Introduction
Lung cancer remains one of the most common
malignancies and leading causes of cancer-related mortality both in
China and worldwide (1,2). Approximately 80–85% of lung cancers are
non-small cell lung cancer (3). In
recent years, huge progress had been made in the treatment of
non-small cell lung cancer (NSCLC) patients harboring EGFR
mutation and ALK rearrangement (4–7). However,
effective therapy specifically targeting KRAS mutation,
which accounts for 25–50% of NSCLC patients in white populations
and 5–10% in Asian populations, has not been developed yet
(8–11).
KRAS is a member of the Ras gene
family, which encodes small G proteins with intrinsic GTPase
activity, contributing to activation of downstream effectors
involved in multiple pathways including apoptosis, proliferation
and differentiation (8,12,13). Point
mutations occurred in tumors result in the loss of intrinsic GTPase
activity and consequently in the deregulation of cell proliferation
signals (13,14). KRAS mutation occurs mainly in
codon 12, 13 or 61. Most common types of KRAS mutation are
G12C, G12V, and G12D (8,9). In addition, in vitro data
reported by Garassino et al suggested that NSCLC cell lines
harboring a G12C, G12V or G12D KRAS mutation had
differential sensitivity to chemotherapeutic agents (15). It appears that various types of
KRAS mutations differ in clinical characters and drug
response (16,17).
As early as 1990, KRAS mutation was already
described as a negative prognostic marker for both overall survival
(OS) and disease-free survival in lung cancer (18). Not until the last decades, more and
more attention has been paid to the clinical significance of
KRAS mutation in NSCLC. When it comes to the first-line
platinum-based chemotherapy for advanced NSCLC patients, some
researchers tend to believe there is no difference between
KRAS mutant and wild-type patients regarding therapeutic
response and prognosis (14,19,20).
However, there were several studies indicated that KRAS
mutation was a predictive factor of worse progression-free survival
(PFS) or OS in advanced NSCLC patients treated with platinum-based
chemotherapy (21–24). Considering the discrepant role of
KRAS and its subtypes on effect of chemotherapy, the aim of
this study was to investigate the predictive significance of
KRAS mutation and its subtypes on clinical response and PFS
in advanced NSCLC patients treated with first-line platinum-based
chemotherapy.
Materials and methods
Study design
In this retrospective study, patients received
KRAS mutation detection between August 2014 and June 2016 at
Shanghai Pulmonary Hospital affiliated to Tongji University School
of Medicine were included. We retrospectively reviewed patients'
medical records to evaluate clinicopathological features and
treatment regimens. All eligible patients' clinical data including
age, sex, smoking status, histological type, TNM stage, Eastern
Cooperative Oncology Group (ECOG) performance status (PS),
treatment regimens, response to treatment, date of first diagnosis,
date of starting chemotherapy, and date of disease progression or
date of last contact. Pathological diagnosis was made by
pathologists. Staging was carried out according to the staging
system of the 2009 International Association for the Study of Lung
Cancer (version 7) (25). Nonsmokers
were defined as patients with the smoking dose of <100
cigarettes in their lifetime. Clinical response was evaluated by at
least two clinicians according to the Response Evaluation Criteria
in Solid Tumors (RECIST) version 1.1 (26). Inclusion criteria were: Adult (age≥18
years old) patients; recurrent or IIIB/IV NSCLC patients; patients
received first-line platinum-based chemotherapy. Exclusion criteria
were: Unknown mutational status; detected EGFR mutation or
ALK rearrangement; no complete documentation; no response
evaluation; adjuvant chemotherapy or radiochemotherapy. The study
was approved by the Ethics Committees of Shanghai Pulmonary
Hospital. Informed consent was obtained from all individual
participants included in the present study. This study was
conducted according to the Declaration of Helsinki, 7th
version.
KRAS mutation analysis
Total DNA was extracted from tissue samples using
AmoyDx DNA kit (Amoy Diagnostics Co., Ltd., Xiamen, China). The
quality and quantity of extracted DNA were measured by NanoDrop
2000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA).
KRAS mutation was identified by an AmoyDx® Human
KRAS gene 7 Mutations Fluorescence Polymerase Chain Reaction
(PCR) Diagnostic kit (Amoy Diagnostics Co., Ltd.). The real-time
PCR conditions were as previously described (27–29).
β-actin was used as an internal reference gene to ensure the
quality of the extracted DNA and KRAS mutant DNA was used as
positive control.
Statistical analysis
The relation between categorical parameters was
tested using Pearson's χ2 test (Fishers exact test was
used when n≤5). Kaplan-Meier curve was used to estimate the
distribution of survival and log-rank test was used to analyze
differences between groups. PFS was defined as the first day of
treatment until either tumor progression or death. We used cox
proportional hazards models for univariate and multivariate
analysis to estimate clinicopathological features, KRAS
mutation types and treatment regimens for their associations with
PFS. Independent variables with P<0.10 in the univariate
analysis were enrolled in multivariate analysis. P-values <0.05
were defined statistically significant. Confidence intervals were
calculated at a 95% CI. Statistical tests were carried out using
SPSS 20.0 software (IBM Corporation, Armonk, NY, USA).
