Introduction
Hepatocellular carcinoma (HCC) is one of the most
deadly types of cancer worldwide (1).
Administration of chemotherapeutic agents is the primary approach
in cancer treatment in the clinic; however, severe side effects
limit their use and further development (2). Immunomodulatory agents, which stimulate
the host immune system, may be a possible method to inhibit tumor
growth without harming the host (3,4).
Therefore, the identification of novel antitumor treatments with
immunomodulatory characteristics is required.
Melittin is the primary toxic component in the venom
of the European honeybee (Apis mellifera) (5). Previous studies have demonstrated that
melittin, which contains 26 amino acid residues, is a cationic and
hemolytic peptide (6). Melittin has
been revealed to exhibit a number of biological effects, including
anti-bacterial, anti-inflammatory, and anti-tumor effects (5,7–9). The anti-tumor effects of melittin are
based on directly killing cancer cells via disruption of the cell
membrane or inducing the apoptosis of tumor cells (10–11).
Previous studies have reported that melittin exhibits a stimulatory
effect on the immune system, indicating its immunomodulatory
potential in treating cancer (12,13). In
the present study, based on the amphipathic structure of melittin,
an analog of melittin (Mel-P15) consisting of 15 amino acids was
designed and synthesized. The effects of Mel-P15 on hepatocellular
carcinoma were then investigated in vitro and in
vivo.
The results from the present study indicated that
Mel-P15 directly promotes the natural killer (NK) activity of
splenocytes in vitro. Additionally, Mel-P15 inhibited tumor
growth in vivo and stimulated the immune system by promoting
splenocyte proliferation, increasing the spleen and thymus indices,
enhancing NK cell cytotoxicity and upregulating the secretion of
interleukin (IL)-2, interferon (IFN)-γ and tumor necrosis factor
(TNF)-α in the serum. Mel-P15 exhibited anti-tumor effects in
BEL-7402-bearing nude mice, which was abrogated by the selective
depletion of the NK cell population via intraperitoneal injection
of an anti-asialo-GM-1 antibody. Taken together, these data
indicate that the anti-hepatocellular carcinoma activity of Mel-P15
is mediated by promoting NK cell cytotoxicity in vivo.
Materials and methods
Cell lines, reagents and animals
Mel-P15 (amino acid sequence, GLPALISWIKRKRQQ) was
synthesized by GL Biochem, Ltd. (Shanghai, China) via stepwise
solid phase methodology (14). Then
the peptide was purified using a Sephadex™ gel column.
The purity was >98% as detected by HPLC.
The mouse H22 and human BEL-7402 hepatocellular
carcinoma cell lines were purchased from the American Type Culture
Collection (Manassas, VA, USA), the YAC-1 mouse lymphoma cell line
was obtained from Nanjing KeyGEN Biotech Co., Ltd. (Nanjing,
China). Cells were cultured at 37°C in a humidified atmosphere
containing 5% CO2 in RPMI-1640 medium with 10% fetal
bovine serum, 100 U/ml penicillin and 100 U/ml streptomycin (all
from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Female 6-week-old ICR mice (n=20, 18–22 g) and
4-week-old nude mice (n=24, 16–18 g) were purchased from the
Laboratory Animal Center of Yangzhou University (Yangzhou, China).
The animals were housed in a rodent facility at 22±1°C with a 12 h
light-dark cycle and provided with continuous standard rodent chow
and water. All procedures involving animals and their care in the
present study were in accordance with protocols approved by the
Ethics Committee of Provincial Hospital Affiliated to Shandong
University (Jinan, China).
Hemolytic activity assay
The hemolytic activity assay was performed as
previously described (15) with
modifications. Briefly, sheep blood cells were harvested and washed
with PBS. Then, the packed cell volume was used to prepare a 10%
(v/v) suspension in PBS. The cell suspension (100 µl) was
transferred to tubes and mixed with 100 µl of peptide solution.
Tubes were incubated at 37°C for 1 h and centrifuged at 300 × g for
5 min at 4°C. Cells incubated with PBS alone were treated as the
negative control. Red blood cells lysed using 0.1% Triton X-100
served as a positive control (100% lysis). The absorbance of the
supernatant was measured at 540 nm to monitor blood cell lysis. The
percentage of hemolysis was calculated as follows: [(A540 in the
peptide solution-A540 in PBS)/(A540 in 0.1% Triton X-100-A540 in
PBS)] ×100%.
Cytotoxic activities of NK cells
The cytotoxic activities of NK cells were determined
using a CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega
Corporation, Madison, WI, USA) as previously described (16). Briefly, splenocytes in each group were
treated with YAC-1 cells at effector:target (E:T) ratios of 40:1,
20:1, and 10:1. Following incubation for 4 h, the cytotoxicity was
measured.
H22 xenograft mouse model and Mel-P15
administration
In the present study, the H22 xenograft mouse model
was established as previously described (17,18).
Briefly, seven-day-old H22 ascites (0.1 ml; 5×106 cells)
were transplanted subcutaneously into the right axilla of each ICR
mouse. A total of 7 days following tumor implantation, the mice
were randomly divided into four groups and administered the
following: the control group was administered normal saline; the
three Mel-P15 groups were administered Mel-P15 (0.5, 1 or 2 mg/kg).
