Introduction
The temporomandibular joint (TMJ) is the movable
joint that connects the skull and mandible, and plays an important
role in occlusion and mastication. TMJ is composed by the
mandibular condyle fitting into the glenoid fossa of the temporal
bone with the lining of the articular disc. This disc attaches to
the medial and lateral poles of the condyle and facilitates the
smooth movements of the mandible. During mouth opening, the condyle
rotates underneath the disc, whereas the condyle-articular disc
complex moves in a sliding movement relative to the temporal bone
to articular eminence. Thus, the TMJ serves as a ginglymoarthrodial
joint, and allows both wide vertical opening movement as well as
lateral excursive movement of the mandible (1).
In the surgery of a mandibular tumor, resection with
TMJ disarticulation and condylar removal are occasionally required
to achieve an adequate surgical margin. However, because of the
complex anatomic and functional features of the TMJ, the ideal
restoration of the condyle is still subject to controversy, and
reconstruction is a challenging issue for surgeons. The use of the
vascularized fibular flap is one of the standard treatment choices
for the reconstruction of the mandible. The method of condylar
reconstruction with direct placement of a vascularized fibular flap
into the glenoid fossa has been reported (2–13);
however, few data have been accumulated about the consequence of
TMJ restoration. In some reported cases, the unsuitable shape or
location of the graft disturbed TMJ function (7), and then affected the patients' quality
of life.
Recently, computer-aided technology has become
available and has given the surgeon the ability to plan the bony
reconstruction and to prepare intraoperative guidance
preoperatively, which can assist the surgeon (14). Although mandibular reconstruction
remains a challenging aspect that depends on the surgeon's
experience, intraoperative judgment, and technical speed, the
stereolithographic model can reduce learning curve associated with
neomandibular countering, enhance the levels of accuracy, and
accelerate a time-consuming operative step, which then allows a
more predictable reconstruction (15).
In this study, we report a stereolithographic
model-assisted reconstruction of the mandibular condyle with direct
placement of a vascularized free fibula into the glenoid fossa. The
stereolithographic model was used for an anatomical template for
mandibular condylar reconstruction. To investigate the
postoperative the morphological and functional outcomes of the TMJ,
clinical and radiographic examinations were performed. We also
administered a food scale questionnaire (16) to evaluate the masticatory function.
Furthermore, we reviewed the previous reports of condylar
reconstruction with a fibular flap and discussed the ideal
restoration of the condyle in the cases where the condyles are
resected.
Materials and methods
Five patients underwent mandibular resection
including the condyle and immediate reconstruction with a
vascularized fibular flap at our hospital from September 2013 to
July 2015 (Table I). There were two
men and three women whose ages ranged from 44 to 74 years (mean
age, 61 years). The lesions of five cases were located in the
posterior mandible; four cases were squamous cell carcinomas, and
one case was keratocystic odontogenic tumor.
 | Table I.Summary of the patients. |
The computed tomography (CT) scanner used in this
study was SOMATOM Definition AS+ (Siemens Healthcare Diagnostics,
Tokyo, Japan) or Discovery CT750 HD (GE Healthcare, Tokyo Japan).
All patients underwent CT scanning (1-mm slice thickness) of the
whole mandible and 5 cm above the glenoid fossa before surgery. The
data from CT scanning in Digital Imaging and Communications in
Medicine (DICOM) format were sent to a medical modeling company
(AHEAD Laboratories, Tokyo, Japan), and the stereolithography model
of the mandible was reconstructed. The resin template was also
fabricated from the stereolithography model in our dental
laboratory and sterilized for intraoperative use.
In all patients, a hemimandibulectomy was performed
with margins to isolate the tumor. Four patients also underwent
neck dissection. In all patients, the masseter and the internal
pterygoid muscles were resected, and the TMJ disc was preserved.
The defect patterns were classified into four cases of
CRBSH and one case of CRB; the letters C, R, B, and S
indicate defects of the condyle, ramus, body and symphyseal regions
and the superscript ‘H’ indicate a hemisymphyseal defect (17). The mean bony defects was 129 mm
(range, 93–141 mm).
All cases were reconstructed with fibular
osteocutaneous flaps. A 3D resin model was used to determine the
length of segments and the angle of bony reconstruction. A single
osteotomy of the fibula was performed to recreate the mandibular
shape. In all cases, the fibular end was placed into the glenoid
fossa under the TMJ disc. The residual mandibular segment was
temporarily fixed with maxillomandibular fixation (MMF), and the
fibular graft was fixed to the residual mandible with titanium
plates and screws. Then, the outer contour of the mandible
(inferior-lateral mandibular border) was restored. In all patients,
splints or dentures were utilized to keep occlusion during MMF.
Microvascular anastomoses were completed using a microscope. The
postoperative MMF was applied for 10–21 days (mean, 15.8 days).
After the release of MMF, the patients were allowed to start
masticating a soft diet, and they began active jaw mobilization
with a rehabilitation device of mouth opening. Four cases received
radiation therapy preoperatively and/or postoperatively. Panoramic
radiograph examination was performed during the follow-up
period.
The flap survival rates were examined and the
postoperative complications were reviewed. The facial contour was
evaluated by two of the authors. To investigate the postoperative
morphological and functional outcomes of the TMJ, radiographic and
clinical examinations were performed. Evaluation was performed at
least one year after the initial reconstruction. In the
radiographic evaluation, the morphologic changes of reconstructed
condyle were traced using panoramic radiographs, and the presence
of rounding, abnormal bone resorption, dislocation, and ankylosis
were examined. To evaluate the abnormal bone resorption, the
measurments were made between the same fixation point on the lower
border of the flap and the medial point on the fibular end
(7).
In the clinical evaluation, occlusion, maximum mouth
opening (MMO), mouth opening pattern (midline deviation), and TMJ
symptoms including pain, muscle pain with palpation (muscles of
mastication and others), jaw joint noises (clicking or crepitation)
and closed or open locking of the jaw of the patients were
examined.
To evaluate masticatory performance, tooth condition
was examined, and a food scale questionnaire (16) was performed (Table II). The number of natural teeth, and
the prior use of prostheses were examined. Tooth-to-tooth contact
was evaluated by occluding paper, and the pairs of occluding teeth
were noted. The food scale questionnaire used in this study has
demonstrated sufficient sensitivity for the evaluation of
functional differences between head and neck cancer patients
(16,18).
 | Table II.Food scale questionnairea. |
Table II.
