Introduction
Endometrial cancer poses a threat for the health of
females, with ~320,000 newly diagnosed cases each year worldwide
(1). A total of 76,000 patients
eventually succumb to this malignancy per year, making it the sixth
most common type of cancer in females (2). In view of the increasing incidence,
understanding how this malignancy is initiated and progresses is of
great importance, and this knowledge is required. It is now well
established that in addition to genetic changes, epigenetic
alterations, including DNA methylation and post-translational
histone modifications, which control chromatin accessibility and
gene activity, are associated with the aberrant expression level of
oncogenic or tumor-suppressor genes. Chromatin-modifying enzymes
can catalyze reversible modifications of histones, in which lysine
methylation serve an important role.
A repression marker, histone H3 lysine 27
trimethylation (H3K27me3), and two activating markers, histone H3
lysine 4 dimethylation (H3K4me2) and histone H3 lysine 4
trimethylation (H3K4me3), were selected as they have been revealed
to possess associations with cancer (3–5). These,
and other modifications, generate a combinatorial histone code that
demarcates chromatin regions for transcription activation or
repression. Recently, cellular patterns of histone modifications
have been demonstrated to be prognostic indicators for numerous
types of tumors, including prostate (6), kidney (7),
lung (8), gastric (9), ovarian (10) and breast cancer (11); however, little is known about the
global alteration of histone status during tumorigenesis, and
cancer progression in endometrial cancer. The present study
therefore aimed to assess the clinical significance of selected
histone modifications in endometrial tissues, including the normal
endometrium, precancerous lesions and endometrial cancer. The
association between histone modifications and the
clinicopathological data was analyzed in the present study.
Materials and methods
Research subject
After obtaining approval from the Obstetrics and
Gynecology Hospital of Fudan University (Shanghai, China) Ethics
Committee, 99 endometrial tissues (including 24 normal endometrial
samples, 18 precancerous lesions, and 44 Type 1 and 13 Type 2
endometrial cancer samples) were obtained from patients treated in
the Obstetrics and Gynecology Hospital of Fudan University between
June 2008 and December 2012. The mean age of all patients enrolled
was 51.7±10.2 years. The mean ages of the normal group,
precancerous group, and type 1 and type 2 cancer groups were
47.5±7.2, 44.3±7.7, 56.4±8.8, and 55.15±12.4 years, respectively.
Written informed consent was obtained from all patients prior to
enrollment in the present study. Normal tissues, including 12
proliferative, 9 secretory and 3 atrophic endometrial tissues, were
obtained from patients who had benign disease, including
adenomyosis or myoma, and 18 precancerous lesions, including 9
simple hyperplasia, 2 complex hyperplasia and 7 atypical
hyperplasia tissues from patients who had irregular bleeding.
Hematoxylin and eosin (H&E) (hematoxylin for 5 min and eosin
for 10 sec at temperature between 20–24°C) stained tissue sections
adjacent to the tissue sections obtained for immunohistochemical
staining of all specimens were reviewed by an experienced
pathologist to confirm the diagnosis. For patients with endometrial
cancer, the clinicopathological parameters, including International
Federation of Gynecology and Obstetrics (FIGO) stage, tumor grade,
depth of myometrial invasion and p53, as well as estrogen receptor
(ER), progesterone receptor (PR) and lymph-vascular space invasion
(LVSI) evaluations, were collected. The surgical pathology stage
was determined in accordance with the 2009 FIGO guidelines
(12).
Immunohistochemistry
Antigen retrieval and antibody dilutions were
optimized prior to the initiation of the study (Mingrui Biotech,
Shanghai, China). Diagnosis of each case was made by two
experienced pathologists without discrepancy. To ensure uniformity,
all tissue sections were processed synchronically. The H&E
tissue sections (4 µm) adjacent to the sections used for
immunohistochemistry assessment were used for pathological
diagnosis. The working dilutions of anti-H3K27me3 antibodies (cat.
no. CST 9733s; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-H3K4me2 antibodies (cat. no. Ab32356; Abcam, Cambridge, UK)
and anti-H3K4me3 antibodies (cat. no. Ab8580; Abcam) were 1:100.
Endogenous peroxidase activity was blocked using 0.3%
H2O2. Slides were washed and incubated with
the biotinylated secondary antibody (polyclonal goat anti-rabbit;
Histostain-Plus IHC kit; Mingrui Biotech, Shanghai, China) for 45
min at 37°C and washed with PBS.
Procedures were performed using an
immunohistochemistry kit (MR Biotech Ltd., Shanghai, China),
according to the manufacturer's protocol. Tissue staining
intensities were assessed using double-blinded quantitative
scoring. For the semi-quantitative analysis, 5 fields of each slide
were randomly selected under magnification, ×10 and further read
under magnification, ×40 using light microscope. Positive staining
refers to dark brown granules within the nuclear region, with or
without brown granules in the cytoplasm. The percentages of
positive cells were assessed independently by two observers and the
mean values of their results was used for evaluation.