Each group contained 10 mice. All of the solutions were dissolved
in normal saline, filtered through a 0.22-µm filter (EMD Millipore,
Billerica, MA, USA) and administered daily via intraperitoneal
injection (200 µl) for 7 days. A total of 24 h after the last drug
administration, all animals were weighed and sacrificed by cervical
dislocation. The weights of spleens, thymus, and tumor tissues in
each group were measured, and the sera were collected to detect
IL-2, IFN-γ and TNF-α levels using murine ELISA kits (R&D
Systems Inc., Minneapolis, MN, USA).
BEL-7402 xenograft mouse model and
Mel-P15 administration in vivo
Human hepatocellular carcinoma BEL-7402 cells were
collected at the logarithmic phase of growth and diluted with
normal saline, then the cell suspension (5×106 cells)
was transplanted subcutaneously into the right axilla of each nude
mouse. When the tumors had grown to 100–300 mm3, the
mice were randomly divided into four groups: a control group
(normal saline) and three Mel-P15-treated groups (0.5, 1 or 2 mg/kg
of body weight). Each group contained six mice. Drugs were
administered by intraperitoneal injection three times per week.
During the three weeks of treatment, the tumor volume of each mouse
was measured every three days. Following the final administration,
the mice from all of the groups were sacrificed by cervical
dislocation 24 h after the final administration. The tumor weights
of the mice from each group were measured.
Depletion of the NK cell population in
mice
The depletion of NK cells in mice was conducted
using an anti-asialo-GM-1 antibody (Wako Pure Chemical Industries,
Ltd., Osaka, Japan; catalogue numbe 986-10001) as previously
described (19). Briefly,
tumor-bearing nude mice were administered 50 µl of anti-asialo-GM-1
antibody by intraperitoneal injection 3 days prior to drug
treatment, followed by repeated injection every four days for the
following three weeks. The control group received isotype normal
rabbit IgG (PeproTech Inc., Rocky Hill, NJ, USA; catalogue number
500-P00) injections. The NK cell populations from the spleens of
these mice were analyzed using flow cytometry.
In vivo anti-tumor activity assay
The in vivo antitumor activity was expressed
as an inhibitory rate percentage and calculated as follows:
[(A-B)/A] ×100%, where A and B were the average weights of the
tumors from the control and experimental groups, respectively.
The tumor volume (TV) was measured and calculated
using the following formula: TV=1/2 × a × b2, where a
and b were the long and short diameters of the tumors in each
mouse, respectively.
The spleen or thymus index was calculated as the
spleen (or thymus) weight/body weight.
Splenocyte proliferation assay
Mouse spleens were collected from mice in each group
under aseptic conditions, then were gently homogenized and passed
through a 300-mesh sieve to obtain single-cell suspensions.
Following treatment with erythrocyte lysis buffer (Beyotime
Institute of Biotechnology, Haimen, China), the cells were washed
with normal saline and resuspended in RPMI-1640 medium, which
contained 10% fetal bovine serum. Then the cell suspension
(5×106 cells/ml) was seeded in a 96-well flat-bottomed
microplate in triplicate at 100 µl/well, and either 5 µg/ml
concanavalin A (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany;
catalogue number L6397) or 4 µg/ml lipopolysaccharide
(Sigma-Aldrich; Merck KGaA; catalogue number L2637) was added to
the wells. The cells were then cultured for 44 h at 37°C in a
humidified, 5% CO2 atmosphere and further incubated for
4 h with MTT solution (20 µl/well; 5 mg/ml). The cells were lysed,
and the purple formazan crystals were solubilized using
dimethylsulfoxide (Sigma-Aldrich; Merck KGaA) for detection at 570
nm as measured by a microplate reader (Model 6800; Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All of the experiments in the present study were
performed at least three times. The data are presented as the mean
± standard deviation. Statistical analysis was performed using a
one-way analysis of variance (followed by Dunnett's test) or a
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
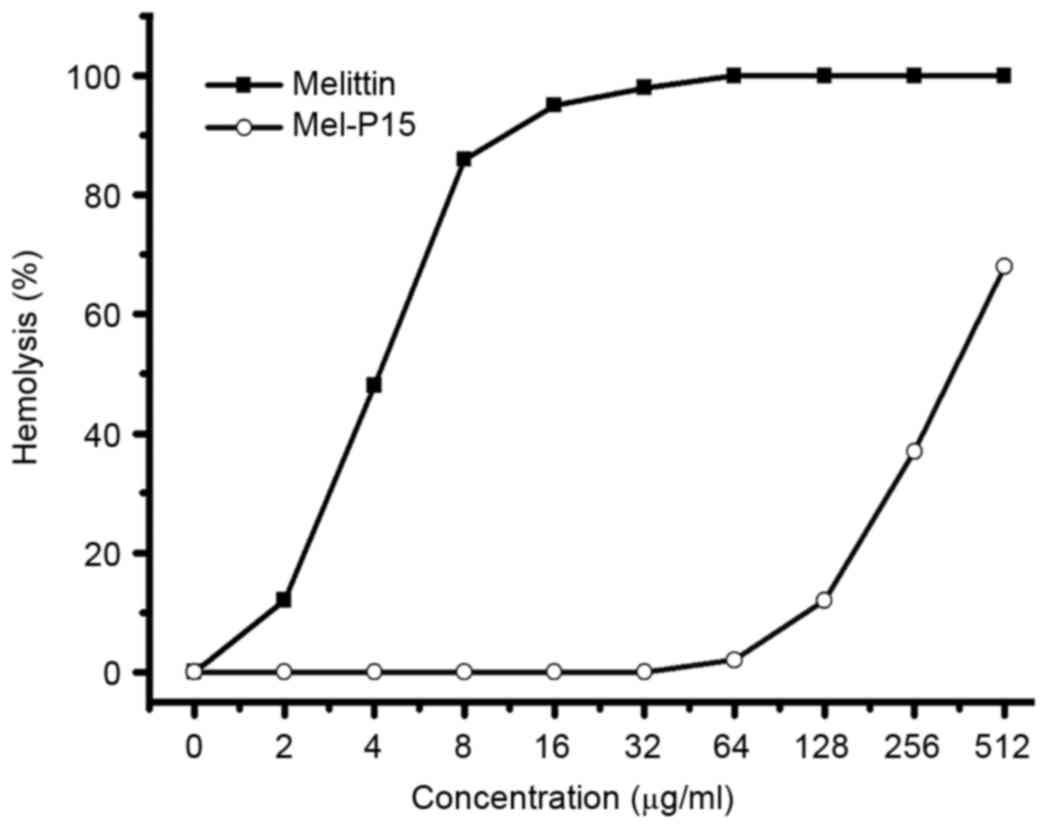
Hemolytic activity of Mel-P15
The hemolytic activity of a peptide against red
blood cells is typically used as a primary assay to test the
toxicity of a peptide (20). Mel-P15
exhibited a relatively low hemolytic activity within the range of
0–128 µg/ml, whereas melittin exhibited clear hemolytic effects
even at low concentrations (Fig.