Food scale questionnairea.
| Rating | Most difficult food
patient is able to masticate |
|---|
| 100 | Full diet (no
restrictions) |
| 90 | Peanuts |
| 80 | All meat |
| 70 | Carrots,
celery |
| 60 | Dry bread and
crackers |
| 50 | Soft, chewable
foodsb |
| 40 | Soft foods
requiring no chewingc |
| 30 | Pureed foods (in
blender) |
| 20 | Warm liquids |
| 10 | Cold liquids |
| 0 | Nonoral feeding
(tube fed) |
The study was approved by the Institutional Research
Board (Ethical Committee of the University of Fukui, Faculty of
Medical Sciences; no. 20170056). The patients provided informed
consent for the usage of the data in this study.
Surgical technique
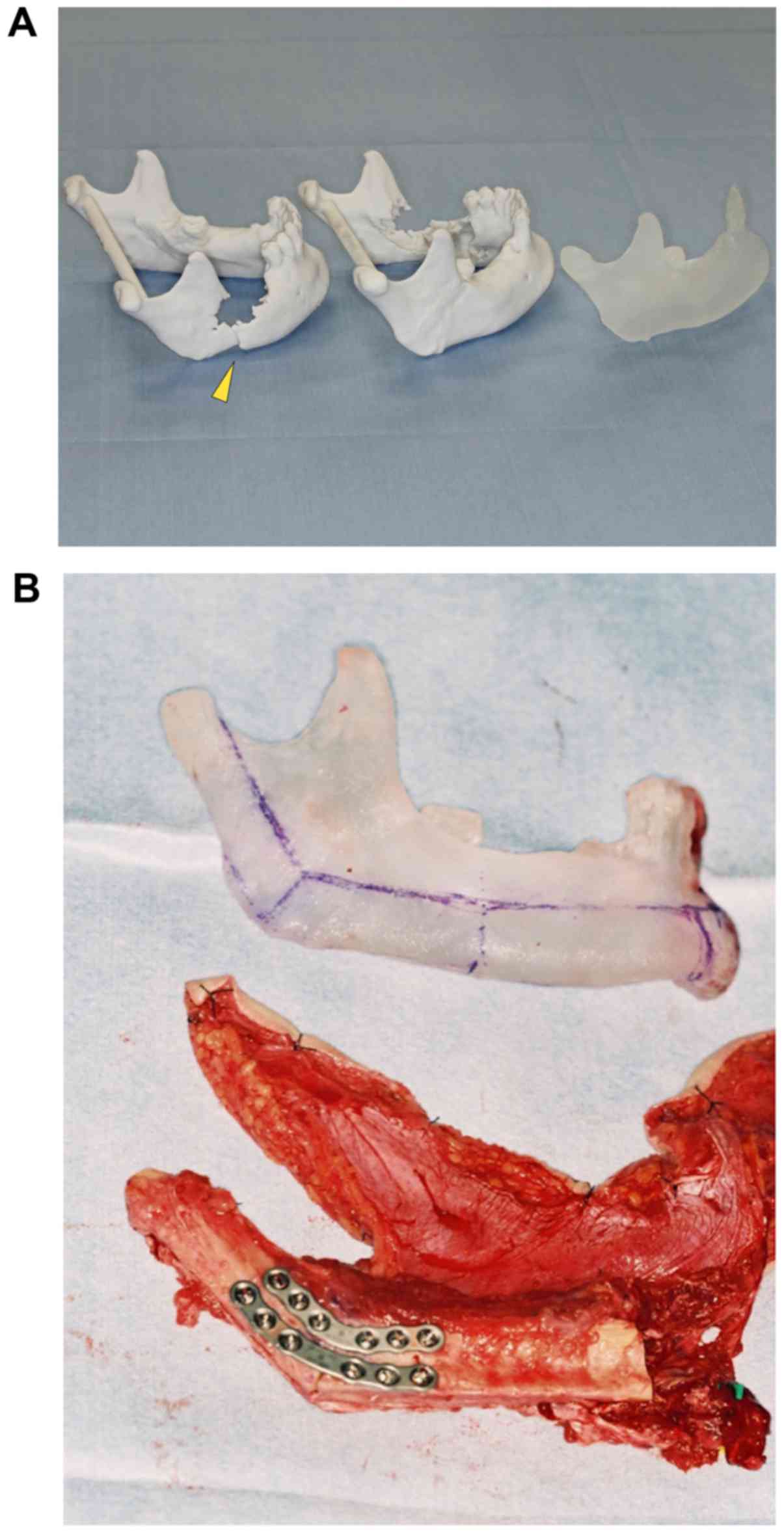
The stereolithography model was made before the
operation. The resin template was also fabricated from the
stereolithography model in the dental laboratory (Fig. 1A). Intermaxillary fixation (IMF)
screws (Dual-Top Auto Screw; Jeil Medical Corporation, Seoul, South
Korea) were used during MMFs. MMF was once performed to recognize
the maxillomandibular relationship and occlusion before the
resection of the mandible. A splint or denture was applied in
patients who were partially or full edentate. Next, the MMF was
released, and a hemimandibulectomy was performed to isolate the
tumor. The temporalis and masseter muscles were resected; the
medial and lateral pterygoid muscles were resected; and the
remaining soft tissue surrounding the condyle was removed. In all
cases, the temporomandibular disc was preserved. Then, a fibular
osteocutaneous flap was harvested and adjusted to the defect. A 3D
resin model was used to determine the length of segments and the
angle of bony reconstruction. The straight fibula bone was
osteotomied to match the form of the mandible. The fibular end was
adjusted to a round shape to fit into the glenoid fossa (Fig. 1B). The residual mandibular segments
were repositioned by MMF (Fig. 1C).
The fibular end was placed into the glenoid fossa under the TMJ
disc. During the operation, care was taken to place the residual
mandible and the lower edge of the transplanted fibula at the same
level. The distance from the angle of the mandible to the angle of
the fibular flap was corrected to be the same length as the 3D
model. The fibular graft was secured to the residual mandible with
titanium plates and screws (Fig. 1D).
A 1.0–1.5 mm thick straight or curved-shaped plate and 5 or 6 mm
screws (MatrixMANDIBLE; DePuy Synthes Co., Tokyo, Japan) were used.
After positioning the fibula, the lateral pterygoid muscle was not
secured to the fibula with sutures. The masseter muscle was also
not sutured to the internal pterygoid muscle to actively seat the
flap into the glenoid fossa. Next, microvascular anastomoses were
performed using a microscope. Then, the MMF was released, and
mandibular movement, dental occlusion, and the position of the
neocondyle were confirmed. Patients underwent postoperative MMF for
10–21 days, followed by mobilization and jaw exercises.