Results
Patient characteristics
In total, 2,183 patients received KRAS
mutation detection at Shanghai Pulmonary Hospital between August
2014 and June 2016 were enrolled into this study and 218 (10.0%)
cases harbored KRAS mutation. Distribution of different
types of KRAS mutation found within 218 patients are listed
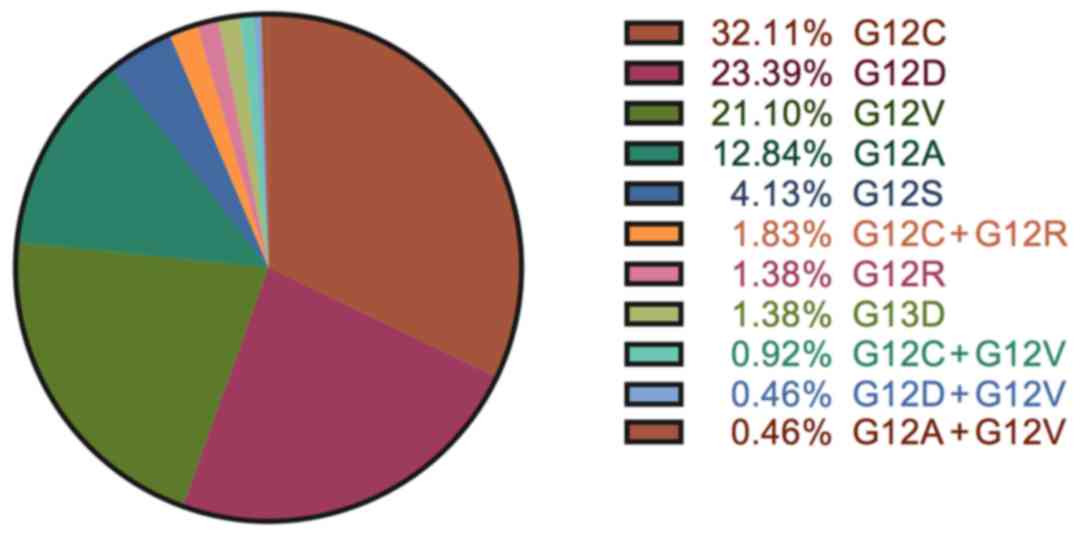
in Fig. 1. Three most common
KRAS mutations were G12C (32.1%), G12D (23.4%) and G12V
(21.1%). Other codon 12 mutations including G12A (12.8%), G12S
(4.1%) and G12R (1.4%) were found in 20% of the patients. 3
patients had codon 13 G13D mutation. Four types of double mutations
were found in 8 patients: G12C + G12R (4 patients), G12C + G12V (2
patients), G12D + G12V (1 patient) and G12A + G12V (1 patient).
Based on our inclusion and exclusion criteria, we further analyzed
100 EGFR/ALK/KRAS wild-type and 70 KRAS mutant
patients. The median age of whole study group was 61 years old
(range 28–78). In total, 84.1% of patients were stage IV disease at
diagnosis, and 77.6% of patients displayed histology of
adenocarcinoma. The patient characteristics were listed in Table I. The patient basic characteristics
were well-matched between KRAS mutant and wild-type groups
except for sex (P=0.035). As for the treatment regimens, 74.1% of
all patients received first-line chemotherapy with
carboplatin-based chemotherapy, with a higher percentage of
wild-type KRAS patients (78.0%) receiving carboplatin-based
doublet comparing with mutant KRAS patients (68.6%).
Numerically more KRAS mutant patients received a
cisplatin-based chemotherapy when compared with KRAS
wild-type patients (28.6% vs. 21.0%, respectively). However, there
seems to be more patients in the KRAS wild-type group
received platinum/pemetrexed treatments (68.0% in KRAS
wild-type group vs. 57.1% in KRAS mutant group). Whereas
patients with wild-type KRAS were as likely as patients with
mutant KRAS to receive platinum/gemcitabine chemotherapies.
Of note, 6 patients within the KRAS mutant group received
platinum/docetaxel whereas only 1 patient within the KRAS
wild-type group received platinum/docetaxel treatments.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
| KRAS mutant
(n=70) | KRAS
wild-type (n=100) | P-value |
|---|
| Mean age at
diagnosis, mean ± SD | 61±7.34 | 60±9.31 | 0.334 |
| Sex, n (%) |
|
|
|
|
Male | 60 (85.7) | 72 (72.0) | 0.035 |
|
Female | 10 (14.3) | 28 (28.0) |
|
| Smoking history, n
(%) |
|
| 0.302 |
|
Smoker | 42 (60.0) | 52 (52.0) |
|
|
Non-smoker | 28 (40.0) | 48 (48.0) |
|
| Histology, n
(%) |
|
| 0.826 |
|
Adenocarcinoma | 55 (78.6) | 77 (77.0) |
|
|
Squamous | 6 (8.6) | 12 (12.0) |
|
|
Other | 0 (0.0) | 1 (1.0) |
|
|
NSCLC-NOS | 9 (12.9) | 10 (10.0) |
|
| Stage, n (%) |
|
|
|
|
IIIB | 6 (8.6) | 12 (12.0) | 0.475 |
| IV | 64 (91.4) | 88 (88.0) |
|
| Platinum, n
(%) |
|
|
|
|
Cisplatin | 20 (28.6) | 21 (21.0) | 0.287 |
|
Carboplatin | 48 (68.6) | 78 (78.0) |
|
|
Other | 2 (2.9) | 1 (1.0) |
|
| Platinum doublets,
n (%) |
|
|
|
|
Platinum/pemetrexed | 40 (57.1) | 68 (68.0) | 0.029 |
|
Platinum/gemcitabine | 23 (32.9) | 31 (31.0) |
|
|
Platinum/docetaxel | 6 (8.6) | 1 (1.0) |
|
|
Other | 1 (1.4) | 0 (0.0) |
|
Effect of KRAS mutation on response
rate and PFS
None of the patients reached complete response.
