Introduction
Lung cancer is one of the most common types of
cancer worldwide and hasone of the highest mortality rates
(1). Despite advances in imaging
technologies, which have substantially improved the accuracy of
preoperative staging of lung cancer, the extent of the disease
remains underestimated, and radical surgical resection may not be
feasible following exploratory surgery. The present study aimed at
investigating intraoperative patients with residual cancer.
Implantation of 125I may control residual disease, and
reduce the risk of surgery and postoperative complications.
Otherwise, post-surgical radiotherapy is required to control the
residual site of tumor growth (2).
Previous studies have indicated that the rate of exploratory
surgery decreases gradually with time to between 1 and 2%, or even
lower (2,3). However, the incidence of macroscopic
residual disease (R2) following resection of non-small cell lung
cancer (NSCLC) remains at ~4% (3).
Intra-tumor 125I implantation is a localized
radiotherapy and any adverse effects on normal tissues are confined
to the immediate vicinity (1).
Furthermore, the intra-tumor 125I may reach the
prescribed radioactivity between 110 and 160 Gy locally, which is
considered to be a curable radiation dose and lasts for a longer
period of time, compared with conventional external beam radiation
to eliminate the tumor cells (4).
External irradiation is generally administered following surgery,
although the efficacy of this process is suboptimal, without any
marked improvement in survival (3).
Intraoperative implantation of irradiative particles increases the
local control in patients with locally advanced lung cancer.
In the present study, 12 patients with macroscopic
residual disease following exploratory surgery received radical
surgical resection plus intraoperative implantation of
125I seeds between March 2010 and May 2014. The duration
of treatment, local recurrence, median survival time and median
progression free survival (PFS) were evaluated.
Materials and methods
Clinical data
Between March 2010 and May 2014, 23 patients with
NSCLC (17 males and 6 females) were included, from General Hospital
of Chengdu Military Region, in the present study. 12 patients in
the radioactive seed implant group and 11 patients the conventional
radiotherapy group. The clinical data of the patients are shown in
Table I. The indications for
125I particle implantation were as follows: i) Stage T4
disease, according to the 7th Japan Joint Committee of Lung
Cancer/Union for International Cancer Control Tumor Node Metastasis
staging system for NSCLC (5). was
identified during treatment and radically resected; ii) the
location of the lesion was at the pulmonary hilus. The residual
lesion infiltrated the major blood vessels, which prevents safe
resection; iii) the lesions involved the mediastinum, trachea,
esophagus, aorta, superior vena cava or pericardium; and iv) tumor
invasion of the thoracic walls or spine preventing complete
removal. The following exclusion criteria was applied: i) Mortality
within 30 days' post-surgery; ii) aged >80 years; and iii) lung
tumors were non-primary lesions. The treatment methods were agreed
upon by the patients, and informed consent was provided from all
patients. The present study was approved by the Ethics Committee of
the General Hospital of Chengdu Military Region (approval no.
10-00253) (Chengdu, China), and written informed consent was
obtained from each participant.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Radioactive seed
implant group (n=12) | Conventional
radiotherapy group (n=11) | P-value |
|---|
| Age |
|
| 0.263 |
| Range,
years | 44–69 | 37–73 |
|
| Mean ±
SD, years | 57.92±7.57 | 53.00±12.55 |
|
| Sex |
|
| 0.901 |
| Male | 9 | 8 |
|
|
Female | 3 | 3 |
|
| Histology |
|
| 0.624 |
|
Squamous | 4 | 6 |
|
|
Adenocarcinoma | 6 | 4 |
|
|
Adenosquamous | 2 | 1 |
|
|
Adenosquamous | 0 | 0 |
|
| Carcinoma |
|
|
|
| TNM
classificationa |
|
| 0.572 |
|
IIA | 2 | 3 |
|
|
IIB | 1 | 2 |
|
|
IIIA | 9 | 6 |
|
| Classification of
tumor |
|
| 0.879 |
| T-R2
type | 4 | 4 |
|
| N-R2
type | 8 | 7 |
|
| Size of
tumorb,
cm3 | 66.42±70.41 | 100.45±208.03 | 0.598 |
| Chemotherapy
regimen |
|
| 0.481 |
|
GEM+DDP | 3 | 5 |
|
| PC | 5 | 3 |
|
|
Other | 4 | 3 |
|
Materials
125I radioactive seeds with
22.4–29.6MBq/particle were obtained from Shanghai GMS
Pharmaceutical Co., Ltd. (Shanghai, China). An enclosed rotatory
implanter and implant needles [Hakko International Trading
(Shanghai) Co., Ltd., Shanghai, China] were used to implant the
radioactive seeds.