Results
The mean follow-up duration was 23.4 months (range,
18–40 months) (Table I). In all
cases, the tumor was completely removed with a clear margin. There
was no instance of vascular compromise, and the flap survival rate
was 100%. There was no extrusion of hardware, breakage of the
titanium plate, or screw looseness. All patients had a stable
mandibular union and good to moderate facial contour. No patients
experienced facial nerve paresis. All patients maintained
intelligible speech.
A radiographic assessment showed that the neocondyle
remained in the glenoid fossa, and there was no dislocation or
ankylosis in any of the patients (Table
III). Remodeling of the end of the neocondyle was found, and
the shape was changed to resemble a round shape. Twelve months
after the surgery, measurement of length between the fixed point on
the lower border of the flap and the median point on the upper side
of the neocondyle showed a decrease of less than 5% of the early
postoperative measurements. Thus, there were no abnormal bone
resorptions.
 | Table III.Morphological and functional
evaluation of the temporomandibular joint. |
Table III.
Morphological and functional
evaluation of the temporomandibular joint.
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|
| Morphological
evaluation |
|
|
|
|
|
|
Dislocation | No | No | No | No | No |
|
Ankylosis | No | No | No | No | No |
| Change
into round-shape of fibular end | Yes | Yes | Yes | Yes | Yes |
|
Abnormal bone resorption | No | No | No | No | No |
| Functional
evaluation |
|
|
|
|
|
|
Occlusion | Centric | Centric | Centric | Centric | Centric |
| MMO
(early postoprative period, mm)a | 23 | 13 | 20 | 15 | 18 |
| MMO
(late postoperative period, mm)b | 40 | 21 | 25 | 38 | 32 |
| Mouth
opening pattern | Minimal
deviation | Minimal
deviation | Minimal
deviation | Minimal
deviation | Minimal
deviation |
| Lateral
movement (affected side, mm) | 8 | 1 | 2 | 4 | 5 |
| Lateral
movement (non-affected side, mm) | 6 | 1 | 2 | 3 | 3 |
|
Protrusive movement, mm | 7 | 0 | 0 | 4 | 5 |
| TMJ
pain | No | No | No | No | No |
| Muscle
pain with palpation | No | No | No | No | No |
| Jaw
joint noise | No | No | No | No | No |
| Closed
or open locking of the jaw | No | No | No | No | No |
In all patients, the mandible was placed in centric
occlusion. The mean MMO at the time of MMF release was 17.8 mm
(range, 13–23 mm), and at least 12 months postoperatively, it was
31.2 mm (range, 21–40 mm). All patients showed an improvement of
MMO of more than 5 mm during the follow-up period. All the patients
had a slight lateral shift from the midline to the affected side
during mouth opening. None of the patients had any TMJ pain, muscle
pain with palpation, jaw joint noise, or closed and open locking of
the jaw.
Masticatory function is presented in Table IV. Before surgery, the average
numbers of natural maxillary and mandibular teeth were 6.8 (range,
0–13) and 8.4 (range, 0–15). After surgery, the average numbers of
natural maxillary and mandibular teeth were 6.4 (range, 0–13) and
4.8 (range, 0–9). As a result of tumor resection, an average of 3.6
natural mandibular teeth (range, 0–6) were lost. Four of the five
patients (80%) used dentures as prostheses, and no patients were
underwent the treatment of dental implants. The average number of
occlusal teeth (including denture) was 9.2 preoperatively and 10.8
postoperatively. The mean food scale rating was 86 (range, 50–100)
preoperatively, and 88 (range, 70–100) postoperatively. Therefore,
all patients recovered their ability to ingest nearly the same
foods that could be ingested before surgery.
 | Table IV.Assessment of masticatory
function. |
Table IV.
Assessment of masticatory
function.
| Status | Patient | 1 | 2 | 3 | 4 | 5 | Average |
|---|
| Preoperative
status | Number of maxillary
natural teeth | 0 | 10 | 0 | 11 | 13 | 6.8 |
|
| Number of
mandibular natural teeth | 0 | 11 | 7 | 9 | 15 | 8.4 |
|
| Use of prostheses
(removable dentures) | No | Yes | Yes | Yes | No | 60% (usage
rate) |
|
| Number of contacted
teetha | 0 | 13 | 6 | 14 | 13 | 9.2 |
|
| Food scale
rating | 50 | 100 | 80 | 100 | 100 | 86 |
| Postoperative
status | Number of natural
maxillary teeth | 0 | 10 | 0 | 9 | 13 | 6.4 |
|
| Number of natural
mandibular teeth | 0 | 8 | 2 | 5 | 9 | 4.8 |
|
| Use of prostheses
(removable dentures) | Yes | Yes | No | Yes | Yes | 80% (usage
rate) |
|
| Number of contacted
teetha | 14 | 13 | 2 | 12 | 13 | 10.8 |
|
| Food scale
rating | 100 | 90 | 70 | 80 | 100 | 88 |
Case report (case no. 1)
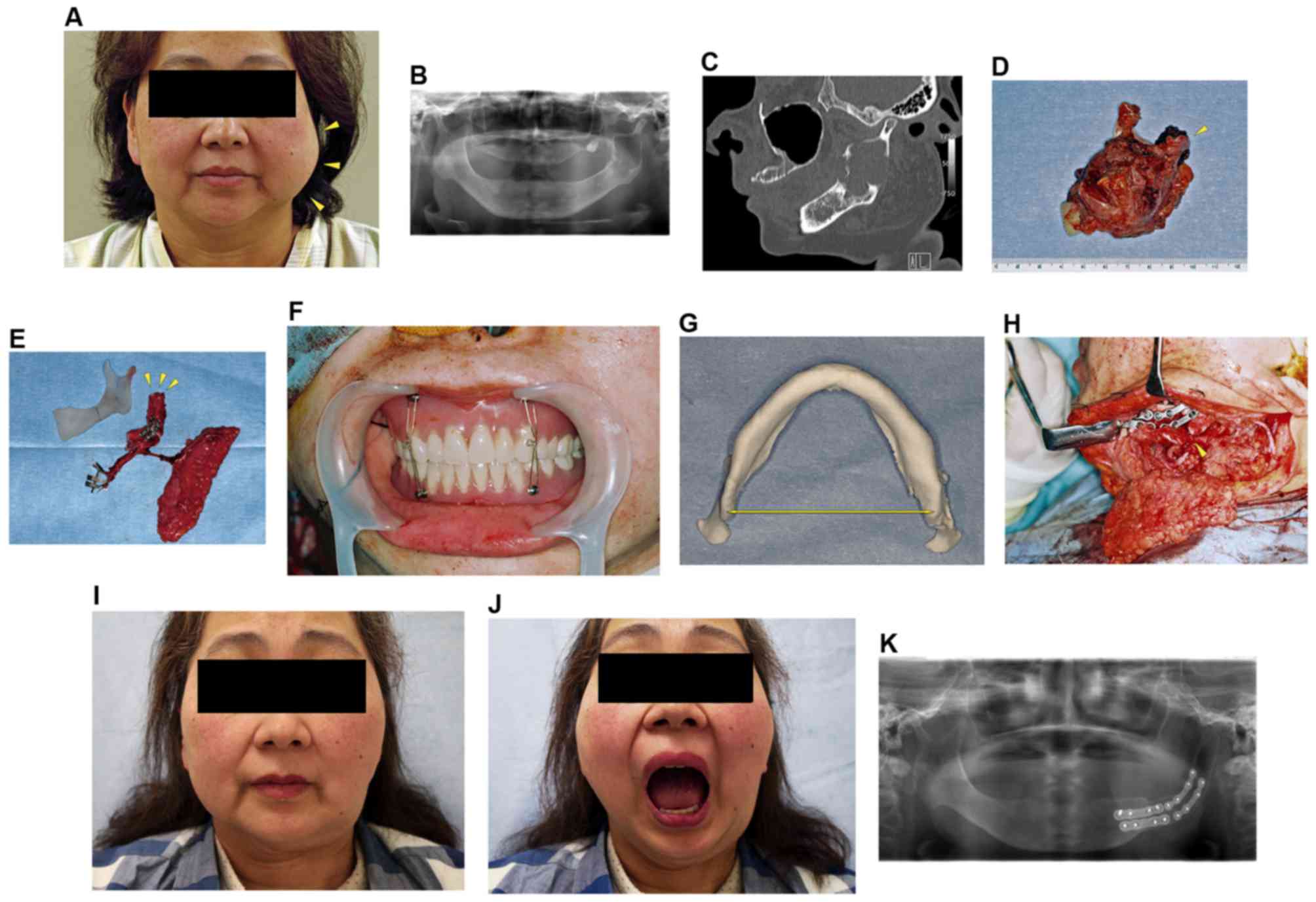