Statistical analysis
All data were statistically analyzed by an
independent-sample t-test (Students' t-test) or one-way analysis of
variance (ANOVA, Bonferroni used as post-hoc test) using SPSS
version 20.0 (IBM Corp., Armonk, NY, USA). The data were presented
as mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results
Samples from 24 normal endometrial tissues, 18
precancerous lesions, and 44 type 1 and 17 type 2 endometrial
cancer tissues were retrieved. The present study observed the
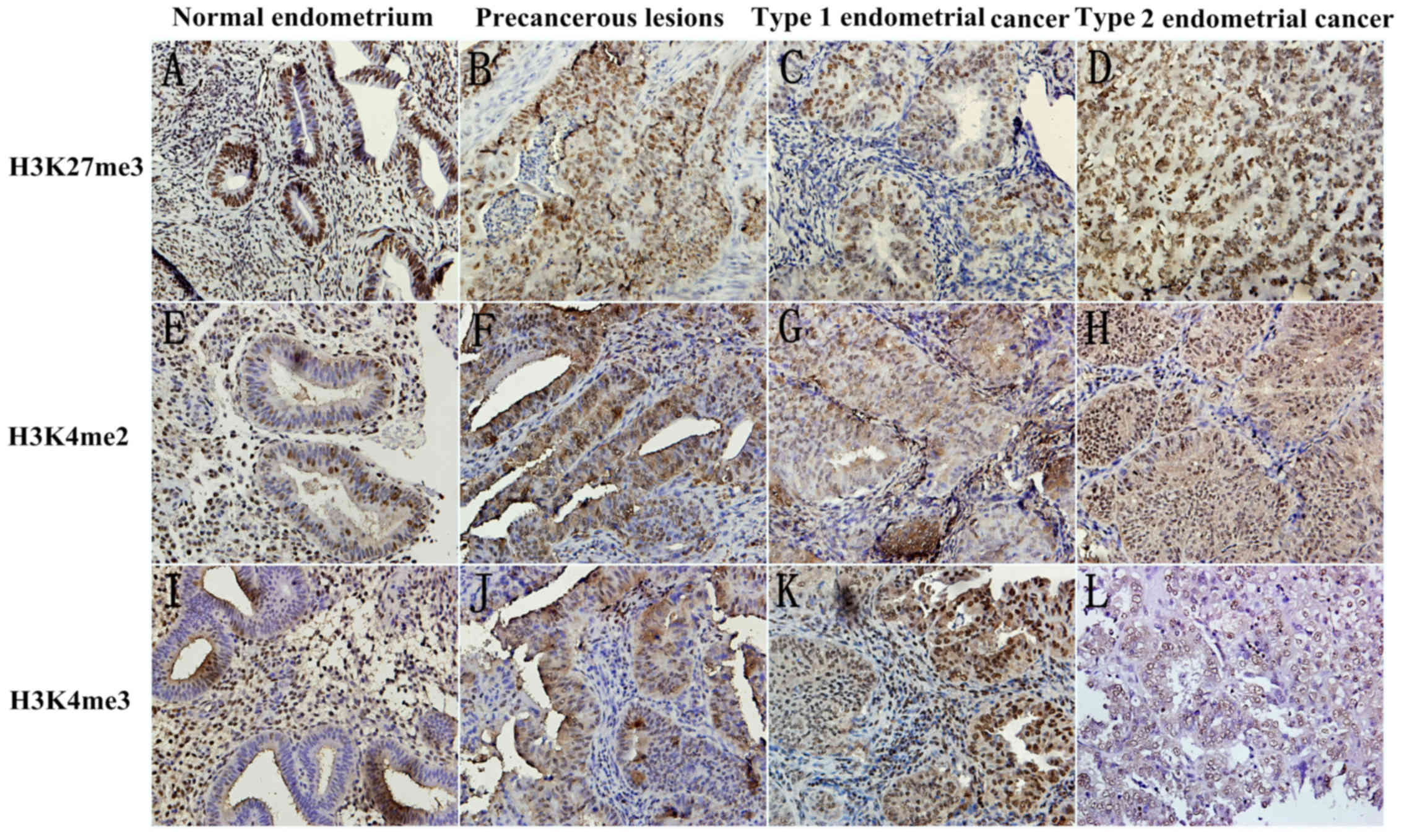
methylation expression level of three markers, H3K27me3, H3K4me2
and H3K4me3, by immunohistochemistry in the epithelial, and stromal
compartments of tissues. Fig. 1
Presents the representative images.