1).
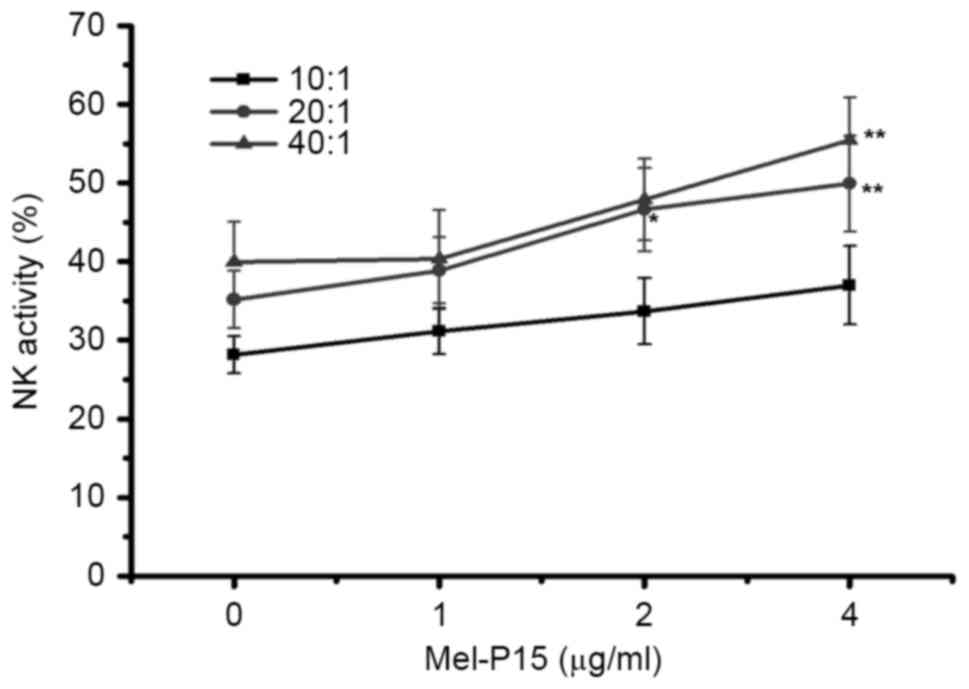
Mel-P15 directly stimulates the
cytotoxicity of NK cells in vitro
When cells were mixed at an E:T ratio of 20:1 or
40:1, Mel-P15 significantly promoted the activity of NK cells in
vitro when administered at a concentration of 4 µM compared
with the control (0 µM) group (P<0.01; Fig. 2). Following treatment with 4 µg/ml
Mel-P15, NK cytotoxicity was increased to 35.72, 49.92 and 55.45%
at E:T ratios of 10:1, 20:1 and 40:1, respectively.