A 52-year-old female visited our hospital due to the
expansion and pain in the left mandible (Fig. 2A). Physical examination revealed no
paresthesia in the left mental region. Oral examination showed a
swelling of the left retromolar region without mucosal alteration.
A panoramic radiograph revealed the radiolucency throughout the
mandibular ramus with extension near the condyle (Fig. 2B). CT scanning showed a multilocular
cystic lesion occupying the mandibular ramus and a loss of cortical
bone (Fig. 2C). A biopsy confirmed
the diagnosis of keratocystic odontogenic tumor (KCOT).
Considering the progression of the lesion, a
mandibular resection, including condyle and reconstruction, was
planned under general anesthesia. Before the mandibular resection,
MMF with denture was carried out to evaluate the maxillomandibular
relationship and released. The resection was performed with the
soft tissue that covered the lesion. The condyle was disarticulated
and the articular disc was preserved (Fig. 2D). The fibular osteocutaneous flap was
harvested, and osteotomy was accomplished to shape the flap as a
substitute ramus and condyle in accordance with the 3D resin model
(Fig. 2E). The distal fibular end was
rounded to allow it to fit the temporomandibular disc in the
glenoid fossa. The residual mandibular segment was repositioned by
MMF with a denture (Fig. 2F). The
width of the mandibular angle was measured in the 3D model
(Fig. 2G), and the distance from the
angle of the mandible to the angle of the fibular flap was adjusted
to be the same length (Fig. 2H).
Then, the fibular osteocutaneous flap was fixed with titanium
plates and screws.
The microvascular anastomosis was completed using a
surgical microscope, and the wound was closed. MMF was finally
released 10 days after the operation, and the patient was
discharged on 37th postoperative day.
Forty months after the mandibular reconstruction,
the patient showed good aesthetic results (Fig. 2I). She had excellent joint mobility
and the MMO was 40 mm (Fig. 2J). The
postoperative panoramic radiograph confirmed the optimal position
of the grafted bone in the glenoid fossa, and showed the remodeled
fibular segment in the condylar fossa (Fig. 2K).
Discussion
The condyle articulates with the glenoid fossa of
the temporal bone and plays an important role in the proper
function of the TMJ. The presence of an extensive tumor in the
mandibular ramus or direct invasion of the condyle may require
resection of the mandible with disarticulation of the TMJ. Because
of the unique and complex features of the TMJ, the reconstruction
of the condylar defect is one of the most challenging issues.
At present, several procedures are used for the
reconstruction of the condyle, including costochondral graft,
attachment of the resected condyle to the end of a graft, titanium
TMJ prosthesis, and placement of the distal end of the vascularized
graft directly into the glenoid fossa (9). Costochondral graft is one of the best
methods for the transplantation of bone and choral components
(19), but it is not large enough to
replace bone after a complete hemimandibulectomy. Replacement with
the resected condyle provides an excellent surface and fit for the
joint, but concern exists regarding the oncologic risk, the
condylar preservation during hemimandibulectomy may predispose the
patient to local recurrence even with negative margins (20). Furthermore, these two types of grafts
are devascularized, and the drawback is the possibility of abnormal
resorption (5). The concern with the
prosthetic condyle is the exposure of the prosthesis, risk of
infection and erosion of the prosthesis into the middle cranial
fossa (21,22). Recently, the fibular graft has become
the preferred procedure for mandibular reconstruction, because it
is simple and can be easily fitted. Its use for the reconstruction
of the condyle has been reported (Table
V) (2–13). It seems particularly well suited for
condylar reconstruction, because of its tubular shape and
adaptability to the glenoid fossa. However, in some cases,
complications were noted including poor mouth opening, mandible
deviation, dislocation, abnormal bone resorption, ankylosis, and
the need for additional surgical treatment (7). Thus, consensus has yet to be reached
regarding the best method for reconstruction of the condyle with a
fibular flap. In addition, little is reported about the outcome of
the fibular flap in reconstructing the mandibular condyle.
 | Table V.Repoorted procedures of
reconstruction of the condyle with vascularized fibular flap and
its outcome. |
Table V.