The patients with type 2 cancer were postmenopausal;
therefore, the present study compared the methylation levels of the
markers in two sessions: Type 1 cancer with a normal endometrium
and hyperplasia tissue; and type 2 cancer with an atrophic
endometrium. For normal endometrium, the present study used one-way
ANOVA to determine whether there were significant differences among
the proliferative, secretory and atrophic endometrium tissues. As
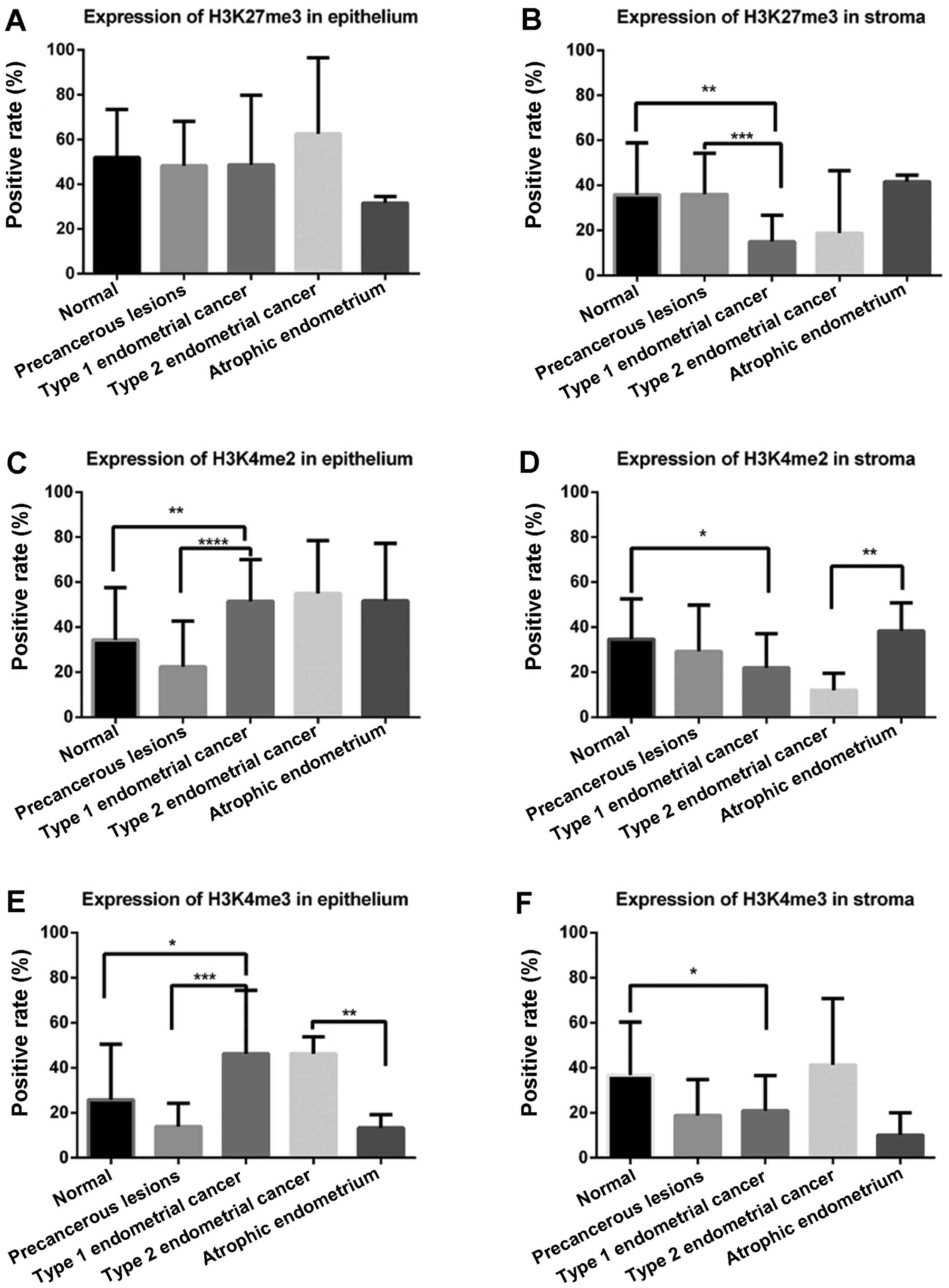
presented in Fig. 2A, in glandular
epithelium, H3K27me3 demonstrated no difference in cancerous
tissues and noncancerous tissues; however, in stromal tissue, the
expression level of H3K27me3 was lowest in the stroma of Type 1
endometrial cancer compared with in the normal endometrium
(P=0.002) and precancerous lesions (P=0.001; Fig. 2B).
H3K4me2 was highly expressed in the glandular
epithelium of Type 1 endometrial cancer compared with in the normal
endometrium (P=0.007) and precancerous lesions (P<0.001;
Fig. 2C). Conversely, in the stroma,
a lower expression level of H3K4me2 was identified in Type 1
endometrial cancer compared with precancerous lesions (P=0.012;
Fig. 2D). Similarly, a lower
expression level of H3K4me2 in the stroma was demonstrated in Type
2 endometrial cancer compared with intheatrophic endometrium
(P=0.009).
In glandular epithelium (Fig. 2E), the expression level of H3K4me3 was
higher in Type 1 endometrial cancer compared within the normal
endometrium (P=0.02) and precancerous lesions (P<0.001), which
was similar to the expression level of H3K4me2. The H3K4me3
expression levels were also higher in epithelial cells of Type 2
endometrial cancer compared within the atrophic endometrium
(P=0.002). Lower expression levels of H3K4me3 in the stroma were
revealed in Type 1 endometrial cancer compared with in the normal
endometrium (P=0.03; Fig. 2F).
Subsequently, the present study investigated the
association between the expression levels of histone modification
markers and clinicopathological features in endometrial cancer.