Partial response was similar between two groups (21.4% in
KRAS mutant patients vs. 19.0% in KRAS wild-type
patients). Comparatively, stable disease was observed more in
wild-type KRAS patients than in mutant KRAS patients
(67.0% vs. 44.3%, respectively). However, numerically more disease
progressed in patients with mutant KRAS than wild-type
KRAS (34.3% vs. 14.0%). There were no statistically
significant differences in the objective response rate (ORR). In
contrast, disease control rate (DCR) of KRAS wild-type
patients to platinum-based chemotherapy was obviously higher than
KRAS mutant patients (86.0% vs. 65.7%, P=0.002; Table II). In Table II, we also listed clinical outcomes
of three most common KRAS mutation subtypes and other rare
mutations. Among them, although G12V has the lowest DCR for 55.6%,
response to platinum-based chemotherapy had no statistically
significant differences between mutation subtypes.
 | Table II.Response to first line chemotherapy
in KRAS mutant vs. KRAS wild-type NSCLC patients. |
Table II.
Response to first line chemotherapy
in KRAS mutant vs. KRAS wild-type NSCLC patients.
|
|
| KRAS mutant
(n=70) |
|
|---|
|
|
|
|
|
|---|
|
| KRAS
wild-type (n=100) | Total (n=70) | G12C (n=23) | G12V (n=18) | G12D (n=9) | Rare (n=20) |
P-valuea |
P-valueb |
|---|
| Response |
|
|
|
|
|
|
|
|
| CR | – | – | – | – | – | – |
|
|
| PR | 19 | 15 | 6 | 4 | 1 | 4 |
|
|
| SD | 67 | 31 | 12 | 6 | 5 | 8 |
|
|
| PD | 14 | 24 | 5 | 8 | 3 | 8 |
|
|
| ORR | 19.0% | 21.4% | 26.1% | 22.2% | 11.1% | 20.0% | 0.893 | 0.697 |
| DCR | 86.0% | 65.7% | 78.3% | 55.6% | 66.7% | 60.0% | 0.442 | 0.002 |
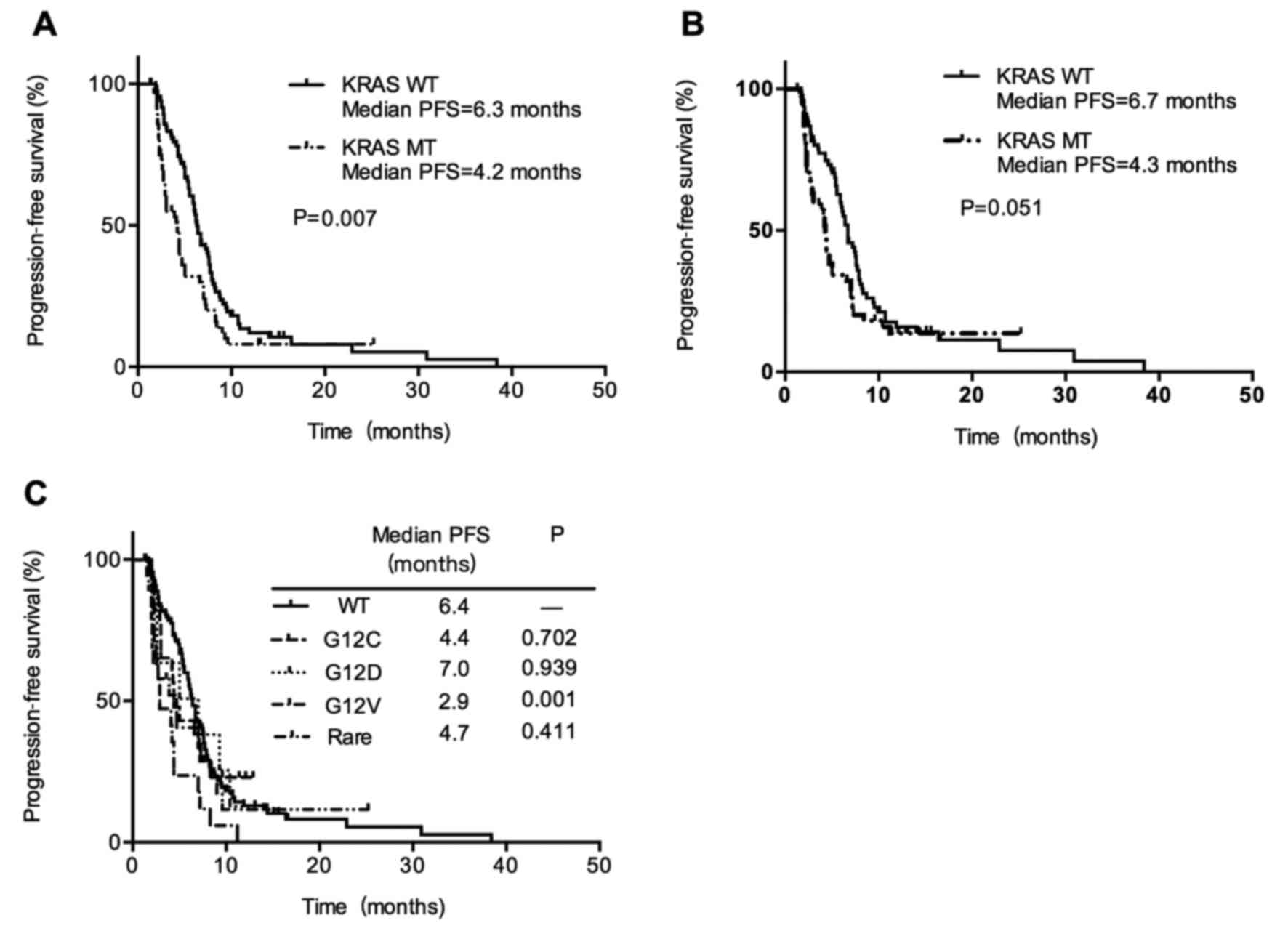
A total of 140 (82.4%) patients had progressed
disease during the study period, with a median PFS for all subjects
of 5.9 months (95% CI, 4.9–6.9 months). In all included patients
with metastatic NSCLC at diagnosis, PFS was shorter in the
KRAS mutant group vs. wild-type group (4.2 vs. 6.3 months;
P=0.007; Fig. 2A). In addition, there
was a shorter but only marginally statistically significant PFS in
KRAS mutant patients with adenocarcinoma histology patients
(4.3 months vs. 6.7 months; P=0.051; Fig.