Methods
Tumors of the 23 patients scheduled for thoracotomy
were resected. The lymph nodes with residual disease were validated
by frozen section examination (Fresh tissue frozen for 5 min under
−20 degrees Celsius, 4% formaldehyde fixation for 10–15 sec,
hematoxylin staining for 30 sec, eosin staining for 3 sec after
hydrochloric alcohol differentiation for 1 sec, rinse water,
neutral resin sheet. Observe under OLYMPUS microscope (X10). From
tissue sections removed during surgery. Once the sites of residual
disease were identified, the particles were implanted according to
the Radiotherapy Treatment Planning System (TPS) plan with a dose
of 120 Gy (1). Postoperative external
irradiation (40–60 Gy at a dose of 2 Gy/day for 20–30 days) was
used in patients in the conventional treatment group within two
months of treatment.
Intraoperative particle
implantations
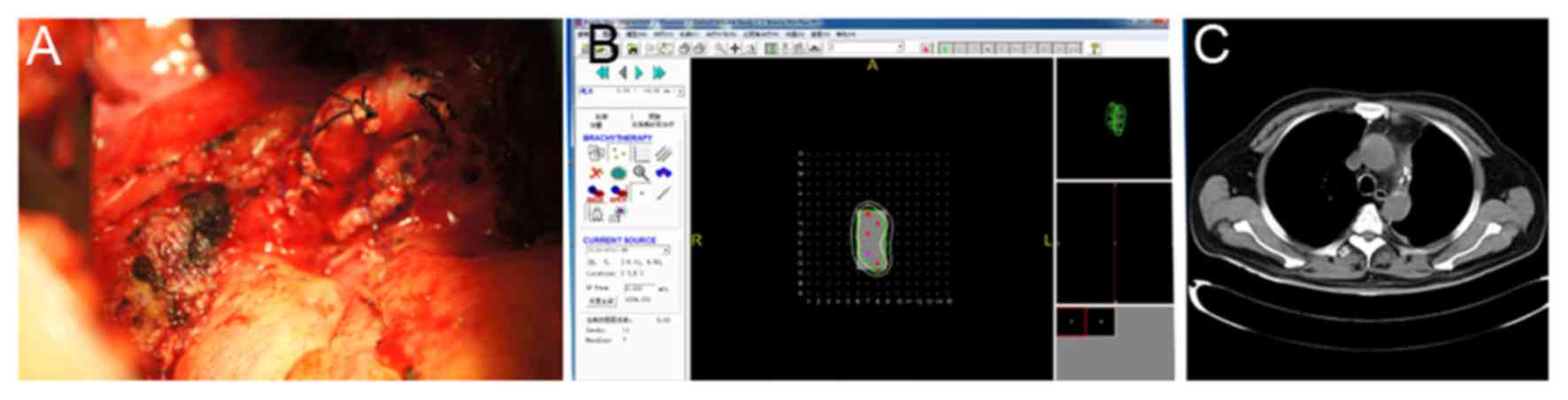
Images of the areas of residual disease were
captured and the thickness of the residual lymph nodes was
measured. This data were subsequently inputted into the TPS system
for 3D reconstruction. The overall dosage and the number of
particles required for tumor control were calculated. The volume of
the residual tumor was estimated and recorded in the TPS system to
simulate the size of the lymph node (Fig.
1). A particle was implanted every 0.5–1.0 cm, and the target
dose was 120 Gy. The volume of the residual lesions were estimated,
and the 22.4–29.6MBq/particles were implanted 0.5–1.0 cm to
maintain identical distances between the particles. For tumor
tissues with relatively low thickness, a gelatin sponge was used as
the particles were not implanted deeply enough. The particles were
distributed at a pitch of 1 cm in the form of gelatin sponge, and
were attached to the residual surface (6) For the cancerous residual disease tissues
on the bronchial stump, a fine thread was used to directly suture
the particles onto the tumor tissues. In the 12 patients, a total
of 4 to 30 125I particles were implanted, with an
average of 10 per patient. A total of 6 patients received 2–6
cycles of postoperative chemotherapy. Appropriate TPS plans were
developed according to the shape and size of residual lesions, so
that the particle implant dose distribution was more uniform
between the particles.
Follow-up
All 23 patients entered the follow-up phase
immediately following resection. The intended follow-up period was
40 months with visits at 1 month, 3 months and every 3 months
thereafter. Anteroposterior and lateral chest images and computed
tomography (CT) scans were conducted one week following the surgery
to assess the distribution of particles. The patients were
re-examined at 1, 2, 6 and 12 months and every 6 months thereafter.
Following surgery, clinical examination, blood sampling and CT
examination of the chestwere performed. No patients were lost to
follow-up. Follow-up chest CT scans were obtained to evaluate
response following surgery.