As presented in Tables
I and II, there were no
significant differences between the H3K27me3 expression levels and
clinical characteristics, including FIGO stage, tumor grade, depth
of myometrial invasion, P53, ER, PR, and LVSI. In the epithelial
elements of Type 1 endometrial cancer, a low expression level of
H3K4me2 was associated with an early FIGO stage (P=0.006). In the
stroma of Type 2 endometrial cancer, a low expression level of
H3K4me3 was associated with P53 negativity (P=0.032).
 | Table I.Association between the expression
levels of histone modification markers and clinicopathological
features in glandular epithelium of endometrial cancer. |
Table I.
Association between the expression
levels of histone modification markers and clinicopathological
features in glandular epithelium of endometrial cancer.
| Clinicopathological
characteristic | H3K27me3 |
P-valuea | H3K4me2 |
P-valueb | H3K4me3 |
P-valuec |
|---|
| FIGO stage |
| 0.325 |
| 0.006 |
| 0.814 |
| I | 0.58±0.22 |
| 0.42±0.18 |
| 0.47±0.27 |
|
|
II–IV | 0.49±0.31 |
| 0.60±0.20 |
| 0.49±0.30 |
|
| Tumor grade |
| NS |
| NS |
| NS |
| G1 | 0.56±0.27 |
| 0.47±0.14 |
| 0.47±0.29 |
|
| G2 | 0.63±0.16 |
| 0.61±0.26 |
| 0.48±0.21 |
|
| G3 | 0.43±0.33 |
| 0.54±0.17 |
| 0.43±0.33 |
|
| Type
2 | 0.63±0.34 |
| 0.55±0.23 |
| 0.46±0.08 |
|
| Endometrial
infiltration |
| NS |
| NS |
| NS |
| Limited
to endometrium | 0.58±0.39 |
| 0.33±0.15 |
| 0.29±0.30 |
|
|
<1/2 | 0.58±0.19 |
| 0.44±0.17 |
| 0.44±0.29 |
|
|
≥1/2 | 0.47±0.34 |
| 0.60±0.20 |
| 0.32±0.33 |
|
| P53 |
| 0.105 |
| 0.339 |
| 0.631 |
|
Negative | 0.57±0.23 |
| 0.48±0.19 |
| 0.39±0.30 |
|
|
Positive | 0.76±0.13 |
| 0.58±0.21 |
| 0.46±0.40 |
|
| ER |
| 0.193 |
| 0.078 |
| 0.723 |
|
Negative | 0.40±0.33 |
| 0.61±0.20 |
| 0.37±0.34 |
|
|
Positive | 0.61±0.20 |
| 0.46±0.19 |
| 0.42±0.30 |
|
| PR |
| 0.773 |
| 0.653 |
| 0.460 |
|
Negative | 0.53±0.39 |
| 0.60±0.43 |
| 0.30±0.32 |
|
|
Positive | 0.49±0.29 |
| 0.47±0.17 |
| 0.42±0.31 |
|
| LVSI |
| 0.182 |
| 0.682 |
| 0.234 |
| No | 0.60±0.24 |
| 0.51±0.17 |
| 0.42±0.28 |
|
|
Yes | 0.48±0.26 |
| 0.48±0.21 |
| 0.28±0.28 |
|
 | Table II.Association between the expression
levels of histone modification markers and clinicopathological
features in the stroma of endometrial cancer. |
Table II.
Association between the expression
levels of histone modification markers and clinicopathological
features in the stroma of endometrial cancer.
| Clinicopathological
characteristic | H3K27me3 |
P-valuea | H3K4me2 |
P-valueb | H3K4me3 |
P-valuec |
|---|
| FIGO stage |
| 0.514 |
| 0.910 |
| 0.437 |
| I | 0.18±0.16 |
| 0.20±0.15 |
| 0.27±0.17 |
|
|
II–IV | 0.15±0.13 |
| 0.20±0.07 |
| 0.22±0.17 |
|
| Tumor grade |
| NS |
| NS |
| NS |
| G1 | 0.16±0.12 |
| 0.22±0.19 |
| 0.22±0.15 |
|
| G2 | 0.08±0.03 |
| 0.17±0.05 |
| 0.26±0.18 |
|
| G3 | 0.18±0.15 |
| 0.27±0.12 |
| 0.15±0.15 |
|
| Type
2 | 0.19±0.28 |
| 0.12±0.08 |
| 0.41±0.30 |
|
| Endometrial
infiltration |
| NS |
| NS |
| NS |
| Limited
to endometrium | 0.15±0.18 |
| 0.08±0.06 |
| 0.34±0.27 |
|
|
<1/2 | 0.15±0.14 |
| 0.18±0.15 |
| 0.22±0.17 |
|
|
≥1/2 | 0.10±0.10 |
| 0.19±0.09 |
| 0.13±0.15 |
|
| P53 |
| 0.394 |
| 0.112 |
| 0.032 |
|
Negative | 0.16±0.15 |
| 0.19±0.14 |
| 0.20±0.15 |
|
|
Positive | 0.10±0.07 |
| 0.09±0.08 |
| 0.36±0.25 |
|
| ER |
| 0.217 |
| 0.818 |
| 0.395 |
|
Negative | 0.09±0.11 |
| 0.18±0.09 |
| 0.16±0.16 |
|
|
Positive | 0.16±0.15 |
| 0.19±0.15 |
| 0.23±0.19 |
|
| PR |
| 0.096 |
| 0.877 |
| 0.163 |
|
Negative | 0.03±0.04 |
| 0.20±0.13 |
| 0.10±0.14 |
|
|
Positive | 0.16±0.15 |
| 0.19±0.14 |
| 0.23±0.19 |
|
| LVSI |
| 0.853 |
| 0.731 |
| 0.203 |
| No | 0.14±0.15 |
| 0.17±0.14 |
| 0.22±0.18 |
|
|
Yes | 0.15±0.09 |
| 0.19±0.11 |
| 0.13±0.16 |
|
Discussion
Histone is the key component of nucleosomes and
serves a significant role in epigenetics. Numerous epigenetic
studies have investigated the role of DNA methylation (13–15), but
there are few reports regarding histone methylation and its
significance in endometrial cancer. To the best of our knowledge,
the present study analyzed the expression of H3K4me2, H3K4me3 and
H3K27me3 in endometrial tissues by immunohistochemistry for the
first time, assessing the potential association between endometrial
cancer progression, and the expression levels of these three
markers.