Repoorted procedures of
reconstruction of the condyle with vascularized fibular flap and
its outcome.
|
|
|
| Treatment
method | Outcome |
|---|
|
|
|
|
|
|
|---|
| Author | Year | Case no. | Template of bone
defect | Rounding off of
fibular end | Maxillo-mandibular
fixation | Stay sutures | Disc
preservation | Additional
technique | Maximum mouth
opening | Occlusion |
Complicationsa | Masticatory ability
(oral diet) | Follow-up
period | (Refs.) |
|---|
| Iñigo et
al | 1997 | 5 (details
unknown) | ND | ND | Yes (intra and
postoperatively) | Yes (disc to
fibular end if possible) | ND | ND | ND | 4 good, 1 not
good | ND | 5 yes (everyone
could chew) | More than 12
months | (2) |
| Wax et
al | 2000 | 17 (details
unknown) | Plate | No | Yes (details
unknown) | Yes (disc to
fibular end or anterior lip of fossa to fibular end) | 15 preserved, 1
partially removed, 2 extirpated with tumor | ND | ND | ND | 3 trismus, 2
dyslocation of neocondyle | 13 yes (2 regular
food, 7 soft food, 4 liquid, 4 tube feed) | 41.3 months (1–64
months) | (3) |
| Guyot et
al | 2004 | 11 (5 benign tumors
or cysts, 4 osteoradi-onecrosis, 2 ramus hypoplasia) | Bone plate | No | No | ND | Yes | ND | 34.6 mm (25–43
mm) | ND | 3 trismus (12–60
months) | ND | 31.1 months | (4) |
| Engroff | 2005 | 1 (1 benign) | Bone plate | Yes | Yes (only intraoper
atively) | Yes (masseter
muscle to the angle of reconst-ruction plate) | Yes | ND | ND | 1 good | No | ND | ND | (5) |
| Khariwala et
al | 2007 | 9 (5 malignancy, 3
osteoradi-onecrosis, 1 trauma) | Template | ND | ND | No | ND | Covering of fibular
end with acellular dermal matrix | 38 mm (20–40
mm) | 7 good, 2 not
good | 1 trismus | 9 yes (soft diet,
no NG tube diet) | 13.1 months (6–24
months) | (6) |
| González-García
et al | 2008 | 6 (2 malignancy, 3
benign, 1 osteoradi-onecrosis) | Bone plate | Yes | Yes (only
intraope-ratively) | Yes (masseter
muscle to the pterygoid muscle) | Yes | ND | 40 mm | ND | 1 trismus, 1
ankylosis of neocondyle, 2 dyslocation of neocondyle | ND | 36 months (15–84
months) | (7) |
| Thor et
al | 2008 | 4 (1 malignancy 3
benign) | Template | ND | ND | Yes (fibular end to
the zygomatic arch and lateral pterygoyd muscle) | Yes | ND | 16–55 mm | 4 good | 1 trismus | ND | 9–36 months | (8) |
| Moore and
Hamilton | 2012 | 7 (6 malignancy, 1
osteomyelitis) | Thermoplastic
sheeting | Yes | Yes [intra and
postoperatively (1 week)] | ND | 1 preserved, 6
extirpated with tumor | Covering of fibular
end with soft tissue and flexor hallucis longus muslce | 40 mm | 6 good, 1 open
bite | No | 7 yes (1 soft diet,
no NG tube diet) | 16 months (8–30
months) | (9) |
| Wang et al | 2013 | 10 (10 benign) | 3D model | ND | Yes [intra and
postoperatively (2 weeks)] | ND | Yes | Double-barrel
vascularized fibular flap | ND | 10 good | ND | ND | 2–18 months | (10) |
| Bredell et al | 2014 | 5 (4 malignancy, 1
osteomyelitis) | ND | ND | ND | ND | 4 preserved, 1
removed | ND | ND | ND | ND | ND | ND | (11) |
| Chao et al | 2014 | 5 (1 malignancy, 3
benign, 1 osteomyelitis) | 3D model | ND | Yes [intra and
postoperatively 3 cases, (20–42 days)] | Yes (2 cases, disc
to fibular end) | ND | ND | 32 mm (5-45mm) | 5 good | 2 trismus | 5 yes (4 regular
food, 1 soft food) | 10.2 months (6–15
months) | (12) |
| Hoang et al | 2015 | 1 (1 benign) | ND | Yes | Yes [intra and
postoperatively (4 weeks)] | Yes (masseter
muscle to the angle of reconstruction plate) | Yes | ND | ND | 1 good | No | ND | 24 months | (13) |
| Present study | 2017 | 5 (4 malignancy, 1
benign) | 3D model | Yes | Yes [intra and
postoperatively (10–21 days)] | No | Yes | No | 31.2 mm (21–40
mm) | 5 good | 2 trismus | 5 yes (normal
diet)b | 23.4 months (18–40
months) |
Because of the unique feature of the TMJ, which is
related to both the dentition and the contralateral TMJ,
reproducing the optimal relationship between both the condyles
(including neocondyle) and the glenoid fossa, and maintaining the
correct occlusion are critical. Some authors reported the technique
of using a bone plate for a template of the bone defect for the
reconstruction of the mandibular condyle (3–5,7). However, this technique uses the outer
surface of the mandibular bone as a template, and thus, it cannot
be used when the lesion involves the outer cortex of the mandible.
The use of a stereolithography model based on CT data has been also
reported (10,12). In this method, fabrication of surgical
template can also be prepared by the mirror-image model of the
unaffected side (Fig. 3A and B). This
method is useful when intraoperative adaptation of the plate is
impossible due to the size or position of the tumor. Using a 3D
model, preoperative measurements can be made to determine the bone
osteotomy for pathological resection, as well as to know the
appropriate dimensions for bone reconstruction. By recognizing the
defect dimensions before surgery, flap design and reliability,
operative safety, and planning of the osteotomies can be improved.
Furthermore, there is the possibility of reducing intraoperative
decision-making, and thus potentially contributing to a significant
shortening of the operative time, which is associated with
complications (23). Thus, a 3D model
can improve patient outcomes with a reduction in complications.
Nahabedian et al reported that the technical
limitations of condylar reconstruction with a fibular flap made it
difficult to reproduce the 3D shape of the native condyle (24). However, in our cases, contouring the
fibular pole was accomplished to shape the flap as a substitute
condyle in accordance with the 3D resin model. It may also
facilitate the appropriate fit in the glenoid fossa and make a
functional condyle. Although the cost of a 3D model is not
negligible (at the time of the preparation of this article, the
costs of creating a 3D model began at ¥20,000), it is decreasing
and becoming more readily available.
