Introduction
The pancreas serves an essential role in the
digestive system, including producing numerous digestive enzymes
(1–3).
In 2013, pancreatic cancer (PC) was reported to be the fourth
leading cause of cancer-associated mortality worldwide (4). The majority of patients with PC present
with locally advanced or metastatic disease at initial diagnosis
and the proportion of patients who can proceed with curative intent
surgery is <20% (1,3). Patients with advanced (A)PC have a poor
prognosis (5–9). Among patients with metastatic PC, the
5-year survival rate is reported to be ~2% (10). Current Japanese guidelines for
systemic chemotherapy in patients with APC recommend the use of
gemcitabine monotherapy, S-1 monotherapy, gemcitabine and S-1
combination therapy, nab-paclitaxel and gemcitabine combination
therapy, or a combination chemotherapy regimen consisting of
oxaliplatin, irinotecan, fluorouracil and leucovorin, based on the
baseline and tumor status of each patient (5–8).
Skeletal muscle is considered to be a large
endocrine organ, which accounts for ~50% of an individual's body
weight and possesses the capacity for high metabolic activity
(11). In general, skeletal muscle
mass is regulated depending on the balance between protein
synthesis and protein catabolism (12). Sarcopenia, defined as decreased
skeletal muscle mass (DSMM) and muscle strength, has become a
relevant clinical feature for understanding the effects of aging on
clinical outcomes (13). Sarcopenia
is a commonly observed disorder in aged populations and is
associated with disability, functional decline and frailty
(13,14). Age-associated sarcopenia is defined as
primary sarcopenia, whilst advanced malignancies, as well as
chronic inflammatory diseases including renal, heart and liver
diseases, can be the causes of secondary sarcopenia (12–16).
Severe underlying diseases can lead to sarcopenia and cachexia,
which involves body weight loss and muscle wasting. Furthermore,
substantial skeletal muscle wasting is an important predictor in
patients with solid malignancies, although the precise mechanisms
by which DSMM increases the risk of mortality remain unclear
(17,18). Knowledge of the underlying mechanisms
in advanced malignancies associated with skeletal muscle wasting
may lead to the development of novel therapeutic drugs. Thus, in
recent years, this clinical area has attracted much attention among
oncologists.
A number of studies have demonstrated that DSMM
could be an adverse predictor for patients with PC who were treated
with surgical resection (16,19–23).
However, to the best of our knowledge, there are few reports
regarding the impact of DSMM on survival in patients with
unresectable APC undergoing systemic chemotherapy (24,25).
Therefore, it is imperative to address these issues. Thus, the aims
of the present study were to investigate the impact of DSMM prior
to systemic chemotherapy on the clinical outcomes of patients with
unresectable APC.
Patients and methods
Patients and indications for systemic
chemotherapy
Between February 2008 and November 2015, 80
consecutive patients diagnosed with unresectable APC undergoing
systemic chemotherapy were admitted to Hyogo College of Medicine
(Nishinomiya, Hyogo, Japan). There were 31 male and 30 female
patients with a median age of 72 years (range, 39–89). All patients
were treatment naive for APC. Cases with distal common bile duct
cancer, ampulla of Vater carcinoma or neuroendocrine carcinoma of
the pancreas were excluded. Of these, 19 patients with unknown
clinical outcomes (succumbed to disease or surviving) due to loss
of follow-up were excluded from the current analysis. Thus, a total
of 61 patients with APC who underwent systemic chemotherapy were
analyzed in the present study. Clinical stage for APC was
determined based on Union for International Cancer Control (UICC)
classification system (26). In cases
with local APC without distant metastases, indication for surgery
was reviewed in each case through discussion with oncologists and
surgeons (27–31). In principal, systemic chemotherapy was
recommended for patients with PC with the following
characteristics, as determined by radiological findings: Dynamic
computed tomography (CT), magnetic resonance imaging and endoscopic
ultrasonography (EUS). This was following informed consent from
each patient. The presence of distant metastases and/or the
presence of tumor vascular invasion was judged as unresectable PC.
Patients with poor performance status [PS; Eastern Cooperative
Oncology Group (ECOG) classification ≥3] were not
recommended for systemic chemotherapy (32). The presence of ascites was not
contraindicated for systemic chemotherapy.
Definition of DSMM and the study
protocol
Assessment of muscle mass was performed using CT
scans obtained prior to systemic chemotherapy. The third lumber
(L3) level was selected as a standard. Bilateral psoas muscles at
the L3 level were identified on the CT images. Cross-sectional
areas (cm2) of these muscles were measured by manual
tracing on the CT images, and their sum was calculated. These sums
were normalized for each patient to provide a psoas muscle index
value (PMI; cm2/m2) (33,34). The
median PMI value was calculated for males and females separately.
Patients with a PMI of more than each median value were defined as
the PMI-High (H) group and those with a PMI of less than each
median value as the PMI-Low (L) group. This is due to the optimal
cut-off point of PMI for DSMM in Japanese patients with PC having
not yet been well established.
The primary endpoint was overall survival (OS) in
the present study. Baseline characteristics and OS in the PMI-H and
PMI-L groups were retrospectively compared, and factors associated
with OS were investigated using univariate and multivariate
analyses. The current study was performed in accordance with the
Declaration of Helsinki and with approval from the Ethics Committee
of Hyogo College of Medicine (approval no. 2117).
Diagnosis for pancreatic cancer and
systemic chemotherapy
PC was diagnosed primarily based on the current
guidelines (35). Briefly, abdominal
US and dynamic CT of the pancreas was routinely performed prior to
initiating systemic chemotherapy (33). In cases with atypical radiological
findings for PC, tumor biopsy or EUS-guided fine needle aspiration
was considered (36). In the present
study, the pathological diagnosis was confirmed in 15 cases
(24.6%).