The trimethylation of H3K27 is associated with the
transcriptional inhibition of genes. Enhancer of zeste 2 polycomb
repressive complex 2 subunit, a methyl-transferase for H3K27, is
upregulated in a variety of tumors and serves an essential role in
tumor promotion (2,16); our previous study reached the same
conclusion (17). Certain studies
have widely associated H3K27me3 with gene silencing and
transcriptional inhibition (6,18);
however, these studies have not achieved a consensus on the level
of H3K27me3 and the significance of its aberrant expression in
human tumorigenesis. Previous studies have suggested that the low
expression level of H3K27me3 in tumor tissues may enhance the
expression of oncogenes and consequently promote tumor growth
(19–22); however, Nakazawa et al
(23) revealed no significant
differences when comparing the expression levels of H3K4me2 and
H3K27me3 in 85 cases of colorectal cancer with the paired normal
colorectal tissues. Conversely, high expression levels of H3K27me3
were detected in other neoplasms (24,25). In
the present study, the expression level of H3K27me3 in the
endometrial stroma was significantly lower in Type 1 endometrial
cancer compared within the normal endometrium (P=0.043) and
precancerous lesions (P<0.001). A low expression level of
H3K27me3 may predict a more aggressive biological behavior in
endometrial carcinoma; however, there were no significant
differences in glandular epithelium of cancerous tissues and
noncancerous tissues, which may be due to insufficient sample
sizes. Stroma-tumor communication serves an important role in the
genesis of neoplasia (26). The
endometrium is composed of epithelium and lamina propria. The
epithelium comprises columnar epithelial cells (endometrial
epithelial cells) with a secretary function. Lamina propria
consists of endometrial stromal cells (ESCs), immune cells,
reticular fibers, matrix, blood vessels and nerves, forming the
microenvironment of the epithelial cells. Tan et al
(27) demonstrated that the
epithelial-to-mesenchymal transition of prostate cancer cells was
inhibited by adiponectin (ADN), which is also inversely correlated
with the risk of endometrial cancer (28), with decreasing expression levels of
H3K27me3 at the ADN promoter in 22RV1cells (a human prostate cancer
cell line). To date, to the best of our knowledge, no previous
study has focused on the association between adiponectin and
H3K27me3 in stromal cells of endometrial cancer. Future studies are
required to assess its potential role as a prognostic marker.
Presently, there is no definite understanding of
H3K4me2 in cancer tissues. Previous studies suggested that it was
positively associated with a poor prognosis in tumors (29,30),
whereas others held converse opinions (31,32) or
revealed no difference between the H3K4me2 expression levels and
prognoses (23). In the present
study, the H3K4me2 expression levels increased with the malignant
degree of endometrial tissues in the epithelium, indicating that
H3K4me2 was involved in the oncogenesis of endometrial cancer.
Furthermore, low expression levels of H3K4me2 in glandular
epithelium of endometrial cancer were significantly associated with
a clinical early FIGO stage (P=0.006). This finding indicated that
endometrial cancer with high expression levels of H3K4me2 tended to
be more invasive. Lei et al (33) and Liu et al (34) demonstrated that LIM-only protein 3 and
PR domain containing 16 were associated with a poor prognosis in
patients with astrocytoma and glioma. Both were indirectly
inhibited by the tumor suppressor microRNA-101 with decreased
H3K4me2 expression levels (33,34).
Cannuyer et al (35) revealed
that MAGE family member A1 demethylation and activation in melanoma
cells were associated with decreased expression levels of H3K9me2,
and increased expression levels of H3ac and H3K4me2, where they
encode tumor-specific antigens. Conversely, the stromal expression
level of H3K4me2 was significantly lower in type 1 and type 2
endometrial cancer compared within the normal endometrium (all
P=0.005). Different expression levels of H3K4me2 in the epithelium
and stroma may indicate diverse mechanisms, and influences. Further
studies are required to explore the role of histone modifications
in the stroma in order to elucidate the interactive effect between
the microenvironment and tumor cells.