Currently, some surgeons prefer to use cutting
guides to perform the osteotomy and fibula shaping. This method is
faster and more reliable surgical results (25). However, one of the concerns of the
cutting guide is the unpredictability of the surgical margins in
some cases (26). The changing the
extent of resection may reduce the usefulness of this method by the
need of adjustments in bone segment length, number of osteotomies,
and the shape of the titanium plate, potentially increasing the
operation time and decreasing the reconstructive accuracy (26). In addition, since the device is
designed according to the location of the fibular bone, it may be
difficult to use a cutting guide if soft tissue is included with
the fibula for oral or facial reconstructions.
Lim et al reported that the maximum error was
observed in the condylar region when only a fibula-cutting guide
was used (27). This error was
thought to be related with the movement of the condylar head in the
TMJ (27). They suggested that it may
be more helpful to adjust the anterior-posterior position of the
fibular bone by referring to the 3D model rather than using a
cutting guide (27).
During the reconstruction of mandible, the optimal
correlation of the condyles in the glenoid fossae and accurate
occlusion are important. When a defect of hemimandible occurs, the
masticatory muscles attached to the remaining segment will shift
the segment into an unusual position. Therefore, the residual
mandible should be relocated during the reconstruction.
Intraoperative MMF is helpful for maintaining the occlusal and
maxillomandibular relationship during reconstruction (28). In the case of edentulous or partially
edentulous patients, dentures or splints are useful for reinforcing
the remaining teeth and maintaining the dimensions of the
maxillomandibular relationship (29,30). In
this study, five patients were edentate, or poor dentition, and
dentures or splints were used to restore the occlusal relationship
during MMF.
It is also a surgical challenge to maintain the
fibular end in the glenoid fossa during and after reconstruction.
In our cases, the use of MMF allows direct appliance of the fibula
to the temporomandibular disc in the glenoid fossa. The distance
from the angle of the mandible to the angle of the fibular flap was
adjusted to be the same length as the 3D model. Then, the fibular
graft was fixed to the residual mandible with titanium plates and
screws. In this method, smooth insetting, anastomosis, and the
planning of flap design, can be performed, and it may decrease the
complication rate. In our cases, no instances of vascular
compromise or fistula formation was observed. The 3D model could
accurately and safely reproduce the 3D shape and the anatomical
location of the original condyle with the aid of MMF.
Some investigators have reported the suture
fixation, such as the suture of the fibular end to the preserved
disc (2,3), the suture of the fibula or the
reconstruction plate to the masseter or pterygoid muscles (5,8,13), and the suture of the masseter muscle
to the pterygoid muscle (7). However,
suspension of the fibular flap in the fossa with sutures is
sometimes difficult and inaccurate (5). In addition, the remaining masseter or
pterygoid muscles is occasionally not enough for suture due to the
tumor resection. The displacement of the fibular flap has been
reported in cases with suture fixation (7). In our cases, we chose to perform MMF for
2–3 weeks followed by mobilization in a manner similar to
rehabilitation. In this method, MMF can maintain occlusal status
and the maxillomandibular relationship, and hold the reconstructed
mandible during the healing process. None of our patients
experienced displacement of the fibula out of the condylar fossa.
Chao et al also reported the use of postoperative MMF to
condylar reconstruction with a fibular flap for 20–42 days and
showed good results (12). These
results indicate that MMF may contribute to the functionality of
reconstruction through the recovery of accurate biting.
The temporomandibular disc is a biconcave sheet of
avascular fibrous connective tissue. The disc divides the joint
into a superior and inferior joint space and prevents direct
contact of the condylar head to the mandibular fossa. The previous
study showed that the preservation of the temporomandibular disc
influences the postoperative recovery of normal function (12). Furthermore, the temporomandibular disc
is important to control the form of the mandibular condyle
(31). Guyot et al reported on
11 patients undergoing reconstruction with a fibular flap following
condylar resection without the removal of the disc. In all cases,
the fibular end was insetted directly into the glenoid fossa under
the disc without contouring (4). In
their cases, TMJ function was preserved, and there were no cases of
ankylosis. They also found the tendency of bone remodeling. In our
cases, the temporomandibular disc was preserved in all patients,
and the round-off of the fibular ends without ankylosis were found.
The most likely reason for these changes is considered to be the
preservation of TMJ disc.
In a previous study, González-García et al
(7) showed six patients who underwent
the mandible and condyle resection without disc resection and
reconstruction with fibular flap. They found that five patients
achieved good postoperative function, but one patient experienced
severe ankylosis. The temporomandibular disc blends with fibers of
the lateral pterygoid muscle at its anterior margin, and attaches
to looser connective tissue containing nerves and is lined
posteriorly with the synovial membrane. Hamada et al
performed the magnetic resonance imaging (MRI) and arthroscopy in
the TMJ of two patients who experienced hemimandibulectomy without
disc removal (32). The MRI of the
affected TMJs showed that the intermediate zone of the disc was
positioned anteriorly to the summit of the articular eminence.
Arthroscopically, the fibrous adhesions were also observed in the
superior joint compartment of the affected TMJs. They suggested
that the disc was pulled anteriorly by the lateral pterygoid
muscle, and they also thought that surgical trauma during
mandibulectomy brought synovitis or hematoma, subsequent
immobilization of the affected TMJs, and aroused intraarticular
fibrous adhesion. As shown in other previous reports, not only the
disc removal, but also the disc displacement or articular damage
was associated with the development of ankylosis (33–36). Thus,
the possibility of ankylosis must be kept in mind even in the case
of disc preservation. In cases where disc removal is necessary due
to disease progression, covering the contoured end of the fibula
with adequate vascularized soft tissue could overcome this problem
(9). Moore and Hamilton reported that
none of their six patients had ankylosis in this technique
(9).
Generally, vascularized bone graft suffers less
resorption than non-vascularized bone graft (5). We performed length measurements using
panoramic radiographs and revealed that no abnormal resorption of
the neocondyle occurred. Chao et al reported the usage of a
resected condyle to obtain adequate TMJ function, but the native
condyle became avascular necrosis and was removed (12). In our opinion, it would seem
unnecessary to attach the native (original) condyle to the fibular
flap.
To objectively evaluate treatment outcomes, we
assessed the TMJ and dental condition, conducted a food scale
questionnaire (12), and examined
masticatory status. In our study, the MMO of three patients was
greater than 30 mm, but two patients exhibited postoperative
trismus with less than 30 mm of MMO. Radiation therapy commonly
influences a patient's mandibular movements (37). These two patients received
radiotherapy and developed limitation of mandibular sliding
mobility and mouth opening. It was considered to be an adverse
effect of radiation therapy. The forward movement is caused by the
contraction of the inferior head of the lateral pterygoid muscle.