The selection of chemotherapeutic agents was
determined by each attending physician. For patients with no
evident risk factors, the recommended initial dosage of each
chemotherapeutic agent (gemcitabine, S-1, nab-paclitaxel, and
5-fluorouracil) was administered (5,37). The
reduced initial dosage was administered to certain patients based
on clinical features, including age, body weight, ECOG-PS and
laboratory data. During systemic chemotherapy, each attending
physician adjusted the dosage of chemotherapeutic agents according
to the grade of adverse events. In patients with adverse events,
systemic chemotherapy was discontinued until the clinical symptoms
resolved to grade 1 or 2, and other alternative chemotherapeutic
regimens were considered. In patients with poor response to initial
chemotherapy, other alternative chemotherapeutic regimens were also
considered.
In principle, the treatment efficacy for systemic
chemotherapy was assessed every 2–4 months following the initiation
of chemotherapy, according to the Response Evaluation Criteria in
Solid Tumors (RECIST; version 1.1) using radiological findings
and/or the levels of various tumor markers, including
carcinoembryonic antigen (CEA) and carbohydrate antigen (CA) 19-9
(38). Patients continued systemic
chemotherapy until the development of any of the following
conditions: Unacceptable drug toxicity, tumor progression or the
patient's request to stop treatment. Chemotherapy-associated
adverse events were evaluated using the Common Terminology Criteria
for Adverse Events (CTCAE; version 3.0) (39).
Evaluation of treatment response
during chemotherapy
The most improved treatment response achieved during
chemotherapy was determined according to the RECIST criteria
(version 1.1), as previously described (7,38). The
most improved treatment response was graded using the following
four categories: (1) Complete
response (CR); (2) partial response
(PR); (3) stable disease (SD);
(4) progressive disease (PD)
(38). The objective response rate
(ORR) was defined as the proportion of patients with the most
improved treatment response rates when considering CR and PR. The
disease control rate (DCR) was defined as the proportion of
patients with the most improved treatment response rates when
considering CR, PR and SD.
Statistical analysis
The categorical parameters in the PMI-H and PMI-L
groups were analyzed using Fisher's exact test, while the numerical
parameters were analyzed either with an unpaired Student's t-test
or with a Mann-Whitney U test as appropriate. OS curves were
created using the Kaplan-Meier estimator method and compared using
the log-rank test. Variables that were considered significant
following univariate analysis were entered into the multivariate
analysis with Cox's proportional hazards model. For the purpose of
analyzing the significance of predictors in multivariate analyses,
analyzed variables were divided by the median values for all cases
(n=61) and treated as dichotomous covariates. OS was defined as the
time interval from the initiation of systemic chemotherapy until
mortality (due to any cause) or to the final follow-up visit. Data
are presented as the median values (range) unless otherwise stated.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analyses were performed using JMP
software (version 11.0; SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
The baseline characteristics of the analyzed patient
cohort (n=61) are presented in Table
I. Of these, 13 were stage IVA and 48 were stage IVB, as
determined using the UICC classification system. Maximum tumor size
in patients ranged between 1.4 and 9.4 cm (median, 3.6 cm). The
median PMI in males was 4.3 cm2/m2 (range,
1.6–8.2 cm2/m2), whereas in females it was
2.3 cm2/m2 (range, 0.7–6.1
cm2/m2). Patients were predominantly PS-0
(70.5%; 43/61). As for initial chemotherapeutic regimens,
gemcitabine monotherapy was performed in 44 patients, S-1
monotherapy in 10, gemcitabine and S-1 combination therapy in 5,
nab-paclitaxel and gemcitabine combination therapy in 1, and
5-fluorouracil monotherapy in 1.
 | Table I.Baseline characteristics of patients
with unresectable advanced pancreatic cancer undergoing systemic
chemotherapy (n=61). |
Table I.
Baseline characteristics of patients
with unresectable advanced pancreatic cancer undergoing systemic
chemotherapy (n=61).
| Variable | Value (range) |
|---|
| Age, years | 72 (39–89) |
| Gender,
male/female | 31/30 |
| ECOG-performance
status, 0/1/2 | 43/15/3 |
| Psoas muscle index,
cm2/m2, male | 4.3 (1.6–8.2) |
| Psoas muscle index,
cm2/m2, female | 2.3 (0.7–6.1) |
| Body mass index,
kg/m2 | 21.2
(15.1–31.6) |
| Pancreatic cancer
stage, IVA/IVB | 13/48 |
| Maximum tumor size,
cm | 3.6 (1.4–9.4) |
| Primary site, uncus
or head/body or tail | 33/28 |
| Total bilirubin,
mg/dl | 0.7 (0.3–6.6) |
| Serum albumin,
g/dl | 3.5 (1.8–4.4) |
| Prothrombin time,
% | 86.3
(47.5–127) |
| Platelet count,
×104/mm3 | 20.8
(7.1–45.9) |
| White blood cell,
×103/µl | 6.05
(2.54–29.76) |
| Hemoglobin,
g/dl | 11.6
(7.5–15.7) |
| Serum creatinine,
mg/dl | 0.65
(0.28–7.41) |
| C reactive protein,
mg/dl | 0.6 (0–22.0) |
| AST, IU/l | 26 (11–265) |
| ALT, IU/l | 30 (8–289) |
| ALP, IU/l | 361 (139–1929) |
| GGT, IU/l | 98 (11–747) |
| Amylase, IU/l | 66 (7–357) |
| CEA,
IU/la | 3.95
(1.4–286.1) |
| CA19-9, IU/l | 408
(0.6–42414) |
Comparison of baseline characteristics
between the PMI-H and the PMI-L group
The proportion of patients with PS-0 in the PMI-H
group was significantly higher compared with that in the PMI-L
group [83.3% (25/30) vs. 58.1% (18/31); P=0.0486]. Body mass index
(BMI) in the PMI-H group was significantly higher compared with
that in the PMI-L group (P=0.0154). As for other baseline
characteristics, no significant differences were identified between
the two groups (Table II).
 | Table II.Comparison of baseline
characteristics between the PMI-H and the PMI-L group. |
Table II.