Statistical analysis
SPSS software (version 16.0; SPSS, Inc., Chicago,
IL, USA) was used to perform statistical analysis. Qualitative data
are described as frequency and percentage, and qualitative data are
expressed as the mean ± standard deviation. χ2 test was
used to compare the qualitative data, while an unpaired t-test was
used for the comparison of quantitative data between the
implantation and conventional groups. The Kaplan-Meier method was
used to perform survival analysis, and log-rank test was used to
compare the survival time between the two groups. P<0.05 was
considered to indicate a statistically significant difference. The
survival rate, PFS, median survival time, and the percentage of
T-/N-residual disease were compared between the two groups.
Results
Patient survival
The characteristics of the patients are listed in
Table I. The median follow-up time
was 18 months for all patients and 24 months for the patients in
the 125I particle implant group (Table II). In total, the patients were
followed up for 1 to 4 and, 2 years after surgery, no tumor
recurrence was observed in the patients who underwent
125I particle implantation. The 2-year local control
rate was 100%. The local control rate in the 125I
particle implantation group was markedly higher compared with
patients who underwent postoperative external irradiation,
irrespective of T- or N2-residual disease. The median survival time
was 12 months and the local recurrence rate was 54.4%.
 | Table II.Profile of patients with non-small
cell lung cancer. |
Table II.
Profile of patients with non-small
cell lung cancer.
| A, Intraoperative
125I implantation group |
|---|
|
|---|
| Patient no. | Number of the
particles 22.4–29.6MBq/particle/dose of external irradiation
(Gy) | Time until local
recurrence (months) | Time until distant
metastasis (months) | Overall survival
(months) | Duration of
follow-up (months) | Site of residual
disease | Lymph node
metastasis | Post-operative
radio-/chemo-therapy | Cause of
mortality |
|---|
| 1 | 4/120 | 0 | 36 | 36 | 36 | Resection margin in
the right upper lobe, residual cancer of the failure bronchial
stump (T residual) | No metastasis | 2 cycles of
DDP+PTX | Pulmonary |
| 2 | 6/110 | 0 | 18 | 34 | 34 | Left upper hilum,
tumor residuals at the bronchial stump and the surface of the
pulmonary artery (T residual) | Residual cancer of
bronchial stump | 2 cycles of
DDP+PTX | Bone and abdominal
metastases |
| 3 | 12/120 | 0 | 24 | 31 | 31 | Group 5 lymph nodes
were closely adhered to the pulmonary artery Group 6 lymph nodes
covered the phrenic nerve (N residual) | Residual and
fixation of groups 5 and 6 lymph node in the left upper lobe, which
were not resected | 4 cycles of
GEM+DDP | Pulmonary
failure |
| 4 | 4/110 | 0 | 6 | 12 | 11 | Left middle lobe;
tumor invaded the phrenic nerve lymph node (T residual) | 1×2 cm patchy lymph
node residual next to the right phrenic nerve | 4 cycles of
DDP+PTX | Thoracic cavity
metastases, pulmonaryfailure |
| 5 | 7/120 | 0 | 9 | 16 | 16 | Mediastinal lymph
node, ascending aortic, and aortic window lymph node enlargement
and fixation (N residual) | 4R (3/3), group 10
(6/8), group 11 (1/1) | 6 cycles of
GEM+DDP; 1 course of head R-knife | Head and clavicle
metastasis, superior vena cava blockage |
| 6 | 6/110 | 0 | 14 | 16 | 16 | Adhesion between
subcarinal lymph node and the main bronchus (N residual) | Subcarinal lymph
node 2×2.5 cm | 3 cycles of
GEM+DDP | Hydrothorax and
ascites, systemic metastases, pulmonary failure |
| 7 | 19/130 | 0 | 6 | 26 | 26 | Enlargement of the
upper mediastinal lymph node and adhesion to the superior vena cava
and main bronchus (N residual) | Group 4 (1/1) | 6 cycles of PC; 1
course of head R-knife | Head
metastases |
| 8 | 9/120 | 40 | 44 | Survival | 48 | Tumor invasion of
the bronchial stump; enlargement of the peribronchial lymph node
and fixation in the left lower lobe (T and N residual) | Group 4 (1/1),
group 7 (1/1), group 10 (1/1), group 11 (4/11) | 1 cycle of PC, the
patient refused radio-/chemo- therapy following recurrence | Survival |
| 9 | 14/110 | 0 | 0 | Survival | 24 | Partial pericardial
invasion with the lymph node under the aortic arch in the left lung
(N residual) | Pericardial