2B). It suggested that the presence of KRAS mutation may
be associated with a worse response to first-line platinum-based
chemotherapy in advanced NSCLC patients. Next, we compared PFS of
wild-type KRAS patients with three most common KRAS
subtypes G12V, G12C, G12D and other rare mutations. When comparing
patients with G12V mutant vs. wild-type, there was a statistically
significant shorter PFS (2.9 months and 6.4 months, respectively;
P=0.001). While other KRAS subtypes had no differences in
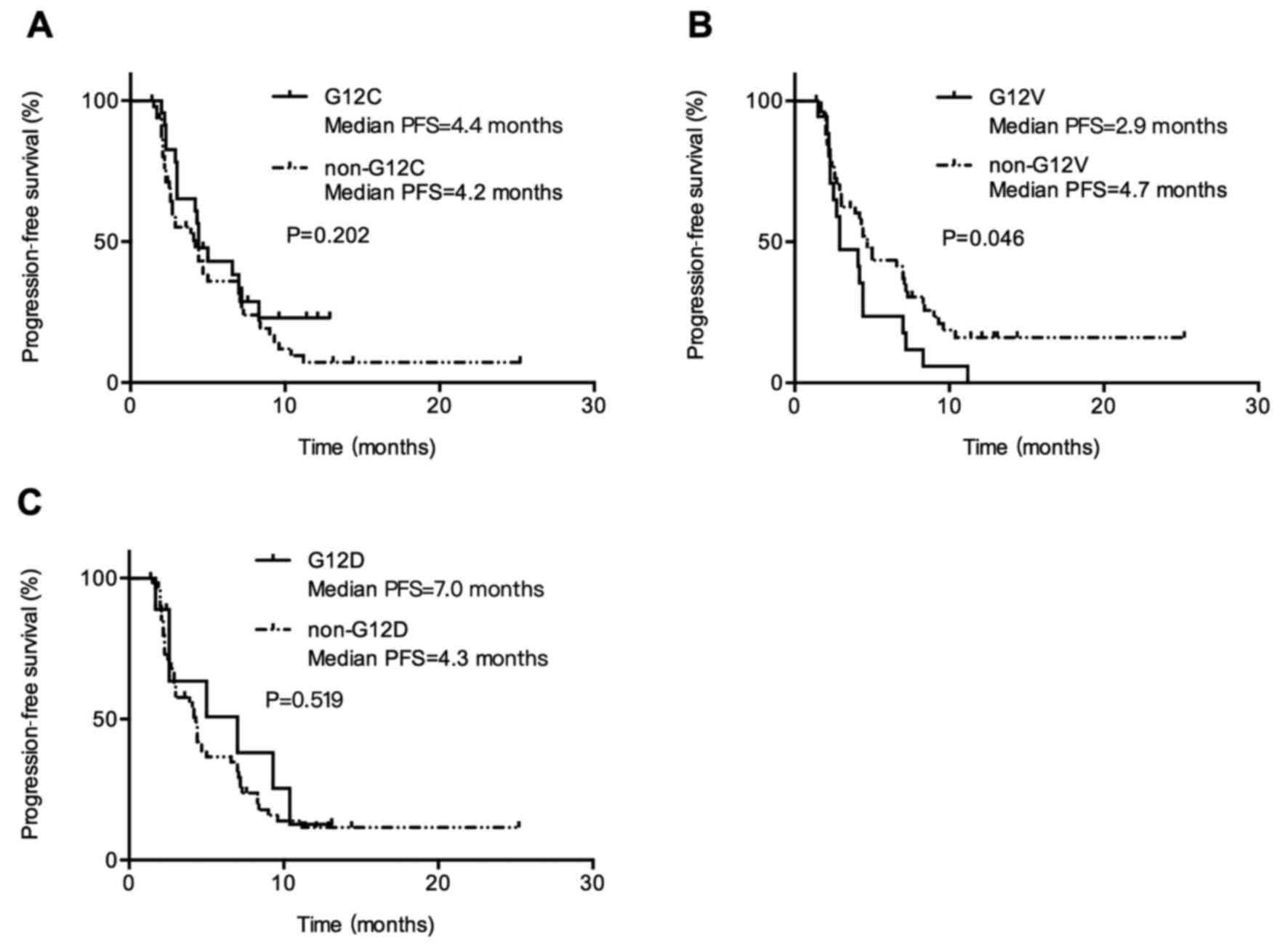
PFS compared with wild-type KRAS (Fig. 2C). Patients with KRAS G12V
mutation had inferior PFS compared with patients with non-G12V
mutation (median PFS, 2.9 vs. 4.7 months; P=0.045; Fig. 3B). When comparing patients with G12C
vs. non-G12C mutation and patients with G12D vs. non-G12D mutation,
there was no differences in PFS, 4.4 months (95% CI, 3.3–5.5) vs.
4.2 months (95% CI, 2.3–6.1; P=0.202; Fig. 3A) and 7.0 months (95% CI, 1.1–12.8)
vs. 4.3 months (95% CI, 3.8–4.8; P=0.519; Fig. 3C). It suggested that response to
chemotherapy is not the same among KRAS mutation subtypes
and patients with KRAS G12V mutation showed the poorest PFS
than those with other KRAS mutant types.