Comparison of baseline
characteristics between the PMI-H and the PMI-L group.
|
| Value (range) |
|
|---|
|
|
|
|
|---|
| Variables | PMI-H | PMI-L | P-value |
|---|
| Age, years | 70 (39–89) | 73 (48–88) | 0.1350 |
| Sex,
male/female | 15/15 | 15/16 | 1.0000 |
| ECOG-performance
status, 0/1/2 | 25/5 | 18/13 | 0.0486 |
| Body mass index,
kg/m2 | 21.6
(17.3–31.6) | 19.9
(15.1–24.8) | 0.0154 |
| Pancreatic cancer
stage, IVA/IVB | 9/21 | 4/27 | 0.1271 |
| Maximum tumor size,
cm | 2.95 (1.4–9.4) | 3.8 (2.2–7.6) | 0.1635 |
| Primary site, uncus
or head/body or tail | 16/14 | 17/14 | 1.0000 |
| Best treatment
response, CR/PR/SD/PD/NE | 0/4/12/11/3 | 0/3/8/13/7 | ORR, 0.7072/DCR,
0.2016 |
| Total bilirubin,
mg/dl | 0.7 (0.3–1.8) | 0.8 (0.3–6.6) | 0.7062 |
| Serum albumin,
g/dl | 3.6 (1.8–4.4) | 3.4 (2.5–4.4) | 0.2806 |
| Prothrombin time,
% | 86.15
(43.5–109.8) | 86.5
(64.7–127) | 0.5345 |
| Platelet count,
×104/mm3 | 21.4
(9.1–45.9) | 19.5
(7.1–35.3) | 0.3124 |
| White blood cell,
×103/µl | 5.92
(3.36–12.75) | 6.34
(2.54–29.76) | 0.5836 |
| Hemoglobin,
g/dl | 12.0
(7.6–15.1) | 11.5
(7.5–15.7) | 0.5342 |
| Serum creatinine,
mg/dl | 0.64
(0.33–1.28) | 0.65
(0.28–7.41) | 0.8062 |
| C reactive protein,
mg/dl | 0.4 (0–22.0) | 0.9 (0–6.9) | 0.3041 |
| AST, IU/l | 31.5 (11–265) | 25 (12–124) | 0.7127 |
| ALT, IU/l | 34 (8–289) | 29 (8–109) | 0.6084 |
| ALP, IU/l | 387.5
(153–1358) | 338.5
(139–1929) | 0.7096 |
| GGT, IU/l | 90.5 (14–747) | 98 (11–467) | 0.8140 |
| Amylase, IU/l | 66 (7–201) | 67 (17–357) | 0.5255 |
| CEA, IU/l | 3.2 (1.5–96.2) | 4.1
(1.4–286.1) | 0.1591 |
| CA19-9, IU/l | 286.4
(0.6–42414) | 801.7
(0.6–31020) | 0.2741 |
Cumulative OS rates for all cases and
comparison of OS rates between the PMI-H and the PMI-L group
The median follow-up period following initial