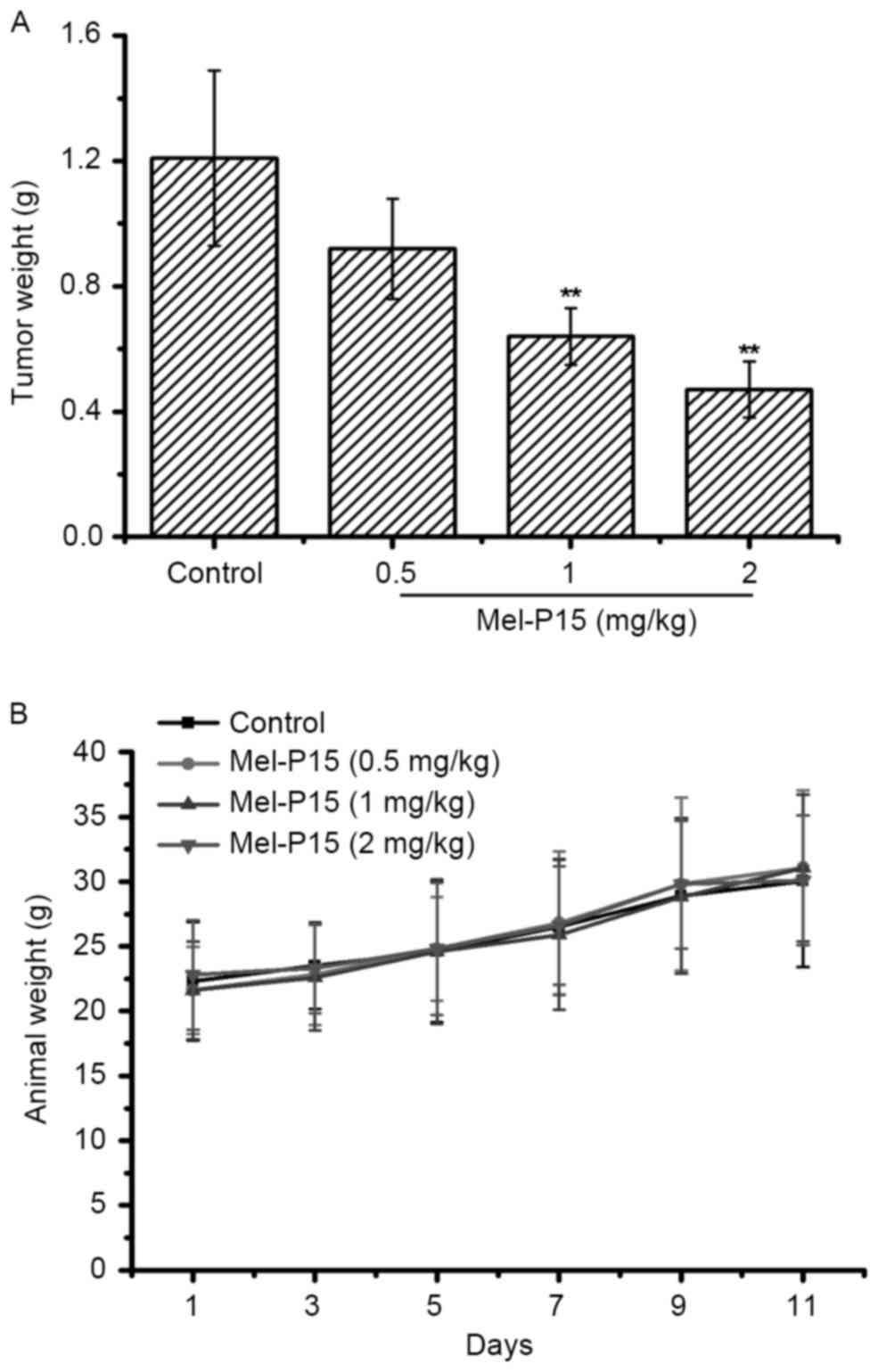
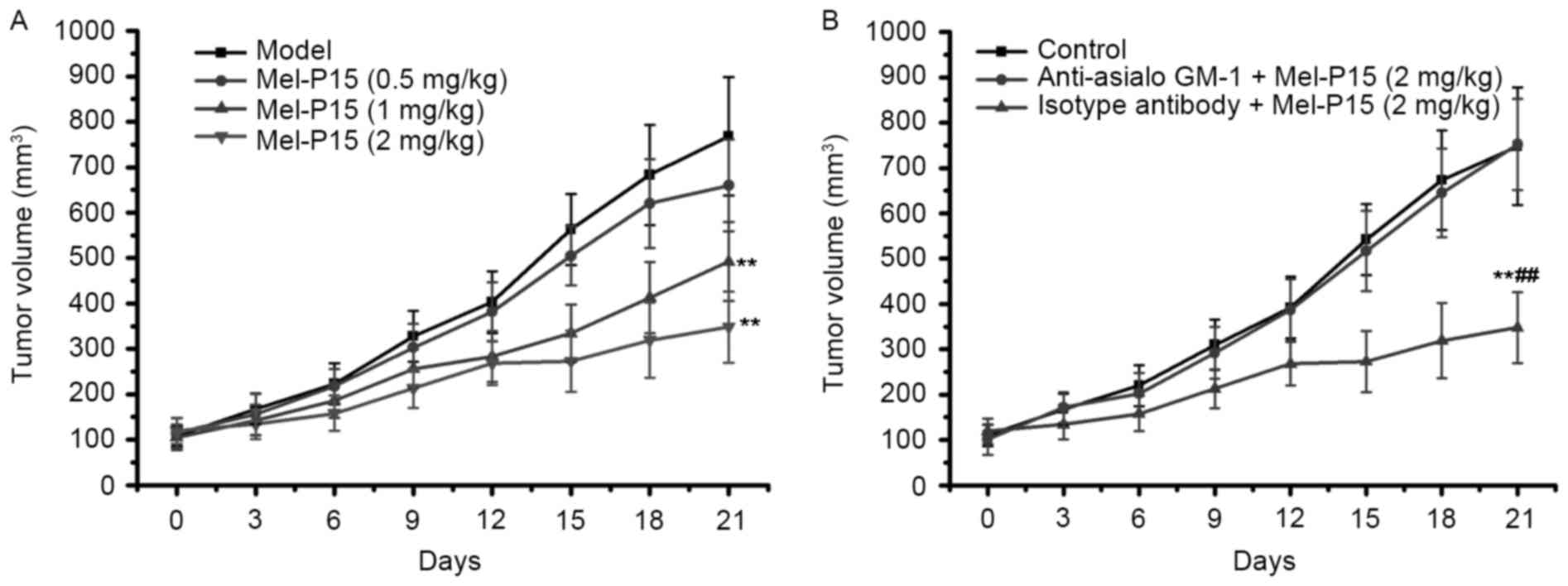
Mel-P15 administration effectively
inhibits the growth of tumors in H22-bearing mice
The tumor weights in each group were measured to
evaluate the inhibitory effect of Mel-P15 on the growth of the
transplanted H22 carcinoma. Mel-P15 significantly inhibited the
growth of H22 hepatomas in vivo in a dose-dependent manner
compared with the control, with an inhibitory rate of 23.97, 47.11
and 61.51% for 0.5, 1 and 2 mg/kg Mel-P15, respectively (P<0.01
for 1 and 2 mg/kg Mel-P15; Fig. 3A).