Univariate and multivariate
analysis
In univariate analysis, sex, smoking history and
KRAS G12V mutation were significantly associated with PFS.
Women had decreased risk of progressed disease when compared with
men (HR, 0.616; 95% CI, 0.405–0.937; P=0.024). Smoking history also
affected PFS (never smokers vs. current/former smokers; HR,0.665;
95% CI, 0.472–0.937; P=0.020). KRAS G12V was associated with
shorter PFS (HR, 2.342; 95% CI, 1.378–3.981; P=0.002). In
multivariate analysis, only KRAS G12V mutation was
associated with shorter PFS (HR, 2.116; 95% CI, 1.211–3.696;
P=0.008; Table III).
 | Table III.Prognostic evaluation of clinical and
histopathological characteristics in whole group and in KRAS
mutant subgroup- progression free survival. |
Table III.
Prognostic evaluation of clinical and
histopathological characteristics in whole group and in KRAS
mutant subgroup- progression free survival.
| Variable | Univariate analyses
1a HR (95% CI)
P-value | Multivariate
analyses 1a HR (95% CI)
P-value | Univariate analyses
1b HR (95% CI)
P-value | Multivariate
analyses 1b HR (95% CI)
P-value |
|---|
| Age, <61 vs. ≥61
years old | 1.170
(0.837–1.634) |
| 1.622
(0.952–2.746) | – |
|
| 0.359 |
| 0.075 | 0.121 |
| Sex, female vs.
male | 0.616
(0.405–0.937) |
| 0.844
(0.399–1.784) |
|
|
| 0.024 |
| 0.657 |
|
| Stage,
IIIB/recurrent vs. IV | 0.811
(0.457–1.438) |
| 0.781
(0.259–1.990) |
|
|
| 0.473 |
| 0.525 |
|
| Smoking, never | 0.665
(0.472–0.937) | – | 0.799
(0.462–1.379) |
|
| vs.
current/former | 0.020 | 0.126 | 0.420 |
|
| Pathology, SQC | 1.301
(0.775–2.183) |
| 0.779
(0.306–1.981) |
|
| vs. ADC | 0.319 |
| 0.599 |
|
| KRAS, mutant vs.
wt | 1.324
(0.942–1.861) |
| – |
|
|
| 0.106 |
|
|
|
| G12C
vs. wt | 1.107
(0.654–1.873) |
| – |
|
|
| 0.705 |
|
|
|
| G12V
vs. wt | 2.342
(1.378–3.981) | 2.116
(1.211–3.696) | – |
|
|
| 0.002 | 0.008 |
|
|
| G12D
vs. wt | 1.031
(0.474–2.239) |
| – |
|
|
| 0.939 |
|
|
|
| G12C
vs. others | – |
| 0.697
(0.395–1.231) |
|
|
|
|
| 0.214 |
|
| G12V
vs. others | – |
| 1.762
(0.992–3.129) | 1.831
(1.025–3.270) |
|
|
|
| 0.053 | 0.041 |
| G12D
vs. others | – |
| 0.774
(0.350–1.714) |
|
|
|
|
| 0.528 |
|
| Chemotherapy,
cisplatin | 1.296
(0.883–1.902) |
| 1.720
(0.982–3.013) | – |
| vs.
carboplatin | 0.186 |
| 0.058 | 0.158 |
| Chemotherapy,
gemcitabine | 1.390
(0.971–1.991) | – | 1.527
(0.852–2.736) |
|
| vs. pemetrexed | 0.072 | 0.335 | 0.155 |
|
In KRAS mutant group, univariate analysis
showed that smoking history did not have impact on outcome for PFS
(HR, 0.799; 95% CI, 0.462–1.379; P=0.420). And there was marginally
statistic difference in outcome of G12V mutant patients vs. other
mutant KRAS patients in univariate analysis (HR, 1.762; 95%
CI, 0.992–3.129; P=0.053). In multivariate analysis based on age,
G12V mutation status and cisplatin- or carboplatin-based
chemotherapy, results showed that G12V mutant patients did have a
shorter PFS than other KRAS mutant types (HR, 1.831; 95% CI,
1.025–3.270; P=0.041; Table
III).
Discussion
Our treatment of NSCLC has been dramatically
improved with the introduction of molecular markers. Targeted
therapies, including tyrosine kinase inhibitors (TKIs), for
EGFR mutation and ALK rearrangement improved PFS in
patients bearing the relevant mutations (4,7,30). However, effective therapy specifically
targeting KRAS mutation has not been developed yet. For
patients with KRAS mutation, platinum-based chemotherapy
remains their first choice. Nevertheless, the predictive value of
KRAS mutation in NSCLC for chemotherapy also remains unclear.
In the last decades, although a large number of
studies had been conducted focusing on KRAS mutation, the
prognostic and predictive value of KRAS in lung cancer is
still a highly debated issue. Considering the enormous discrepancy
of studies in terms of races, tumor stage, histological types and
various treatments, it is difficult to draw a definite conclusion.
Therefore, we analyzed a well-defined Chinese patient cohort with
advanced NSCLC received first-line platinum-based chemotherapy in
our study. KRAS mutation rate in all tested population was
10.0%, which is in accordance with other studies of Asian NSCLC
study cohort (10,11,29,31,32).
Furthermore, we found a ratio of the major subtypes, G12C (32.1%),
G12V (23.4%), G12D (21.1%), which is almost identical with the
previous reports (31–35). We also identified four kinds of
co-mutations in our study group: Four patients with G12C/G12R, two
patients with G12C/G12V, one patient with G12D/G12V and one patient
with G12A/G12V. And no significant differences in PFS between
KRAS co-mutant and other KRAS mutant or wild-type patients
were found (data not shown).