systemic chemotherapy for all cases was 246 days (range, 25–1,304
days). For all cases, the 6 month, 1- and 2-year cumulative
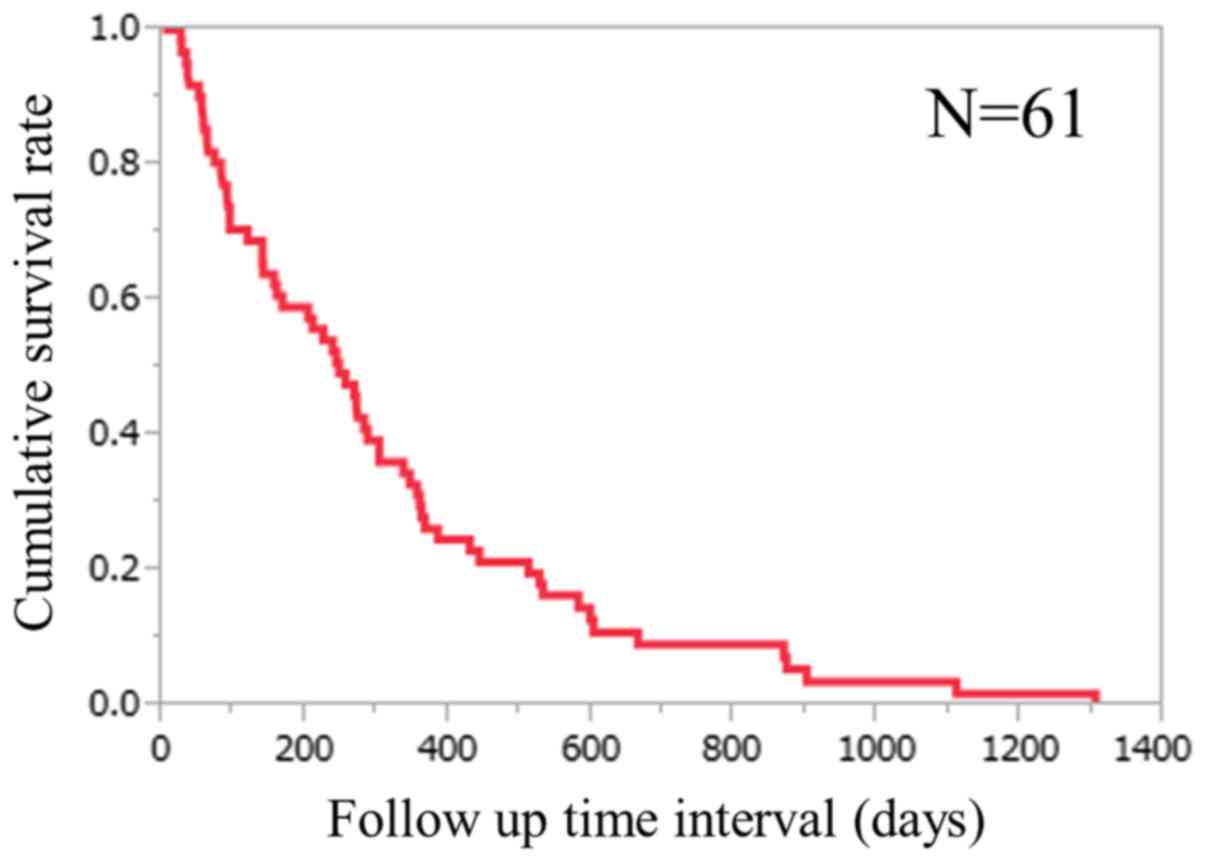
survival rates were 59.0, 27.9 and 9.1%, respectively (Fig. 1). The median follow-up period
following initial systemic chemotherapy was 357 days (range,
25–1,304 days) in the PMI-H group and 155 days (range, 26–900 days)
in the PMI-L group. The 6 month, 1- and 2-year cumulative survival
rates were 73.3, 43.3 and 15.2%, respectively, in the PMI-H group,
and 45.2, 12.9 and 3.2%, respectively, in the PMI-L group
(P=0.0027; Fig. 2).
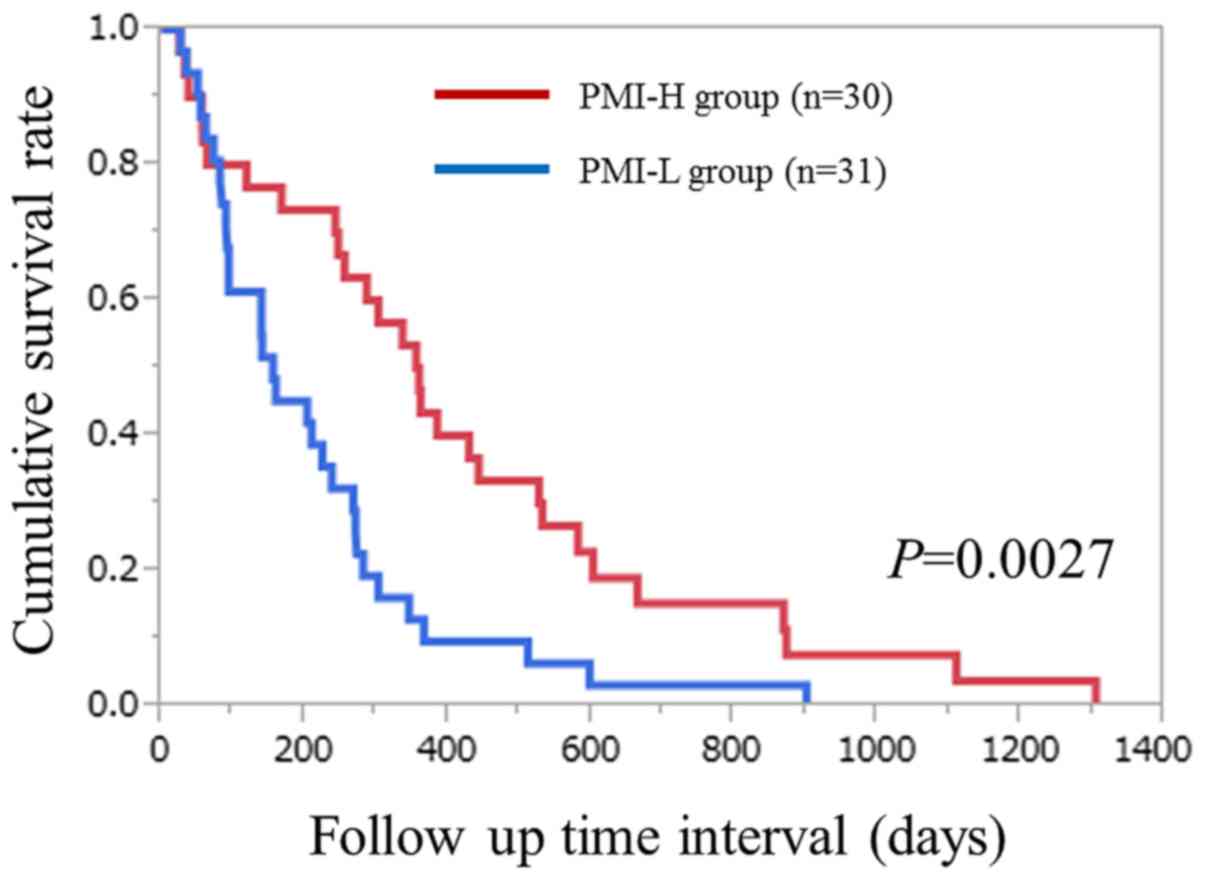 | Figure 2.Cumulative overall survival rates for
patients with unresectable advanced pancreatic cancer undergoing
systemic chemotherapy in each of the PMI-H and PMI-L groups. The
6-month, 1- and 2-year cumulative survival rates were 73.3, 43.3
and 15.2%, respectively, in the PMI-H group, and 45.2, 12.9, and
3.2%, respectively, in the PMI-L group (P=0.0027). PMI, psoas
muscle index; H, high; L, low. |
Comparison of serious adverse events
(SAEs) of grade ≥3 between the PMI-H and the PMI-L groups
The prevalence of chemotherapy-associated SAEs of
grade ≥3, as assessed using CTCAE (version 3.0), were 10.0% (3/30)
in the PMI-H group and 25.8% (8/31) in the PMI-L group (P=0.1822;
data not presented). In the PMI-H group, SAEs of grade ≥3 included
severe vomiting (1 patient), severe neuropathy (1 patient) and
neutropenia (1 patient). In the PMI-L group, SAEs of grade ≥3
included interstitial pneumonia (1 patient), severe anemia (1
patient), thrombocytopenia (2 patients), liver injury (1 patient),
neutropenia (1 patient) and jaundice (2 patients). In the present
study, chemotherapy-associated mortality was not observed (data not
presented).