Additionally, treatment with Mel-P15 did not significantly change
the body weight of tumor-bearing mice compared with the control
mice, suggesting that Mel-P15 does not exhibit marked toxicity
in vivo (Fig. 3B).
Mel-P15 promotes the immune response
in vivo
In order to investigate the effect of Mel-P15 on the
immune system in H22-bearing mice, the spleen and thymus indices
were measured. The results obtained indicated that Mel-P15
significantly increased the indices of spleens and thymuses at
doses of 1 or 2 mg/kg (P<0.05 and P<0.01, respectively for
the spleen and P<0.01 and P<0.01, respectively for the
thymus; Table I). In particular, at a
dose of 2 mg/kg, the spleen and thymus indices were increased 1.47-
and 1.39-fold, respectively, compared with the control group.
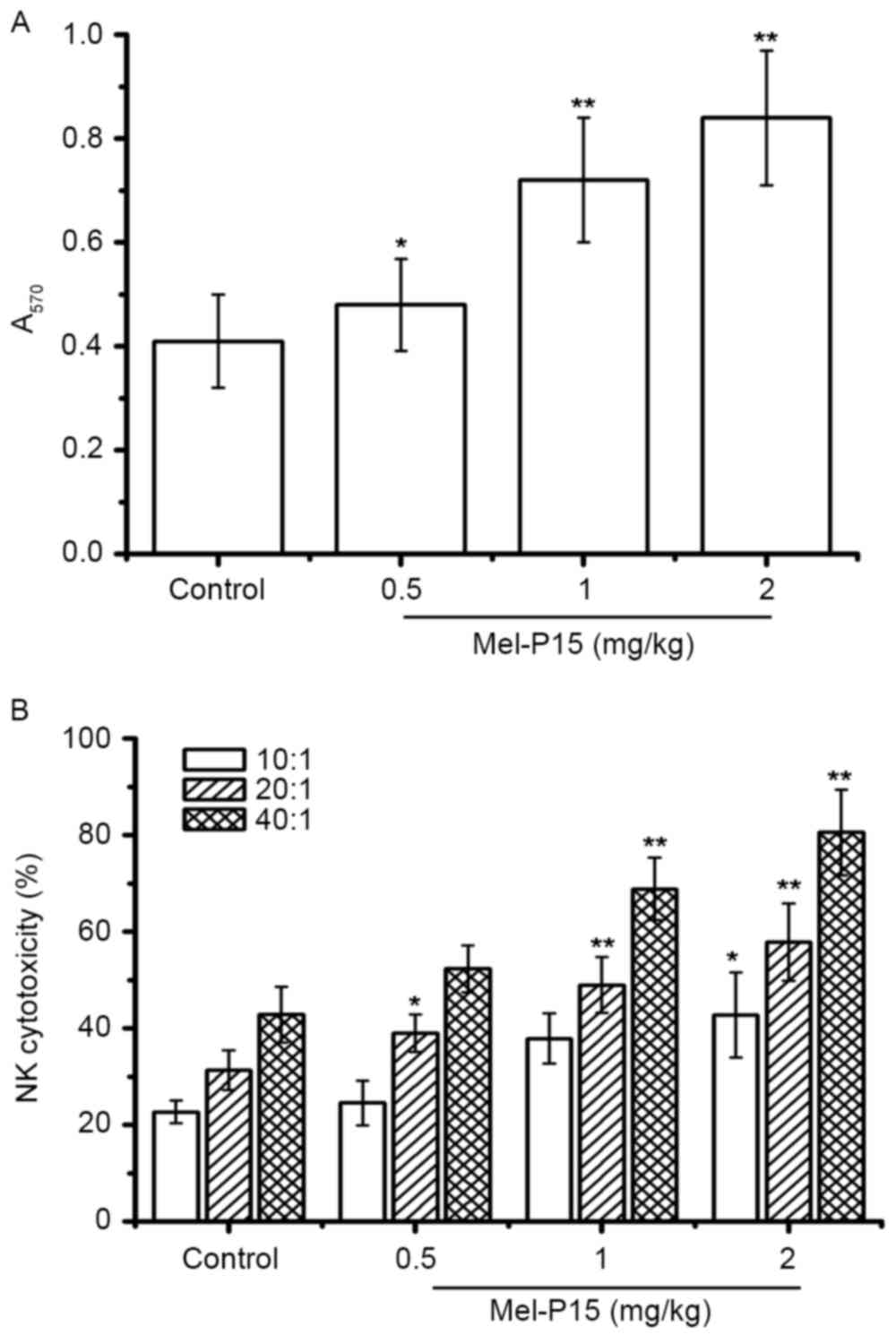
Additionally, Mel-P15 significantly promoted splenocyte
proliferation in vivo in a dose-dependent manner (P<0.05,
P<0.01 and P<0.01 for 0.5, 1 and 2 mg/kg, respectively;
Fig. 4A). The splenocyte
proliferation rates were 1.17-, 1.76- and 2.05-fold following
treatment with 0.5, 1 and 2 mg/kg Mel-P15, respectively, compared
with the control group.
 | Table I.The spleen and thymus indices of
H22-bearing mice following treatment with Mel-P15. |
Table I.