Prior findings indicated patients with KRAS
mutation were preferably to be smokers and have histology of
adenocarcinoma comparing with patients of wild-type KRAS
(36,37). However, in the current study, we noted
that there were no differences in smoking history and pathological
types between two groups of patients. Nevertheless, we observed
KRAS mutation was not exclusively found in patients with
adenocarcinoma. Hence testing all patients with NSCLC for
KRAS mutation is necessary. Although KRAS mutant
patients and KRAS wild-type patients shared similar smoking
habits, smokers had increased risk of shorter PFS compared with
non-smoker in our univariate analysis of whole study group. There
seemed to be more males in the KRAS-mutant group comparing
with the group of patients with wild-type KRAS. But the
significance of this finding was complicated to explain regarding
clinical outcome. Although male sex was dramatically associated
with worse outcomes in our univariate analysis, survival was
similar in whole study group between KRAS mutant and
wild-type groups despite the KRAS cohort had a higher
percentage of males. The majority of patients in the study group
received a cisplatin or carboplatin plus pemetrexed or gemcitabine
chemotherapy. The different choice of chemotherapy regimens did not
affect the PFS both in whole group and in KRAS mutant cohort
in univariate and multivariate analysis.
There were many articles reporting inconsistent
results in regards to the impact of KRAS mutation on
survival of advanced NSCLC patients who received platinum-based
chemotherapy. For example, a retrospective analysis performed by
Mellema et al showed no significant differences in clinical
response to chemotherapy or OS when compared patients with
KRAS mutation with patients without KRAS mutation
(19). Conversely, Metro et al
demonstrated that patients with KRAS mutation had lower
response rates, and shorter PFS compared with EGFR
wild-type/KRAS wild-type patients (23). Besides, Hames et al reported
that the presence of KRAS mutation in advanced NSCLC
patients displayed a worse prognosis of platinum-based chemotherapy
compared with those absence of detectable driver mutations
(21). In the current analysis, our
results suggested that KRAS mutant patients did have lower
DCR compared with KRAS wild-type patients, but not ORR. In
addition, KRAS mutant patients demonstrated a decrease PFS
comparing with wild-type patients, which was in accordance with
prior report (21) and we found more
convincing results in patients with metastatic NSCLC at diagnosis,
PFS was significantly shorter in the KRAS mutant group vs.
wild-type group (4.2 vs. 6.3 months; P=0.007). In addition, there
was a shorter but only marginally statistically significant PFS in
KRAS mutant patients with adenocarcinoma histology patients
(4.3 months vs. 6.7 months; P=0.051). Based on the above results,
we made the conclusion that KRAS mutation was a negative predictive
factor of PFS in Chinese patients with advanced NSCLC received
first platinum-based chemotherapy. Admittedly, this study was
conducted at a single institution and had limited patient samples.
We considered that, to make our findings more convincing, sharing
of more data from multicenter studies, especially those covering
various populations should be encouraged. We will also stay focuced
on this issue and further exploration of the prognostic value of
KRAS and its underlying mechanism is needed. Although recent
research in colorectal cancer reported that G12V mutation
demonstrated poor response to therapy and survival (38), the relevance of specific mutation
subtypes in KRAS and clinical outcome remains controversial
in NSCLC (16,39–41). In
recent studies of advanced NSCLC, effects of KRAS G12V
mutation regrading as either response to chemotherapy or OS were
not obvious (40). However, in our
study, patients with G12V mutant not only responded poorer to
platinum-based chemotherapy, although not statisticly significant,
but also had a significantly shorter PFS than those with other
KRAS mutations. Our finding was in accordance with results
carried out by Ihle et al (16). Downstream signaling of RAS
differed in mutation subtypes. KRAS G12C/G12V preferably
activated RalA/B signaling while KRAS G12D activated Akt
pathway and the former demonstrated decreased survival (42). Taking all our presented results
together, there is reason to believe that, in NSCLC, patients with
different KRAS mutant subtypes may lead to distinct response
to first-line platinum-based chemotherapy. Furthermore,
subtype-specific mutation analysis is necessary in clinical
practice, which may help to identify the most effective treatment
regimens for each individual patient. Despite some of our results
were consistent with previous publication, our study was conducted
among Chinese population. Considering the differences in gene
background between Caucasian and East Asian people (43,44),
whether previous observation is also true among East Asian
population remains uncertain. The conclusions we made in the study
will provide clinicians with more comprehensive evidence when
making clinical decisions for NSCLC patients with KRAS
mutation.
There are several limitations in the present study
that should be acknowledged. First of all, selection bias was
inevitable due to the nature of retrospective studies. Second this
study design was at a single institution. Taking the high cost of
molecular detection into consideration, not all patients in our
hospital received KRAS mutation test, therefore patients
included in our study may not be representative of a general
population. Sufficiency of cancer samples was also one of the
limitations in this study. However, according to previous reports,
in white populations KRAS accounts for 25–50% of NSCLC patients but
KRAS mutations are only found in 5–10% of NSCLC patients in Asian
populations (8–11). When we reviewed relative studies
conducted among Caucasian populations, we found our patient number
was very similar to other studies. In a retrospective analysis
performed by Hames et al and colleagues, they compared 70
patients with pan-mutation negative and 80 patients with
KRAS-mutant advanced NSCLC patients (21). On the other hand, considering the
lower incidence of KRAS mutation among Asian people, we only
focused on whether KRAS mutation was a negative predictive factor
of PFS in Chinese patients with advanced NSCLC received first
platinum-based chemotherapy. Further studies should be done aiming
at the prognostic value of KRAS mutation on chemotherapy and
also comparing responses with different cytotoxic chemotherapy
regimens in patients with advanced NSCLC based on KRAS
mutation and subtypes. Thus, considering the above limitations,
multi-centered, international cooperative and larger number of
NSCLC patients should be analyzed to valid our present
findings.