Most improved tumor treatment response
during chemotherapy
With regard to the most improved treatment response
during chemotherapy, out of all cases CR was achieved in 0, PR in
7, SD in 20, PD in 24 and not evaluated (NE) in 10 patients
(Table II). The ORR and DCR were
calculated to be 11.5% (7/61) and 44.3% (27/61), respectively. In
the analysis of the most improved tumor response in the PMI-H
group, CR was achieved in 0, PR in 4, SD in 12, PD in 11 and NE in
3 patients. The ORR and DCR were calculated to be 13.3% (4/30) and
53.3% (16/30), respectively. In the analysis of the most improved
tumor response in the PMI-L group, CR was achieved in 0, PR in 3,
SD in 8, PD in 13 and NE in 7 patients. The ORR and DCR were
calculated to be 9.7% (3/31) and 35.5% (11/31), respectively. No
significant differences in the most improved treatment response
were identified between the PMI-H and PMI-L groups (ORR, P=0.7072;
DCR, P=0.2016; Table II).
Causes of mortality
During the follow-up period, 60 patients (98.4%)
succumbed to disease. In the PMI-H group, 29 (96.7%) patients
succumbed during the follow-up period. All patients succumbed due
to tumor progression. In the PMI-L group, 31 (100%) patients
succumbed during the follow-up period. All patients succumbed due
to tumor progression.
Univariate and multivariate analyses
of parameters contributing to OS
The univariate analysis identified that the
following factors significantly contributed to OS for all cases
(n=61): PMI (H or L; P=0.0027); tumor stage (IVA or IVB; P=0.0026);
maximum tumor size (>3.6 cm or ≤3.6 cm P=0.0262); prothrombin
time (PT; >86.3% or ≤86.3%; P=0.0052); C reactive protein (CRP;
>0.6 mg/dl or ≤0.6 mg/dl; P=0.0445); gamma glutamyl
transpeptidase (>98 IU/l or ≤98 IU/l; P=0.0175); CA 19-9 >408
IU/l or ≤408 IU/l (P=0.0008) (Table
III). The hazard ratios and 95% confidence intervals determined
by multivariate analysis for the 7 variables (selected based on a
P<0.05 in univariate analysis) are detailed in Table III. On multivariate analysis, PMI (H
or L; P=0.0036), PT (>86.3% or ≤86.3%; P=0.0044) and CA19-9
(>408 IU/l or ≤408 IU/l; P=0.0451) were identified as
significant predictors of OS.
 | Table III.Univariate and multivariate analyses
of factors associated with overall survival for patients with
unresectable advanced pancreatic cancer (n=61). |
Table III.
Univariate and multivariate analyses
of factors associated with overall survival for patients with
unresectable advanced pancreatic cancer (n=61).
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | No. of
patients | Univariate
analysis | Hazard ratio | 95% CI | P-value |
|---|
| Age, >72/≤72
years | 29/32 | 0.4593 |
|
|
|
| Sex,
male/female | 31/30 | 0.3967 |
|
|
|
| ECOG-performance
status, 0–1/2 | 43/18 | 0.6853 |
|
|
|
| PMI, high/low | 30/31 | 0.0027 | 2.446 | 1.340–4.541 | 0.0036a |
| Body mass index,
>21.2/≤21.2 kg/m2 | 30/31 | 0.5700 |
|
|
|
| Pancreatic cancer
stage, IVA/IVB | 13/48 | 0.0026 | 2.147 | 0.934–5.207 | 0.0725 |
| Maximum tumor size,
>3.6/≤3.6 cm | 29/32 | 0.0262 | 0.901 | 0.489–1.648 | 0.7351 |
| Primary site, uncus
or head/body or tail | 33/28 | 0.1758 |
|
|
|
| Total bilirubin,
>0.7/≤0.7 mg/dl | 29/32 | 0.3342 |
|
|
|
| Serum albumin,
>3.5/≤3.5 g/dl | 28/33 | 0.2986 |
|
|
|
| Prothrombin time,
>86.3/≤86.3% | 30/31 | 0.0052 | 2.219 | 1.283–3.874 | 0.0044a |
| Platelet count,
>20.8/≤20.8 ×104/mm3 | 30/31 | 0.0951 |
|
|
|
| WBC,
>6.05/≤6.05×103/µl | 30/31 | 0.4832 |
|
|
|
| Hemoglobin,
>11.6/≤11.6 g/dl | 30//31 | 0.4077 |
|
|
|
| Serum creatinine,
>0.65/≤0.65 mg/dl | 28/33 | 0.6884 |
|
|
|
| CRP, >0.6/≤0.6
mg/dl | 30/31 | 0.0445 | 0.665 | 0.384–1.156 | 0.1474 |
| AST, >26/≤26
IU/l | 29/32 | 0.9588 |
|
|
|
| ALT, >30/≤30
IU/l | 30/31 | 0.1678 |
|
|
|
| ALP, >361/≤361
IU/l | 29/32 | 0.5993 |
|
|
|
| GGT, >98/≤98
IU/l | 30/31 | 0.0175 | 0.808 | 0.425–1.509 | 0.5053 |
| Amylase, >66/≤66
IU/l | 29/32 | 0.7084 |
|
|
|
| CEA, >3.95/≤3.95
IU/l | 29/29 | 0.1372 |
|
|
|
| CA19-9,
>408/≤408 IU/l | 30/31 | 0.0008 | 0.504 | 0.251–0.985 | 0.0451a |
Discussion
The effect of muscle mass depletion on clinical
outcomes in solid malignancies is a relevant topic among
oncologists (17,18). However, as aforementioned, few reports
have addressed this important clinical question in patients with
unresectable APC who are receiving systemic chemotherapy (24,25). The
present study was, therefore, conducted. The data of the present
study revealed that subjects in the PMI-H group survived
significantly longer compared with those in the PMI-L group
(P=0.0027) and additionally, lower PMI was revealed to be an
independent adverse predictor for survival. These results suggest
that pretreatment PMI is useful for predicting outcomes for
unresectable patients with APC undergoing systemic chemotherapy.