The spleen and thymus indices of
H22-bearing mice following treatment with Mel-P15.
| Group | Spleen index
(mg/g) | Thymus index
(mg/kg) |
|---|
| Control | 5.89±0.22 | 1.28±0.09 |
| Mel-P15 (0.5
mg/kg) | 6.17±0.33 | 1.41±0.21 |
| Mel-P15 (1
mg/kg) |
7.26±0.47a |
1.66±0.27b |
| Mel-P15 (2
mg/kg) |
8.63±1.25b |
1.78±0.45b |
Mel-P15 may stimulate NK cells in vitro;
therefore the cytotoxicity of NK cells in vivo was measured.
The results indicated that NK cytotoxicity was increased in an E:T
ratio- and Mel-P15 dose-dependent manner (Fig. 4B). In particular, at an E:T ratio of
40:1, the NK cytotoxicity was 52.43, 68.89 and 80.56% following
treatment with 0.5, 1 and 2 mg/kg Mel-P15, respectively.
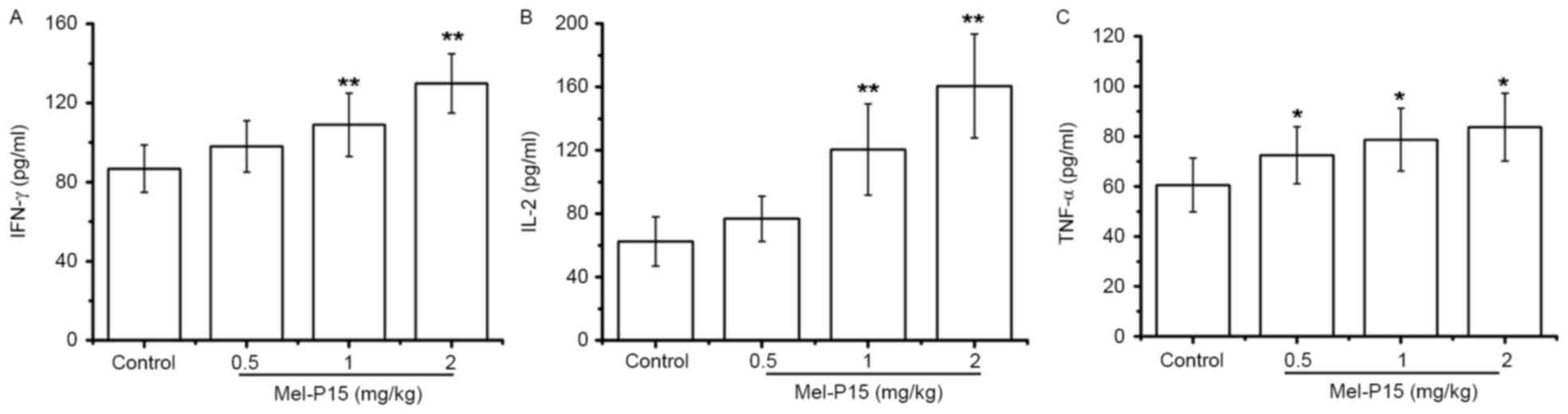
TNF-α, IL-2 and IFN-γ levels in the sera of
H22-bearing mice were significantly increased in a dose-dependent
manner following Mel-P15 administration (Fig. 5A-C). Following treatment with 2 mg/kg
Mel-P15, the levels of TNF-α, IL-2 and IFN-γ were 1.38-, 2.57- and
1.49-fold, respectively, compared with the control group. Taken
together, these results suggested that the inhibition of tumor
growth by Mel-P15 in vivo was mediated partly by stimulating
the immune response.
Depletion of NK cells impairs the
antitumor effects of Mel-P15 in BEL-7402-bearing nude mice
As Mel-P15 treatment significantly prevented the
growth of mouse H22 hepatocarcinoma in vivo, the effect of
Mel-P15 on BEL-7402 tumor growth was further studied in nude mice.
The results demonstrated that Mel-P15 significantly inhibited tumor
growth in BEL-7402-bearing mice in a dose-dependent manner
(Fig. 6A). However, when NK cells
were depleted in BEL-7402-bearing nude mice by intraperitoneal
injection of anti-asialo-GM1 antibody, the effect of Mel-P15 on
BEL-7402 tumor growth was almost completely abolished (Fig. 6B). Taken together, the results
indicated that NK cytotoxicity serves a role in the
anti-hepatocarcinoma effects of Mel-P15.
Discussion
Hepatocarcinoma is one of the most common types of
malignant cancer and has been associated with high fatality rates
in humans (1). In the clinic, one of
the primary treatments is chemotherapy, which causes adverse side
effects (21). Therefore, novel
treatments are required to treat hepatocarcinoma.