The current study suggested that the presence of
KRAS mutation was associated with a worse response in
advanced NSCLC patients received first-line platinum-based
chemotherapy. Responses to cytotoxic chemotherapy are not same
among KRAS mutation subtypes. As the currently available
literatures are still conflicting on the predictive value of
KRAS mutation and its subtypes in advanced NSCLC, future
studies should be done aiming at comparing responses with different
cytotoxic chemotherapy regimens in patients with advanced NSCLC
based on KRAS mutation and subtypes.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81372392 and
81402486) and Shanghai Committee of Science and Technology, China
(no. 134119b1001).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyanaga A, Shimizu K, Noro R, Seike M,
Kitamura K, Kosaihira S, Minegishi Y, Shukuya T, Yoshimura A,
Kawamoto M, et al: Activity of EGFR-tyrosine kinase and ALK
inhibitors for EML4-ALK-rearranged non-small-cell lung cancer
harbored coexisting EGFR mutation. BMC Cancer. 13:2622013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Friboulet L, Li N, Katayama R, Lee CC,
Gainor JF, Crystal AS, Michellys PY, Awad MM, Yanagitani N, Kim S,
et al: The ALK inhibitor ceritinib overcomes crizotinib resistance
in non-small cell lung cancer. Cancer Discov. 4:662–673. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Riely GJ, Marks J and Pao W: KRAS
mutations in non-small cell lung cancer. Proc Am Thorac Soc. 6:pp.
201–205. 2009, View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Piva S, Ganzinelli M, Garassino MC, Caiola
E, Farina G, Broggini M and Marabese M: Across the universe of
K-RAS mutations in non-small-cell-lung cancer. Curr Pharm Des.
20:3933–3943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xia N, An J, Jiang QQ, Li M, Tan J and Hu
CP: Analysis of EGFR, EML4-ALK, KRAS, and c-MET mutations in
Chinese lung adenocarcinoma patients. Exp Lung Res. 39:328–335.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao B, Sun Y, Zhang J, Ren Y, Fang R, Han
X, Shen L, Liu XY, Pao W, Chen H and Ji H: Spectrum of LKB1, EGFR,
and KRAS mutations in chinese lung adenocarcinomas. J Thorac Oncol.
5:1130–1135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malumbres M and Barbacid M: RAS oncogenes:
The first 30 years. Nat Rev Cancer. 3:459–465. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pylayeva-Gupta Y, Grabocka E and Bar-Sagi
D: RAS oncogenes: Weaving a tumorigenic web. Nat Rev Cancer.
11:761–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brady AK, McNeill JD, Judy B, Bauml J,
Evans TL, Cohen RB, Langer C, Vachani A and Aggarwal C: Survival
outcome according to KRAS mutation status in newly diagnosed
patients with stage IV non-small cell lung cancer treated with
platinum doublet chemotherapy. Oncotarget. 6:30287–30294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garassino MC, Marabese M, Rusconi P, Rulli
E, Martelli O, Farina G, Scanni A and Broggini M: Different types
of K-Ras mutations could affect drug sensitivity and tumour
behaviour in non-small-cell lung cancer. Ann Oncol. 22:235–237.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ihle NT, Byers LA, Kim ES, Saintigny P,
Lee JJ, Blumenschein GR, Tsao A, Liu S, Larsen JE, Wang J, et al:
Effect of KRAS oncogene substitutions on protein behavior:
Implications for signaling and clinical outcome. J Natl Cancer
Inst. 104:228–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller MS and Miller LD: RAS mutations and
oncogenesis: Not all RAS mutations are created equally. Front
Genet. 2:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Slebos RJ, Kibbelaar RE, Dalesio O,
Kooistra A, Stam J, Meijer CJ, Wagenaar SS, Vanderschueren RG, van
Zandwijk N, Mooi WJ, et al: K-ras oncogene activation as a
prognostic marker in adenocarcinoma of the lung. N Engl J Med.
323:561–565. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mellema WW, Dingemans AM, Thunnissen E,
Snijders PJ, Derks J, Heideman DA, Van Suylen R and Smit EF: KRAS
mutations in advanced nonsquamous non-small-cell lung cancer
patients treated with first-line platinum-based chemotherapy have
no predictive value. J Thorac Oncol. 8:1190–1195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rulli E, Marabese M, Torri V, Farina G,
Veronese S, Bettini A, Longo F, Moscetti L, Ganzinelli M,
Lauricella C, et al: Value of KRAS as prognostic or predictive
marker in NSCLC: Results from the TAILOR trial. Ann Oncol.
26:2079–2084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hames ML, Chen H, Iams W, Aston J, Lovly
CM and Horn L: Correlation between KRAS mutation status and
response to chemotherapy in patients with advanced non-small cell
lung cancer. Lung Cancer. 92:29–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Campos-Parra AD, Zuloaga C, Manriquez ME,
Avilés A, Borbolla-Escoboza J, Cardona A, Meneses A and Arrieta O:
KRAS mutation as the biomarker of response to chemotherapy and
EGFR-TKIs in patients with advanced non-small cell lung cancer:
Clues for its potential use in second-line therapy decision making.