Since the majority of previous reports have focused on the effect
of skeletal muscle mass on survival for patients undergoing
surgery, the results of the current study may be worth reporting
(16,19–23).
For the baseline PMI values, the median PMI in males
was 4.3 cm2/m2 (range, 1.6–8.2
cm2/m2), whereas in females it was 2.3
cm2/m2 (range, 0.7–6.1
cm2/m2). However, Hamaguchi et al
(34) demonstrated that the PMI
figure below two standard deviations of the mean among 541 healthy
living donors for liver transplantation were 6.36
cm2/m2 for males and 3.92
cm2/m2 for females. When these cut-off values
are applied to the current cohort, 55 patients (90.2%) were
determined to have muscle mass loss. The significant discrepancy
between the present data and the results of Hamaguchi et al
(34) for baseline PMI may be
attributed to the presence of advanced malignancies. Advanced
malignancies themselves can cause severe muscle mass loss (17,18).
Regarding comparison of baseline characteristics between the PMI-H
and PMI-L groups, ECOG-PS and BMI were identified to be significant
factors. DSMM is associated with disability, functional decline,
poorer nutritional status and frailty, which may lead to poorer PS
and lower BMI (13,14). However, aging was not identified to be
a significant factor in the present study. Advanced malignancies,
rather than just aging, may also affect skeletal muscle loss
(17,18). The proportion of SAEs of grade ≥3 in
the PMI-H group was higher, as compared with in the PMI-L group,
although the difference in the two groups did not reach
significance in the present study. The majority of previous studies
demonstrated that DSMM can increase the risk of development of
surgery-associated complications for patients with PC (16,19–23). Thus,
caution for the development of SAEs during chemotherapy should be
exercised, particularly in patients with PC with lower skeletal
muscle mass.
CA19-9 was identified as an independent predictor
for survival in the multivariate analysis. CA19-9 is the pancreatic
cancer biomarker currently recommended for clinical use, and
numerous reports have revealed that elevated CA 19-9 levels are
associated with a worse survival rate, which are concordant with
the results of the current study (35,40). In
addition, CA19-9 levels in stage IVB patients were significantly
higher compared with those in stage IVA patients (P=0.0060), as
determined in the present study, which indicates that this
biomarker effectively reflects tumor status. However, CRP is an
inflammation marker and elevated CRP levels have been demonstrated
to be adverse predictive factors in patients with solid
malignancies (41). Although CRP was
not identified to be significant in the present multivariate
analysis, this marker may be important for predicting outcomes.
Clinical evidence that physical activity is
beneficial for patients with solid malignancies in reducing
chemotherapy-associated symptoms and improving quality of life, as
well as drug tolerance and drug adherence for chemotherapy, has
previously been reported (42).
However, the effects of physical activity in patients with APC
undergoing systemic chemotherapy remain unclear. Currently, a
randomized controlled trial for investigating the impact of
physical activity on outcomes in patients with APC is underway
(43). If positive results are
obtained in the study, treatment strategies for patients with APC
receiving systemic chemotherapy may be altered in the future.
A number of limitations must be acknowledged with
regard to the present study. Firstly, it was a retrospective
observational study and biases inherent to retrospective analyses
could not be completely removed. Secondly, the initial
chemotherapeutic agents differed between the patients and these
therapies could have potentially caused bias for clinical outcomes.
Thirdly, the sample size was relatively small for analysis,
potentially creating bias. Fourth, muscle quality as reflected by
muscle strength was not evaluated in the current analysis. Finally,
the present study population only included Japanese patients with
PC with relatively low body weights compared with patients with PC
in Western countries (18).
Therefore, these results may not be directly applied to different
ethnic populations. However, the results of the present study
demonstrated that DSMM is associated with the clinical outcomes of
patients with APC undergoing systemic chemotherapy.
In conclusion, DSMM as determined by PMI may be a
significant predictor of prognosis in patients with APC receiving
systemic chemotherapy. In such patients, appropriate interventions
may be required for ameliorating the clinical outcome.
Acknowledgements
The authors would like to thank all medical staff at
the Department of Internal Medicine, Hyogo College of Medicine
(Nishinomiyashi, Japan) for their assistance.