The condyle and disc then move forward until they reach a point
just slightly anterior to the crest of the articular eminence
(38). In all our patients, the
lateral pterygoid muscle was partially resected and not sutured
with fibular end; thus, forward movement was considered to be
difficult to achieve. The mouth opening-closing path showed a
tendency to deviate toward the affected side, which was considered
to be due to movement restriction on the affected side. Early
exercise therapy is essential in surgery involving the TMJ
(5). Our patients underwent
mobilization and rehabilitation exercises after the release of MMF,
and MMO improved as jaw physiotherapy progressed. The position of
the condylar axis is related to symptomatology of TMJ (39), and mandibular resection may cause TMJ
disorder (40). In this method, both
condylar positions were kept by MMF, and TMJ disorder was not
observed.
Mastication comprises synchronous interaction
between the orofacial soft and hard tissues to manipulate,
triturate, and consolidate a food bolus prior to deglutition
(37). The mandible plays an critical
role in the competence of mastication (37). After resection of the mandible,
masticatory function can be affected by several factors such as the
number of residual teeth and tooth-to-tooth contacts (37,41,42). To
objectively assess our outcomes, masticatory function was evaluated
with reference to dental status, and was examined using a food
scale questionnaire. In our patients, an average of 3.6 natural
mandibular teeth was lost due to the tumor resection. Four patients
(80%) were treated with removable dentures postoperatively, and
tooth-to-tooth contact was achieved. The food scale questionnaire
showed that the patients recovered their ability to ingest nearly
the same foods that can be ingested before surgery. Tan et
al (43) showed that
postoperative restoration with removable dentures significantly
improved masticatory function when teeth were lost after segmental
mandibulectomy and bone reconstruction. The results of our study
are concordant with the report. The overall efficiency of
mastication was usually performed the patients' questionnaire
(18,42). Wax et al reported on 17
patients who underwent condyle reconstruction with a fibula free
graft and found that four patients needed a liquid diet and four
patients remained on a nasogastric (NG) feeding tube (3). Although our sample size was small, there
were no patients remained on a liquid diet or were NG-tube
dependent. Thus, our patients exhibited almost good food tolerance
and masticatory function after condylar reconstruction.
In this study, we evaluated the morphological and
functional outcome following the stereolithographic model-assisted
reconstruction of the condyle with placement of a vascularized free
fibula into the glenoid fossa. The stereolithography model could
accurately reproduce the 3D shape and anatomical location of the
original condyle with the aid of MMF. MMF could also support in the
determination of a proper position for the bone flap to ensure the
future dental rehabilitation. The correct occlusion and optimal
relationship between the condyle and the glenoid fossa were
achieved. The end of the fibular bone graft recapitulated the
native condylar anatomy and function, and served morphologically
and functionally as a neocondyle. The reservation of the disc and
molding of the end of the fibular graft seemed to be responsible
for the good outcome in terms of function. All the patients showed
good food tolerance and masticatory function after
reconstruction.
Although the type of condylar reconstruction remains
controversial, the stereolithographic model-assisted reconstruction
of the mandibular condyle with a vascularized fibular flap is
useful for reconstructions of the hemimandible, including condylar
defects.
References
|
1
|
Potter JK and Dierks EJ: Vascularized
options for reconstruction of the mandibular condyle. Semin Plast
Surg. 22:156–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iñigo F, Rojo P, Ysunza A and Jimenez Y:
Three different techniques for mandibular reconstruction after
hemimandibulectomy. J Craniofac Surg. 8:58–64. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wax MK, Winslow CP, Hansen J, MacKenzie D,
Cohen J, Andersen P and Albert T: A retrospective analysis of
temporomandibular joint reconstruction with free fibula
microvascular flap. Laryngoscope. 110:977–981. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guyot L, Richard O, Layoun W, Cheynet F,
Bellot-Samson V, Chossegros C, Blanc JL and Gola R: Long-term
radiological findings following reconstruction of the condyle with
fibular free flaps. J Craniomaxillofac Surg. 32:98–102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Engroff SL: Fibula flap reconstruction of
the condyle in disarticulation resections of the mandible: A case
report and review of the technique. Oral Surg Oral Med Oral Pathol
Oral Radiol Endod. 100:661–665. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khariwala SS, Chan J, Blackwell KE and
Alam DS: Temporomandibular joint reconstruction using a
vascularized bone graft with Alloderm. J Reconstr Microsurg.
23:25–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
González-García R, Naval-Gías L,
Rodríguez-Campo FJ, Martínez-Chacón JL and Gil-Díez Usandizaga JL:
Vascularized fibular flap for reconstruction of the condyle after
mandibular ablation. J Oral Maxillofac Surg. 66:1133–1137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thor A, Rojas RA and Hirsch JM: Functional
reconstruction of the temporomandibular joint with a free fibular
microvascular flap. Scand J Plast Reconstr Surg Hand Surg.
42:233–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moore EJ and Hamilton SS: Mandibular
condyle reconstruction with fibula free-tissue transfer. Ear Nose
Throat J. 91:E18–E24. 2012.PubMed/NCBI
|
|
10
|
Wang WH, Zhu J, Deng JY, Xia B and Xu B:
Three-dimensional virtual technology in reconstruction of
mandibular defect including condyle using double-barrel
vascularized fibula flap. J Craniomaxillofac Surg. 41:417–422.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bredell M, Grätz K, Obwegeser J and Gujer
AK: Management of the temporomandibular joint after ablative
surgery. Craniomaxillofac Trauma Reconstr. 7:271–279. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chao JW, Rohde CH, Chang MM, Kutler DI,
Friedman J and Spector JA: Oral rehabilitation outcomes after free
fibula reconstruction of the mandible without condylar restoration.
J Craniofac Surg. 25:415–417. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hoang MP, Nguyen TT, Nguyen LK and Jeng
SF: Ossifying fibroma of the mandible: A case report using
vascularized free fibula flap reconstruction. Plast Reconstr Surg
Glob Open. 3:e4702015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smith M, Williams F and Ward BB: Hard
tissue reconstructionManagement of Complications in Oral and
Maxillofacial Surgery. Miloro M and Kolokythas A: 1st.