Am J Clin Oncol. 38:33–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Metro G, Chiari R, Bennati C, Cenci M,
Ricciuti B, Puma F, Flacco A, Rebonato A, Giannarelli D, Ludovini
V, et al: Clinical outcome with platinum-based chemotherapy in
patients with advanced nonsquamous EGFR wild-type non-small-cell
lung cancer segregated according to KRAS mutation status. Clin Lung
Cancer. 15:86–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Marabese M, Ganzinelli M, Garassino MC,
Shepherd FA, Piva S, Caiola E, Macerelli M, Bettini A, Lauricella
C, Floriani I, et al: KRAS mutations affect prognosis of
non-small-cell lung cancer patients treated with first-line
platinum containing chemotherapy. Oncotarget. 6:34014–34022. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th edition.
Wiley-Blackwell; New York: 2009
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deng LL, Deng HB, Lu CL, Guo Y, Wang D,
Yan CH, Lv X and Shao YX: Mutations of EGFR or KRAS and expression
of chemotherapy-related genes based on small biopsy samples in
stage IIIB and IV inoperable non-small cell lung cancer. J Cancer
Res Clin Oncol. 140:2097–2105. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu C, Zhao C, Yang Y, He Y, Hou L, Li X,
Gao G, Shi J, Ren S, Chu H, et al: High discrepancy of driver
mutations in patients with NSCLC and synchronous multiple lung
ground-glass nodules. J Thorac Oncol. 10:778–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren S, Kuang P, Zheng L, Su C, Li J, Li B,
Chen X, Wang Y, KimCurran V, Liu L, et al: Analysis of driver
mutations in female non-smoker Asian patients with pulmonary
adenocarcinoma. Cell Biochem Biophys. 64:155–160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Costa DB, Shaw AT, Ou SH, Solomon BJ,
Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, et al:
Clinical experience with crizotinib in patients with advanced
ALK-rearranged non-small-cell lung cancer and brain metastases. J
Clin Oncol. 33:1881–1888. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Wu YL, Zhang GC, Zhou Q, Xu CR and
Guo AL: EGFR/KRAS mutations and gefitinib therapy in Chinese NSCLC
patients. Onkologie. 31:174–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li M, Liu L, Liu Z, Yue S, Zhou L, Zhang
Q, Cheng S, Li RW, Smith PN and Lu S: The status of KRAS mutations
in patients with non-small cell lung cancers from mainland China.
Oncol Rep. 22:1013–1020. 2009.PubMed/NCBI
|
|
33
|
Jia XL and Chen G: EGFR and KRAS mutations
in Chinese patients with adenosquamous carcinoma of the lung. Lung
Cancer. 74:396–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim HR, Shim HS, Chung JH, Lee YJ, Hong
YK, Rha SY, Kim SH, Ha SJ, Kim SK, Chung KY, et al: Distinct
clinical features and outcomes in never-smokers with nonsmall cell
lung cancer who harbor EGFR or KRAS mutations or ALK rearrangement.
Cancer. 118:729–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun JM, Hwang DW, Ahn JS, Ahn MJ and Park
K: Prognostic and predictive value of KRAS mutations in advanced
non-small cell lung cancer. PLoS One. 8:e648162013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahrendt SA, Decker PA, Alawi EA, Zhu YRYR,
Sanchez-Cespedes M, Yang SC, Haasler GB, Kajdacsy-Balla A, Demeure
MJ and Sidransky D: Cigarette smoking is strongly associated with
mutation of the K-ras gene in patients with primary adenocarcinoma
of the lung. Cancer. 92:1525–1530. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Riely GJ, Kris MG, Rosenbaum D, Marks J,
Li A, Chitale DA, Nafa K, Riedel ER, Hsu M, Pao W, et al: Frequency
and distinctive spectrum of KRAS mutations in never smokers with
lung adenocarcinoma. Clin Cancer Res. 14:5731–5734. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Imamura Y, Morikawa T, Liao X, Lochhead P,
Kuchiba A, Yamauchi M, Qian ZR, Nishihara R, Meyerhardt JA, Haigis
KM, et al: Specific mutations in KRAS codons 12 and 13, and patient
prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer
Res. 18:4753–4763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shepherd FA, Domerg C, Hainaut P, Jänne
PA, Pignon JP, Graziano S, Douillard JY, Brambilla E, Le Chevalier
T, Seymour L, et al: Pooled analysis of the prognostic and
predictive effects of KRAS mutation status and KRAS mutation
subtype in early-stage resected non-small-cell lung cancer in four
trials of adjuvant chemotherapy. J Clin Oncol. 31:2173–2181. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cserepes M, Ostoros G, Lohinai Z, Raso E,
Barbai T, Timar J, Rozsas A, Moldvay J, Kovalszky I, Fabian K, et
al: Subtype-specific KRAS mutations in advanced lung
adenocarcinoma: A retrospective study of patients treated with
platinum-based chemotherapy. Eur J Cancer. 50:1819–1828. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Izar B, Zhou H, Heist RS, Azzoli CG,
Muzikansky A, Scribner EE, Bernardo LA, Dias-Santagata D, Iafrate
AJ and Lanuti M: The prognostic impact of KRAS, its codon and amino
acid specific mutations, on survival in resected stage I lung
adenocarcinoma. J Thorac Oncol. 9:1363–1369. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Stolze B, Reinhart S, Bulllinger L,
Fröhling S and Scholl C: Comparative analysis of KRAS codon 12, 13,
18, 61, and 117 mutations using human MCF10A isogenic cell lines.
Sci Rep. 5:85352015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Thomas RK, Weir B and Meyerson M: Genomic
approaches to lung cancer. Clin Cancer Res. 12:4384s–4391s. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou W and Christiani DC: East meets West:
Ethnic differences in epidemiology and clinical behaviors of lung
cancer between East Asians and Caucasians. Chin J Cancer.
30:287–292. 2011. View Article : Google Scholar : PubMed/NCBI
|