The association between H3K4me3 expression level and
the prognosis of tumors also remains controversial. In an effort to
assess the cellular expression level of H3K4me3 in HCC and its
association with clinicopathological variables and outcomes,
expression levels of H3K4me3, and histone methyltransferase SET and
MYND domain-containing protein 3were investigated using western
blotting, and immunohistochemistry in cell lines and tumor tissue
microarrays from a well-characterized series of patients with HCC
(n=168) (29). The author compared
two experimental results, and revealed that patients with maximum
tumor diameters of <5 cm, a low tumor-node-metastasis score, no
intravascular invasion and no recurrence of hepatocellular
carcinoma had a lower expression level of H3K4me3 (P<0.05)
(29). It was also demonstrated that
as the expression level of H3K4me3 increased, the prognosis of
patients suffering from HCC worsened (P<0.0001) (36). However, this conclusion has been
disputed by another report (37). In
the present study, the expression levels of H3K4me3 increased with
the malignant degree of endometrial tissues in the epithelium;
however, the specific underlying mechanism remains to be further
explored. The factor p53 has a high level of association with
tumors in humans. The overexpression of p53 is often observed in
malignant tumors and can be a reliable marker for enhanced
proliferation (38). In the present
study, a low expression level of H3K4me3 in the stroma was
associated with p53-negativity (P=0.032), which predicts a benign
prognosis in humans with endometrial cancer. Tang et al
(39) demonstrated that
enhancer/promoter-bound p53, via direct interactions, recruits p300
and SET1 complex (SET1C) to affect associatedH3K4me3 events via
additional p300-SET1C interactions at the contiguous enhancer-core
promoter region. Mungamuri et al (40) revealed that in response to p53
stabilization, its pro-apoptotic target promoters become enriched
with the H3K4me3 epigenetic mark and its readers. Lauberth et
al (41) also demonstrated a
direct effect of H3K4me3 on p53-dependent transcription. It may
serve as a potential therapeutic target for the treatment of
endometrial cancer. Future studies are required to further evaluate
the biological function of H3K4me3 in endometrial tissue and
prospectively assess its potential role as a prognostic marker.
The majority of previous studies have focused on
glands; however, few have paid attention to the stroma in
endometrial cancer. The pathological diagnosis of endometrial
cancer primarily depends on its glandular component in a high state
of dysplasia. The stromal components of tumors (vascular, lymphatic
interstitial and protein) serve an important role during the tumor
growth and development. The roles of the stroma and its properties
have not been studied extensively. ESCs and gland cells maybe
homologous during embryogenesis for development from the Mullerian
duct (42). ESC and gland cells
express steroid receptors, meaning that they are regulated by
steroid hormones in the peripheral blood (43). Furthermore, the development of cancer
of gland cells accompanies the decline of ESCs until they disappear
(44). The present study analyzed the
expression level of histone methylation in the stroma of
endometrial tumors, and proposed that the expression levels of
H3K27me3 and H3K4me2 are low in the stroma of endometrial cancerous
tumors. Low expression levels of H3K4me3 in the stroma of
endometrial cancerous tumors may be associated with poor
prognosis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 30973185 and
81572836) and by the Shanghai Science and Technology Committee
(grant nos. 15140903200 and 16411953500).
Glossary
Abbreviations
Abbreviations:
|
H3K27me3
|
trimethylation of histone 3 lysine
27
|
|
H3K4me2
|
dimethylation of histone 3 lysine
4
|
|
H3K4me3
|
trimethylation of histone 3 lysine
4
|
|
LVSI
|
lymph-vascular space involvement
|
|
HCC
|
hepatocellular carcinoma
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gao Y, Hyttel P and Hall VJ: Regulation of
H3K27me3 and H3K4me3 during early porcine embryonic development.
Mol Reprod Dev. 77:540–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benard A, Goossens-Beumer IJ, van Hoesel
AQ, de Graaf W, Horati H, Putter H, Zeestraten EC, van de Velde CJ
and Kuppen PJ: Histone trimethylation at H3K4, H3K9 and H4K20
correlates with patient survival and tumor recurrence in
early-stage colon cancer. BMC Cancer. 14:5312014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deb M, Kar S, Sengupta D, Shilpi A, Parbin
S, Rath SK, Londhe VA and Patra SK: Chromatin dynamics: H3K4
methylation and H3 variant replacement during development and in
cancer. Cell Mol Life Sci. 71:3439–3463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tao H, Li H, Su Y, Feng D, Wang X, Zhang
C, Ma H and Hu Q: Histone methyltransferase G9a and H3K9
dimethylation inhibit the self-renewal of glioma cancer stem cells.