Wiley-Blackwell; Oxford: pp. 283–316. 2012
|
|
15
|
Antony AK, Chen WF, Kolokythas A, Weimer
KA and Cohen MN: Use of virtual surgery and
stereolithography-guided osteotomy for mandibular reconstruction
with the free fibula. Plast Reconstr Surg. 128:1080–1084. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
List MA, Ritter-Sterr C and Lansky SB: A
performance status scale for head and neck cancer patients. Cancer.
66:564–569. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Urken ML, Weinberg H, Vickery C,
Buchbinder D, Lawson W and Biller HF: Oromandibular reconstruction
using microvascular composite free flaps. Report of 71 cases and a
new classification scheme for bony, soft-tissue, and neurologic
defects. Arch Otolaryngol Head Neck Surg. 117:733–744. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Curtis DA, Plesh O, Miller AJ, Curtis TA,
Sharma A, Schweitzer R, Hilsinger RL, Schour L and Singer M: A
comparison of masticatory function in patients with or without
reconstruction of the mandible. Head Neck. 19:287–296. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vega LG, González-García R and Louis PJ:
Reconstruction of acquired temporomandibular joint defects. Oral
Maxillofacial Surg Clin North Am. 25:251–269. 2013. View Article : Google Scholar
|
|
20
|
Petruzzelli GJ, Cunningham K and
Vandevender D: Impact of mandibular condyle preservation on
patterns of failure in head and neck cancer. Otolaryngol Head Neck
Surg. 137:717–721. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marx RE, Cillo JE Jr, Broumand V and Ulloa
JJ: Outcome analysis of mandibular condylar replacements in tumor
and trauma reconstruction: A prospective analysis of 131 cases with
long-term follow-up. J Oral Maxillofac Surg. 66:2515–2523. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carlson ER: Disarticulation resections of
the mandible: A prospective review of 16 cases. J Oral Maxillofac
Surg. 60:176–181. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosenberg AJ, Van Cann EM, van der Bilt A,
Koole R and van Es RJ: A prospective study on prognostic factors
for free-flap reconstructions of head and neck defects. Int J Oral
Maxillofac Surg. 38:666–670. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nahabedian MY, Tufaro A and Manson PN:
Improved mandible function after hemimandibulectomy, condylar head
preservation, and vascularized fibular reconstruction. Ann Plast
Surg. 46:506–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu LQ, Zhang CP, Poh EH, Yin XL and Shen
SK: A novel fibula osteotomy guide for mandibular reconstruction.
Plast Reconstr Surg. 129:861e–863e. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanasono MM and Skoracki RJ:
Computer-assisted design and rapid prototype modeling in
microvascular mandible reconstruction. Laryngoscope. 123:597–604.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lim SH, Kim MK and Kang SH: Precision of
fibula positioning guide in mandibular reconstruction with a fibula
graft. Head Face Med. 12:72016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mast G: Techniques of mandibulomaxillary
fixation (MMF)Principles of Internal Fixation of the
Craniomaxillofacial Skeleton. Ehrenfeld M, Manson PN and Prein J:
1st. George Thieme Verlag; New York, NY: pp. 115–123. 2012
|
|
29
|
Markowitz BL, Roumanas E and Calcaterra T:
Surgical stents for composite mandible reconstruction. Plast
Reconstr Surg. 96:194–200. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshimura H, Ohba S, Yasuta M, Nakai K,
Fujieda S and Sano K: Infrazygomatico-coronoid fixation in a
segmental mandibular reconstruction with a free vascularized flap:
A simple and correct repositioning method without interfering with
reconstructive and microsurgical procedures. Head Neck.
38:1679–1687. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McDonald F: The condylar disk as a
controlling factor in the form of the condylar head. J Craniomandib
Disord. 3:83–86. 1989.PubMed/NCBI
|
|
32
|
Hamada Y, Kondoh T, Takada N and Seto K:
MRI and arthroscopic findings in the temporomandibular joint after
mandibulectomy including the unilateral condyle. Report of two
cases. Int J Oral Maxillofac Surg. 29:341–343. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miyamoto H, Kurita K, Ishimaru J and Goss
AN: A sheep model for temporomandibular joint ankylosis. J Oral
Maxillofac Surg. 57:812–817. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Porto G, Vasconcelos B and Silva V Jr:
Development of temporomandibular joint ankylosis in rats: A
preliminary experimental study. Int J Oral Maxillofac Surg.
37:282–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dai J, Ouyang N, Zhu X, Huang L and Shen
G: Injured condylar cartilage leads to traumatic temporomandibular
joint ankylosis. J Craniomaxillofac Surg. 44:294–300. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiang GL, Long X, Deng MH, Han QC, Meng QG
and Li B: A retrospective study of temporomandibular joint
ankylosis secondary to surgical treatment of mandibular condylar
fractures. Br J Oral Maxillofac Surg. 52:270–274. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Beumer J III, Marunick MT, Silverman S Jr,
Garrett N, Rieger J, Abemayor E, Penn R, Nabili V, Rezaee R, Curtis
DA, et al: Rehabilitation of tongue and mandibular
defectsMaxillofacial Rehabilitation: Prosthodontic and Surgical
Management of Cancer-Related, Acquired and Congenital Defects of
the Head and Neck. Beumer J III, Marunick MT and Salvatore JE: 3rd.
Quintessence Publishing; Hanover Park: pp. 61–154. 2011
|
|
38
|
Brand RW and Isselhard DE:
Temporomandibular jointAnatomy of Orofacial Structures. 7th. Mosby,
St. Louis: pp. 348–352. 2003
|
|
39
|
Crawford SD: Condylar axis position, as
determined by the occlusion and measured by the CPI instrument, and
signs and symptoms of temporomandibular dysfunction. Angle Orthod.
69:103–116. 1999.PubMed/NCBI
|
|
40
|
Linsen S, Schmidt-Beer U, Fimmers R,
Grüner M and Koeck B: Craniomandibular pain, bite force, and oral
health-related quality of life in patients with jaw resection. J
Pain Symptom Manage. 37:94–106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marunick MT and Mathog RH: Mastication in
patients treated for head and neck cancer: A pilot study. J
Prosthet Dent. 63:566–573. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Boretti G, Bickel M and Geering AH: A
review of masticatory ability and efficiency. J Prosthet Dent.
74:400–403. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tan ZZ, Liu B, Wei JX, Zou H and Zhao YF:
Effects of mandibular odontogenic keratocyst surgery and removable
partial prostheses on masticatory performance. J Prosthet Dent.
97:107–111. 2007. View Article : Google Scholar : PubMed/NCBI
|