SM, Langroudi L, Kouhkan F, Yazdani L,
Koupaee AN, Asgharpour S, Shojaei Z, Bamdad T and Arefian E:
Transcription factor decoy: A pre-transcriptional approach for gene
downregulation purpose in cancer. Tumour Biol. 36:4871–4881. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Barancík M, Bohácová V, Sedlák J, Sulová Z
and Breier A: LY294,002, a specific inhibitor of PI3K/Akt kinase
pathway, antagonizes P-glysoprotein-mediated multidrug resistance.
Eur J Pharm Sci. 29:426–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Zhou L, Li Q, Haibe-Kains B, Tian R,
Li Y, Desmedt C, Sotiriou C, Szallasi Z, Iglehart JD, et al:
Amplification of LAPTM4B and YWHAZ contributes to chemotherapy
resistance and recurrence of breast cancer. Nat Med. 16:214–218.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tan X, Thapa Q, Sun Y and Anderson RA: A
kinase-independent role for EGF receptor in autophagy initiation.
Cell. 160:145–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Zhang Q, Tian R, Wang Q, Zhao JJ,
Iglehart JD, Wang ZC and Richardson AL: Lysosomal transmembrane
protein LAPTM4B promotes autophagy and tolerance to metabolic
stress in cancer cells. Cancer Res. 71:7481–7489. 2011. View Article : Google Scholar : PubMed/NCBI
|