Mol Cell Biochem. 394:23–30. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ngollo M, Lebert A, Dagdemir A, Judes G,
Karsli-Ceppioglu S, Daures M, Kemeny JL, Penault-Llorca F, Boiteux
JP, Bignon YJ, et al: The association between histone 3 lysine 27
trimethylation (H3K27me3) and prostate cancer: Relationship with
clinicopathological parameters. BMC Cancer. 14:9942014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park KC, Heo JH, Jeon JY, Choi HJ, Jo AR,
Kim SW, Kwon HJ, Hong SJ and Han KS: The novel histone deacetylase
inhibitor, N-hydroxy-7-(2-naphthylthio) hepatonomide, exhibits
potent antitumor activity due to cytochrome-c-release-mediated
apoptosis in renal cell carcinoma cells. BMC Cancer. 15:192015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Langevin SM, Kratzke RA and Kelsey KT:
Epigenetics of lung cancer. Transl Res. 165:74–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang WY, Gu JL and Zhen TM: Recent
advances of histone modification in gastric cancer. J Cancer Res
Ther. 10 Suppl:S240–S245. 2014. View Article : Google Scholar
|
|
10
|
Lyu T, Jia N, Wang J, Yan X, Yu Y, Lu Z,
Bast RC Jr, Hua K and Feng W: Expression and epigenetic regulation
of angiogenesis-related factors during dormancy and recurrent
growth of ovarian carcinoma. Epigenetics. 8:1330–1346. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li LL, Xue AM, Li BX, Shen YW, Li YH, Luo
CL, Zhang MC, Jiang JQ, Xu ZD, Xie JH and Zhao ZQ: Erratum to:
JMJD2A contributes to breast cancer progression through
transcriptional repression of the tumor suppressor ARHI. Breast
Cancer Res. 18:1142016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stenchever MA, Rizk DE, Falconi G and
Ortiz OC: FIGO task force on standard guidelines for training
residents and fellows in urogynecology, female urolog: FIGO
guidelines for training residents and fellows in urogynecology,
female urology, and female pelvic medicine and reconstructive
surgery. Int J Gynaecol Obstet. 107:187–190. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jia N, Wang J, Li Q, Tao X, Chang K, Hua
K, Yu Y, Wong KK and Feng W: DNA methylation promotes paired box 2
expression via myeloid zinc finger 1 in endometrial cancer.
Oncotarget. 7:84785–84797. 2016.PubMed/NCBI
|
|
14
|
Yanokura M, Banno K, Adachi M, Aoki D and
Abe K: Genome-wide DNA methylation sequencing reveals miR-663a is a
novel epimutation candidate in CIMP-high endometrial cancer. Int J
Oncol. 50:1934–1946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones A, Teschendorff AE, Li Q, Hayward
JD, Kannan A, Mould T, West J, Zikan M, Cibula D, Fiegl H, et al:
Role of DNA methylation and epigenetic silencing of HAND2 in
endometrial cancer development. PLoS Med. 10:e10015512013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li K, Chen MK, Situ J, Huang WT, Su ZL, He
D and Gao X: Role of co-expression of c-Myc, EZH2 and p27 in
prognosis of prostate cancer patients after surgery. Chin Med J
(Engl). 126:82–87. 2013.PubMed/NCBI
|
|
17
|
Jia N, Li Q, Tao X, Wang J, Hua K and Feng
W: Enhancer of zeste homolog 2 is involved in the proliferation of
endometrial carcinoma. Oncol Lett. 8:2049–2054. 2014.PubMed/NCBI
|
|
18
|
Gnani D, Romito I, Artuso S, Chierici M,
De Stefanis C, Panera N, Crudele A, Ceccarelli S, Carcarino E,
D'Oria V, et al: Focal adhesion kinase depletion reduces human
hepatocellular carcinoma growth by repressing enhancer of zeste
homolog 2. Cell Death Differ. 24:889–902. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei Y, Xia W, Zhang Z, Liu J, Wang H,
Adsay NV, Albarracin C, Yu D, Abbruzzese JL, Mills GB, et al: Loss
of trimethylation at lysine 27 of histone H3 is a predictor of poor
outcome in breast, ovarian, and pancreatic cancers. Mol Carcinog.
47:701–706. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rogenhofer S, Kahl P, Mertens C, Hauser S,
Hartmann W, Büttner R, Müller SC, von Ruecker A and Ellinger J:
Global histone H3 lysine 27 (H3K27) methylation levels and their
prognostic relevance in renal cell carcinoma. BJU Int. 109:459–465.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen Y, Guo X, Wang Y, Qiu W, Chang Y,
Zhang A and Duan X: Expression and significance of histone H3K27
demethylases in renal cell carcinoma. BMC Cancer. 12:4702012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pellakuru LG, Iwata T, Gurel B, Schultz D,
Hicks J, Bethel C, Yegnasubramanian S and De Marzo AM: Global
levels of H3K27me3 track with differentiation in vivo and are
deregulated by MYC in prostate cancer. Am J Pathol. 181:560–569.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakazawa T, Kondo T, Ma D, Niu D,
Mochizuki K, Kawasaki T, Yamane T, Iino H, Fujii H and Katoh R:
Global histone modification of histone H3 in colorectal cancer and
its precursor lesions. Hum Pathol. 43:834–842. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He LJ, Cai MY, Xu GL, Li JJ, Weng ZJ, Xu
DZ, Luo GY, Zhu SL and Xie D: Prognostic significance of
overexpression of EZH2 and H3k27me3 proteins in gastric cancer.