Glossary
Abbreviations
Abbreviations:
|
APC
|
advanced pancreatic cancer
|
|
DSMM
|
decreased skeletal muscle mass
|
|
UICC
|
Union for International Cancer
Control
|
|
CT
|
computed tomography
|
|
EUS
|
endoscopic ultrasonography
|
|
PS
|
performance status
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
L3
|
the third lumber
|
|
PMI
|
psoas muscle index
|
|
H
|
high
|
|
L
|
low
|
|
OS
|
overall survival
|
|
RECIST
|
Response Evaluation Criteria in Solid
Tumors
|
|
CEA
|
carcinoembryonic antigen
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
CTCAE
|
Common Terminology Criteria for
Adverse Events
|
|
CR
|
complete response
|
|
PR
|
partial response
|
|
SD
|
stable disease
|
|
PD
|
progressive disease
|
|
ORR
|
objective response rate
|
|
DCR
|
disease control rate
|
|
SAE
|
serious adverse event
|
|
NE
|
not evaluated
|
|
PT
|
prothrombin time
|
|
CRP
|
C reactive protein
|
References
|
1
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sudo K, Nakamura K and Yamaguchi T: S-1 in
the treatment of pancreatic cancer. World J Gastroenterol.
20:15110–15118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cid-Arregui A and Juarez V: Perspectives
in the treatment of pancreatic adenocarcinoma. World J
Gastroenterol. 21:9297–9316. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malvezzi M, Bertuccio P, Levi F, La
Vecchia C and Negri E: European cancer mortality predictions for
the year 2013. Ann Oncol. 24:792–800. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ueno H, Ioka T, Ikeda M, Ohkawa S,
Yanagimoto H, Boku N, Fukutomi A, Sugimori K, Baba H, Yamao K, et
al: Randomized phase III study of gemcitabine plus S-1, S-1 alone,
or gemcitabine alone in patients with locally advanced and
metastatic pancreatic cancer in Japan and Taiwan: GEST study. J
Clin Oncol. 31:1640–1648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Imaoka H, Kou T, Tanaka M, Egawa S, Mizuno
N, Hijioka S, Hara K, Yazumi S, Shimizu Y and Yamao K: Clinical
outcome of elderly patients with unresectable pancreatic cancer
treated with gemcitabine plus S-1, S-1 alone, or gemcitabine alone:
Subgroup analysis of a randomised phase III trial, GEST study. Eur
J Cancer. 54:96–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaga Y, Sunakawa Y, Kubota Y, Tagawa T,
Yamamoto T, Ikusue T, Uto Y, Miyashita K, Toshima H, Kobayashi K,
et al: Early tumor shrinkage as a predictor of favorable outcomes
in patients with advanced pancreatic cancer treated with
FOLFIRINOX. Oncotarget. 7:67314–67320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boursi B, Finkelman B, Giantonio BJ,
Haynes K, Rustgi AK, Rhim AD, Mamtani R and Yang YX: A clinical
prediction model to assess risk for pancreatic cancer among
patients with new-onset diabetes. Gastroenterology. 152:840–850.e3.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
American Cancer Society, . Cancer Facts
& Figures 2013. American Cancer Society, Inc.; Atlanta, GA:
2013
|
|
11
|
Muller MJ, Wang Z, Heymsfield SB, Schautz
B and Bosy-Westphal A: Advances in the understanding of specific
metabolic rates of major organs and tissues in humans. Curr Opin
Clin Nutr Metab Care. 16:501–508. 2013.PubMed/NCBI
|
|
12
|
Dasarathy S: Consilience in sarcopenia of
cirrhosis. J Cachexia Sarcopenia Muscle. 3:225–237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosenberg IH: Sarcopenia: Origins and
clinical relevance. J Nutr. 127 5 Suppl:990S–991S. 1997.PubMed/NCBI
|
|
14
|
Wang C and Bai L: Sarcopenia in the
elderly: Basic and clinical issues. Geriatr Gerontol Int.
12:388–396. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukushima H, Nakanishi Y, Kataoka M,
Tobisu K and Koga F: Prognostic significance of sarcopenia in
patients with metastatic renal cell carcinoma. J Urol. 195:26–32.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levolger S, van Vugt JL, de Bruin RW and
IJzermans JN: Systematic review of sarcopenia in patients operated
on for gastrointestinal and hepatopancreatobiliary malignancies. Br
J Surg. 102:1448–1458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Prado CM, Lieffers JR, McCargar LJ, Reiman
T, Sawyer MB, Martin L and Baracos VE: Prevalence and clinical
implications of sarcopenic obesity in patients with solid tumors of
the respiratory and gastrointestinal tracts: A population based
study. Lancet Oncol. 9:629–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chindapasirt J: Sarcopenia in Cancer
Patients. Asian Pac J Cancer Prev. 16:8075–8077. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Dijk DP, Bakens MJ, Coolsen MM, Rensen
SS, van Dam RM, Bours MJ, Weijenberg MP, Dejong CH and Damink SW
Olde: Low skeletal muscle radiation attenuation and visceral
adiposity are associated with overall survival and surgical site
infections in patients with pancreatic cancer. J Cachexia
Sarcopenia Muscle. 8:317–326. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carrara G, Pecorelli N, De Cobelli F,
Cristel G, Damascelli A, Beretta L and Braga M: Preoperative
sarcopenia determinants in pancreatic cancer patients. Clin Nutr.
Oct 20–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Onesti JK, Wright GP, Kenning SE, Tierney
MT, Davis AT, Doherty MG and Chung MH: Sarcopenia and survival in
patients undergoing pancreatic resection. Pancreatology.