Among the novel candidate drugs, antimicrobial
peptides have attracted attention due to their decreased likelihood
of drug resistance and low cytotoxicity (22). Melittin, a natural antibacterial
peptide, has been demonstrated to possess broad-spectrum
antimicrobial, antiviral and antitumor effects (7–9). In the
present study, based on the structure of melittin, a new peptide
was designed and named Mel-P15. The whole sequence of Mel-P15 was
GLPALISWIKRKRQQ, which consisted of 15 amino acids. Then the
anti-tumor activity and underlying molecular mechanisms of the
effects of Mel-P15 were investigated.
First, the toxicity of Mel-P15 in vitro was
studied. The hemolytic activity of Mel-P15 indicated that for doses
up to 128 µg/ml, Mel-P15 exhibited low toxicity on eukaryotic
cells. However, for melittin, at the concentration of 10 µg/ml, the
hemolytic activity reached ~70%, which was higher compared with
that of Mel-P15. The data indicated that Mel-P15 exhibited a lower
cytotoxicity on eukaryotic cells compared with melittin.
In a mouse H22 hepatocarcinoma xenograft tumor
model, Mel-P15 inhibited tumor growth in vivo and increased
the spleen and thymus indices. Further investigated indicated that
Mel-P15 activates the immune system by promoting splenocyte
proliferation, NK cell cytotoxicity and cytokine secretion,
including that of IFN-γ, TNF-α and IL-2. These findings suggested
that the anti-H22 effects of Mel-P15 may be the result of enhanced
immune responses in vivo.
The immune system serves an important role in
antitumor defenses via cytotoxic lymphocyte T cells and NK cells
(23–25). NK cells possess a number of biological
activities; NK cells not only directly recognize and lyse cancer
cells, but also initiate anti-tumor immune responses by secreting a
number of cytokines, including IFN-γ and TNF-α (26). In the present study, Mel-P15 directly
stimulated NK cytotoxicity in vitro. In addition, NK
activity was markedly induced in H22-bearing mice following Mel-P15
administration. A BEL-7402-bearing nude mice model, which lacks T
cells (and therefore for which NK cells are important for the
immune response), was established. The results demonstrated that
tumor growth was inhibited in a dose-dependent manner in the
Mel-P15-treated group. Taken together, these results indicate that
NK cells are important in mediating the anti-tumor effects of
Mel-P15. In order to confirm this hypothesis, NK cells were
depleted by pre-treatment with an anti-asialo-GM-1 antibody, which
almost completely impaired the Mel-P15-induced suppression of tumor
growth. These results suggest that Mel-P15-enhances NK cytotoxicity
in tumor bearing mice.
Cytokines serve a vital role in the immune response
to tumor cells, including IFN-γ, TNF-α and IL-2; in particular,
IFN-γ may enhance the activity of NK cells against intracellular
pathogens and tumor cells (27,28).
Additionally, IL-2 may promote the proliferation and cytotoxicity
of NK cells (29). In H22-bearing
mice, the secretion of IFN-γ, TNF-α and IL-2 was elevated following
Mel-P15 administration, suggesting that Mel-P15 increased the
secretion of IL-2 and IFN-γ in tumor-bearing mice, thereby
activating and maintaining the cytotoxicity of NK cells and
eliminating H22 cells.
In conclusion, the data from the present study
demonstrated that Mel-P15, a novel analogue derived from melittin,
not only inhibited tumor growth in vivo, but also stimulated
the immune system by enhancing NK activity, increasing the spleen
and thymus indices and promoting the release of cytokines,
including IL-2, IFN-γ and TNF-α. Additionally, the antitumor effect
of Mel-P15 in vivo was impaired when NK cells were depleted
using an anti-asialo GM-1 antibody. Traditional chemotherapy drugs
exhibit severe side effects; Mel-P15, which activates the immune
system, may decrease the toxicity of chemotherapy in vivo.
In the future, Mel-P15 may potentially be used as a complementary
drug or protective immunotherapeutic agent for the treatment of
cancer.
Acknowledgements
The present study was financially supported by
grants from the Scientific and Technological Support & Social
Development Plan of Shandong Province (grant no. 2014GGB1422).
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu C, Geng X, Wan S, Hou H, Yu F, Jia B
and Wang L: Cecropin-P17, an analog of Cecropin B, inhibits human
hepatocellular carcinoma cell HepG-2 proliferation via regulation
of ROS, Caspase, Bax, and Bcl-2. J Pept Sci. 21:661–668. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McMillin DW and Mitsiades CS:
High-throughput approaches to discover novel immunomodulat
immunomodulatory agents for cancer. Oncoimmunology. 1:1406–1408.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kurtin SE and Bilotti E: Novel agents for
the treatment of multiple myeloma: Proteasome inhibitors and
immunomodulatory agents. J Adv Pract Oncol. 4:307–321.
2013.PubMed/NCBI
|
|
5
|
Fletcher JE and Jiang MS: Possible
mechanisms of action of cobra snake venom cardiotoxins and bee
venom melittin. Toxicon. 31:669–695. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bechinger B: Structure and functions of
channel-forming peptides: Magainins, cecropins, melittin and
alamethicin. J Membr Biol. 156:197–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Raghuraman H and Chattopadhyay A:
Melittin: A membrane-active peptide with diverse functions. Biosci
Rep. 27:189–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi JH, Jang AY, Lin S, Lim S, Kim D,
Park K, Han SM, Yeo JH and Seo HS: Melittin, a honeybee
venom-derived antimicrobial peptide, may target
methicillin-resistant Staphylococcus aureus. Mol Med Rep.