Asian Pac J Cancer Prev. 13:3173–3178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Au SL, Wong CC, Lee JM, Wong CM and Ng IO:
EZH2-mediated H3K27me3 is involved in epigenetic repression of
deleted in liver cancer 1 in human cancers. PLoS One. 8:e682262013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan W, Wang L, Ma Q, Qi M, Lu N, Zhang L
and Han B: Adiponectin as a potential tumor suppressor inhibiting
epithelial-to-mesenchymal transition but frequently silenced in
prostate cancer by promoter methylation. Prostate. 75:1197–1205.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zeng F, Shi J, Long Y, Tian H, Li X, Zhao
AZ, Li RF and Chen T: Adiponectin and endometrial cancer: A
systematic review and meta-analysis. Cell Physiol Biochem.
36:1670–1678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mancuso M, Matassa DS, Conte M, Colella G,
Rana G, Fucci L and Piscopo M: H3K4 histone methylation in oral
squamous cell carcinoma. Acta Biochim Pol. 56:405–410.
2009.PubMed/NCBI
|
|
30
|
Elsheikh SE, Green AR, Rakha EA, Powe DG,
Ahmed RA, Collins HM, Soria D, Garibaldi JM, Paish CE, Ammar AA, et
al: Global histone modifications in breast cancer correlate with
tumor phenotypes, prognostic factors, and patient outcome. Cancer
Res. 69:3802–3809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manuyakorn A, Paulus R, Farrell J, Dawson
NA, Tze S, Cheung-Lau G, Hines OJ, Reber H, Seligson DB, Horvath S,
et al: Cellular histone modification patterns predict prognosis and
treatment response in resectable pancreatic adenocarcinoma: results
from RTOG 9704. J Clin Oncol. 28:1358–1365. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Seligson DB, Horvath S, McBrian MA, Mah V,
Yu H, Tze S, Wang Q, Chia D, Goodglick L and Kurdistani SK: Global
levels of histone modifications predict prognosis in different
cancers. Am J Pathol. 174:1619–1628. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lei Q, Liu X, Fu H, Sun Y, Wang L, Xu G,
Wang W, Yu Z, Liu C, Li P, et al: miR-101 reverses hypomethylation
of the PRDM16 promoter to disrupt mitochondrial function in
astrocytoma cells. Oncotarget. 7:5007–5022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Lei Q, Yu Z, Xu G, Tang H, Wang W,
Wang Z, Li G and Wu M: MiR-101 reverses the hypomethylation of the
LMO3 promoter in glioma cells. Oncotarget. 6:7930–7943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cannuyer J, Loriot A, Parvizi GK and De
Smet C: Epigenetic hierarchy within the MAGEA1 cancer-germline
gene: Promoter DNA methylation dictates local histone
modifications. PLoS One. 8:e587432013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He C, Xu J, Zhang J, Xie D, Ye H, Xiao Z,
Cai M, Xu K, Zeng Y, Li H and Wang J: High expression of
trimethylated histone H3 lysine 4 is associated with poor prognosis
in hepatocellular carcinoma. Hum Pathol. 43:1425–1435. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ellinger J, Kahl P, Mertens C, Rogenhofer
S, Hauser S, Hartmann W, Bastian PJ, Büttner R, Müller SC and von
Ruecker A: Prognostic relevance of global histone H3 lysine 4
(H3K4) methylation in renal cell carcinoma. Int J Cancer.
127:2360–2366. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Soussi T: Role of the p53 gene in human
malignant tumors. A major discovery in oncology. Rev Prat.
43:2531–2535. 1993.(In French). PubMed/NCBI
|
|
39
|
Tang Z, Chen WY, Shimada M, Nguyen UT, Kim
J, Sun XJ, Sengoku T, McGinty RK, Fernandez JP, Muir TW and Roeder
RG: SET1 and p300 act synergistically, through coupled histone
modifications, in transcriptional activation by p53. Cell.
154:297–310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mungamuri SK, Wang S, Manfredi JJ, Gu W
and Aaronson SA: Ash2L enables P53-dependent apoptosis by favoring
stable transcription pre-initiation complex formation on its
pro-apoptotic target promoters. Oncogene. 34:2461–2470. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lauberth SM, Nakayama T, Wu X, Ferris AL,
Tang Z, Hughes SH and Roeder RG: H3K4me3 interactions with TAF3
regulate preinitiation complex assembly and selective gene
activation. Cell. 152:1021–1036. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stewart CA, Wang Y, Bonilla-Claudio M,
Martin JF, Gonzalez G, Taketo MM and Behringer RR: CTNNB1 in
mesenchyme regulates epithelial cell differentiation during
Müllerian duct and postnatal uterine development. Mol Endocrinol.
27:1442–1454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ishikawa A, Kudo M, Nakazawa N, Onda M,
Ishiwata T, Takeshita T and Naito Z: Expression of keratinocyte
growth factor and its receptor in human endometrial cancer in
cooperation with steroid hormones. Int J Oncol. 32:565–574.
2008.PubMed/NCBI
|
|
44
|
Lessey BA, Albelda S, Buck CA, Castelbaum
AJ, Yeh I, Kohler M and Berchuck A: Distribution of integrin cell
adhesion molecules in endometrial cancer. Am J Pathol. 146:717–726.
1995.PubMed/NCBI
|