16:284–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Amini N, Spolverato G, Gupta R, Margonis
GA, Kim Y, Wagner D, Rezaee N, Weiss MJ, Wolfgang CL, Makary MM, et
al: Impact total psoas volume on short- and long-term outcomes in
patients undergoing curative resection for pancreatic
adenocarcinoma: N new tool to assess sarcopenia. J Gastrointest
Surg. 19:1593–1602. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zalite I Ozola, Zykus R, Gonzalez M
Francisco, Saygili F, Pukitis A, Gaujoux S, Charnley RM and Lyadov
V: Influence of cachexia and sarcopenia on survival in pancreatic
ductal adenocarcinoma: A systematic review. Pancreatology.
15:19–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi Y, Oh DY, Kim TY, Lee KH, Han SW, Im
SA, Kim TY and Bang YJ: Skeletal muscle depletion predicts the
prognosis of patients with advanced pancreatic cancer undergoing
palliative chemotherapy, independent of body mass index. PLoS One.
10:e01397492015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park I, Choi SJ, Kim YS, Ahn HK, Hong J,
Sym SJ, Park J, Cho EK, Lee JH, Shin YJ and Shin DB: Prognostic
factors for risk stratification of patients with recurrent or
metastatic pancreatic adenocarcinoma who were treated with
gemcitabine-based chemotherapy. Cancer Res Treat. 48:1264–1273.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trotti A, Colevas AD, Setser A, Rusch V,
Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN and
Rubin P: CTCAE v3.0: Development of a comprehensive grading system
for the adverse effects of cancer treatment. Semin Radiat Oncol.
13:176–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nawaz H, Fan CY, Kloke J, Khalid A,
McGrath K, Landsittel D and Papachristou GI: Performance
characteristics of endoscopic ultrasound in the staging of
pancreatic cancer: A meta-analysis. JOP. 14:484–497.
2013.PubMed/NCBI
|
|
29
|
Zhao WY, Luo M, Sun YW, Xu Q, Chen W, Zhao
G and Wu ZY: Computed tomography in diagnosing vascular invasion in
pancreatic and periampullary cancers: A systematic review and
meta-analysis. Hepatobiliary Pancreat Dis Int. 8:457–464.
2009.PubMed/NCBI
|
|
30
|
Yang R, Lu M, Qian X, Chen J, Li L, Wang J
and Zhang Y: Diagnostic accuracy of EUS and CT of vascular invasion
in pancreatic cancer: A systematic review. J Cancer Res Clin Oncol.
140:2077–2086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Puli SR, Singh S, Hagedorn CH, Reddy J and
Olyaee M: Diagnostic accuracy of EUS for vascular invasion in
pancreatic and periampullary cancers: A meta-analysis and
systematic review. Gastrointest Endosc. 65:788–797. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Doi R, Imamura M, Hosotani R, Imaizumi T,
Hatori T, Takasaki K, Funakoshi A, Wakasugi H, Asano T, Hishinuma
S, et al: Surgery versus radiochemotherapy for resectable locally
invasive pancreatic cancer: Final results of a randomized
multi-institutional trial. Surg Today. 38:1021–1028. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Okumura S, Kaido T, Hamaguchi Y, Fujimoto
Y, Masui T, Mizumoto M, Hammad A, Mori A, Takaori K and Uemoto S:
Impact of preoperative quality as well as quantity of skeletal
muscle on survival after resection of pancreatic cancer. Surgery.
157:1088–1098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamaguchi Y, Kaido T, Okumura S, Kobayashi
A, Hammad A, Tamai Y, Inagaki N and Uemoto S: Proposal for new
diagnostic criteria for low skeletal muscle mass based on computed
tomography imaging in Asian adults. Nutrition. 32:1200–1205. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamaguchi K, Okusaka T, Shimizu K, Furuse
J, Ito Y, Hanada K and Shimosegawa T; Committee for revision of
clinical guidelines for pancreatic cancer of Japan Pancreas
Society, : EBM-based clinical guidelines for pancreatic cancer
(2013) issued by the japan pancreas society: A synopsis. Jpn J Clin
Oncol. 44:883–888. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hewitt MJ, McPhail MJ, Possamai L, Dhar A,
Vlavianos P and Monahan KJ: EUS-guided FNA for diagnosis of solid
pancreatic neoplasms: A meta-analysis. Gastrointest Endosc.
75:319–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishii H, Furuse J, Boku N, Okusaka T,
Ikeda M, Ohkawa S, Fukutomi A, Hamamoto Y, Nakamura K and Fukuda H;
JCOG Gastrointestinal Oncology Study Group, : Phase II study of
gemcitabine chemotherapy alone for locally advanced pancreatic
carcinoma: JCOG0506. Jpn J Clin Oncol. 40:573–579. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Le N, Sund M and Vinci A; GEMS
collaborating group of Pancreas, : 2000 Prognostic and predictive
markers in pancreatic adenocarcinoma. Dig Liver Dis. 48:223–230.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mahmoud FA and Rivera NI: The role of
C-reactive protein as a prognostic indicator in advanced cancer.
Curr Oncol Rep. 4:250–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Speck RM, Courneya KS, Mâsse LC, Duval S
and Schmitz KH: An update of controlled physical activity trials in
cancer survivors: A systematic review and meta-analysis. J Cancer
Surviv. 4:87–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Neuzillet C, Vergnault M, Bonnetain F and
Hammel P: Rationale and design of the Adapted Physical Activity in
advanced Pancreatic Cancer patients (APACaP) GERCOR (Groupe
Coopérateur Multidisciplinaire en Oncologie) trial: Study protocol
for a randomized controlled trial. Trials. 16:4542015. View Article : Google Scholar : PubMed/NCBI
|