12:6483–6490. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu X, Zhao B, Cheng Y, Yang Y, Huang C,
Meng X, Wu B, Zhang L, Lv X and Li J: Melittin induces PTCH1
expression by down-regulating MeCP2 in human hepatocellular
carcinoma SMMC-7721 cells. Toxicol Appl Pharmacol. 288:74–83. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang C, Chen T, Zhang N, Yang M, Li B, Lü
X, Cao X and Ling C: Melittin, a major component of bee venom,
sensitizes human hepatocellular carcinoma cells to tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis
by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha
kinase-NFkappaB. J Biol Chem. 284:3804–3813. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J and Lee DG: Melittin triggers
apoptosis in Candida albicans through the reactive oxygen
species-mediated mitochondria/caspase-dependent pathway. FEMS
Microbiol Lett. 355:36–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oršolić N: Bee venom in cancer therapy.
Cancer Metastasis Rev. 31:173–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dezfuli HT, Shahbazzadeh D, Eidi A,
Bagheri KP, Pakravan N, Amini S, Aghasadeghi MR and Mahdavi M:
Induction of IFN-γ cytokine response against hepatitis B surface
antigen using melittin. Gastroenterol Hepatol Bed Bench. 7:108–117.
2014.PubMed/NCBI
|
|
14
|
Antopolsky M, Azhayeva E, Tengvall U and
Azhayev A: Towards a general method for the stepwise solid-phase
synthesis of peptide-oligonucleotide conjugates. Tetrahedron Lett.
43:527–530. 2002. View Article : Google Scholar
|
|
15
|
Tu J, Wu G, Zuo Y, Zhao L and Wang S:
ZL-2, a cathelicidin-derived antimicrobial peptide, has a broad
antimicrobial activity against gram-positive bacteria and
gram-negative bacteria in vitro and in vivo. Arch Pharm Res.
38:1802–1809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harakeh S, Azar R, Azhar E, Damanhouri GA,
Assidi M, Abu-Elmagd M, Alqahtani MH, Kumosani T, Niedzwiecki A,
Rath M, et al: Pecific nutrient combination effects on tax, NF-κB
and MMP-9 in human T-cell lymphotropic virus-1 positive malignant
T-lymphocytes. BMC Cancer. 15 Suppl 1:S22015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Wu X, Zhang H, Yang G, Hao M, Sheng
S, Sun Y, Long J, Hu C, Sun X, et al: A Huaier polysaccharide
inhibits hepatocellular carcinoma growth and metastasis. Tumour
Biol. 36:1739–1745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang XY, Qiao H, Ni JM, Shi YB and Qiang
Y: Preparation of isoliquiritigenin-loaded nanostructured lipid
carrier and the in vivo evaluation in tumor-bearing mice. Eur J
Pharm Sci. 49:411–422. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huo L, Yao H, Wang X, Wong GW, Kung HF and
Lin MC: Inhibition of melanoma growth by subcutaneous
administration of hTERTC27 viral cocktail in C57BL/6 mice. PLoS
One. 5:e127052010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheng M, Zhao Y, Zhang A, Wang L and Zhang
G: The effect of LfcinB9 on human ovarian cancer cell SK-OV-3 is
mediated by inducing apoptosis. J Pept Sci. 20:803–810. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daniel D and Crawford J: Myelotoxicity
from chemotherapy. Semin Oncol. 33:74–85. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kang W, Liu H, Ma L, Wang M, Wei S, Sun P,
Jiang M, Guo M, Zhou C and Dou J: Effective antimicrobial activity
of a peptide mutant Cbf-14-2 against penicillin-resistant bacteria
based on its unnatural amino acids. Eur J Pharm Sci. 105:169–177.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou Z, Yu X, Zhang J, Tian Z and Zhang C:
TLR7/8 agonists promote NK-DC cross-talk to enhance NK cell
anti-tumor effects in hepatocellular carcinoma. Cancer Lett.
369:298–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McDowell KA, Hank JA, DeSantes KB,
Capitini CM, Otto M and Sondel PM: NK cell-based immunotherapies in
pediatric oncology. J Pediatr Hematol Oncol. 37:79–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woo SR, Corrales L and Gajewski TF: Innate
immune recognition of cancer. Annu Rev Immunol. 33:445–474. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ke M, Wang H, Zhang M, Tian Y, Wang Y, Li
B, Yu J, Dou J, Xi T and Zhou C: The anti-lung cancer activity of
SEP is mediated by the activation and cytotoxicity of NK cells via
TLR2/4 in vivo. Biochem Pharmacol. 89:119–130. 2015. View Article : Google Scholar
|
|
27
|
Schoenborn JR and Wilson CB: Regulation of
Interferon-gamma during innate and adaptive immune responses. Adv
Immunol. 96:41–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Balachandran S and Adams GP:
Interferon-γ-induced necrosis: An antitumor biotherapeutic
perspective. J Interferon Cytokine Res. 33:171–180. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
García-Cuesta EM, López-Cobo S,
Álvarez-Maestro M, Esteso G, Romera-Cárdenas G, Rey M, Cassady-Cain
RL, Linares A, Valés-Gómez A, Reyburn HT, et al: NKG2D is a key
receptor for recognition of bladder cancer cells by IL-2-activated
NK cells and BCG promotes NK cell activation. Front Immunol.
6:2842015.PubMed/NCBI
|