invasion; adhesion and fixation of the lymph node under the aortic
arch | 4 cycles of PC | Survival |
| 10 | 4/120 | 0 | 7 | 12 | 11 | Right lower lobe
bronchial stump, tumor invasion of the enlarged lymph nodes at the
upper lobe (N residual) | Group 10 (2/5),
residual cancer at the bronchial stump lymph node | 3 cycles of
PTX+DDP | Chest, back, limb,
and spine metastases, hydrothorax and ascites |
| 11 | 10/100 | 0 | 0 | Survival | 18 | Enlargement of the
lymph node at 4 cm posterior to the vena cava (N residual) | Fixation of the
groups 4, 5, and 6 lymph node | 4 cycles of PC | Survival |
| 12 | 30//130 | 0 | 3 | 24 | 24 | Paratracheal lymph
node fixation (~4×3×2 cm) inthe right upper lobe (N residual) | Group 3A (1/1),
carinal and paratracheal lymph nodes were not removed | 4 cycles of PC, 1
course of head R-knife and radiotherap for recurrent tumor | Recurrence of the
head metastases |
|
| B, Conventional
postoperative external irradiation group |
|
| 1 | 60 | 0 | 3 | 8 | 8 | Dense paratracheal
adhesion, titanium clip marked stump (N residual) | Groups 4 and 7
(5/5) | 3 cycles of PC
3 | Radiation
pneumonitis, pulmonary failure |
| 2 | 50 | 6 | 0 | 18 | 18 | Residual cancer of
bronchial stump, titanium clip marked stump (T residual) | No lymph node
metastasis | 4 cycles of
GEM+DDP | Local recurrence of
lung cancer, bone metastases |
| 3 | 60 | 0 | 1 | 3 | 3 | Enlargement of the
lymph nodes anterior to the bronchus and invasion of the left
pulmonary artery (N residual) | Group 7 (2/3) | 1 cycle of
GEM+DDP | Intracranial
metastases |
| 4 | 50 | 2 | 0 | 6 | 6 | Residual cancer of
bronchial stump and the vein at the middle lobe (T residual) | No lymph node
metastasis | 1 cycle of
GEM+DDP | Local recurrence,
radiation pneumonitis |
| 5 | 60 | 0 | 24 | 33 | 33 | Frozen-shape of the
superior mediastinal lymph nodes and bronchus (N-residual) | Group 7 (2/2) | 6 cycles of
docetaxel +DDP | Brain metastases,
pulmonary failure |
| 6 | 50 | 3 | 0 | 8 | 8 | Enlargement of the
lymph nodes at the intermediate bronchus and inferior lobar
bronchus (N residual) | Group 11 (2/2) | 4 cycles of
GEM+DDP | Pulmonary failure,
systemic bone metastases |
| 7 | 50 | 0 | 18 | 32 | 32 | Hilar lymph node
metastases (N-residual) | Para- bronchial
stump; group 2 and 4 lymph node metastases (3/4, 7/7, 1/1) | 4 cycles of PC | Pulmonary failure,
systemic bone metastases |
| 8 | 40 | 0 | 24 | Survival | 36 | Dense adhesion of
the lymph nodes at the basal segments of the right lower lobe,
superior segmental bronchus, and right inferior pulmonary vein
(N-residual) | Group 10 (1/2) | 3 cycles of
docetaxel+ DDP | Left lung
metastases 24 months' post-operation |
| 9 | 60 | 6 | 0 | 12 | 12 | Cancer invasion of
the bronchial stump lymph nodes and fibrous tissues
(T-residual) | No lymph node
metastasis | 4 cycles of
docetaxel+ DDP | Systemic pleura,
bone and celiac lymph node metastases |
| 10 | 50 | 0 | 4 | 32 | 32 | Bifurcation of the
middle and lower lobes, carinal lymph node enlargement
(N-residual) | No lymph node
dissection | 4 cycles of PC | Liver,
intracranial, and bone metastases |
| 11 | 55 | 2 | 0 | 7 | 7 | Bronchial stump
(T-residual) | Group 4 (1/3),
group 10 (2/2) | 4 cycles of
GEM+DDP | Local recurrence,
radiochemotherapy complications |
In the 125I particle implantation group,
4 patients succumbed to pulmonary failure, and 3 succumbed to brain
metastases. In addition, 2 patients were found with thoracic,
abdominal and bone metastases. Of the 3 surviving patients,
multiple lymph node metastases around the lesion were observed in 1
patient, with postoperative pathological examination indicating
N2-stage disease. The lymph nodes at the ascending aorta and aortic
window were fixed, and a total of 9 125I particles were
implanted during the operation. Another patient was found to have
brain metastases 3 months following the operation, and was treated
with stereotactic radiotherapy.
In the conventional treatment group, the 11 patients
that underwent conventional treatment for only one cycle, 40–60 Gy
of external irradiation (2 Gy/day for 20–30 days) was used
postoperatively for the target region. The median survival time of
these 11 patients was 12 months. Of the 11 patients, 1 survived, 4
succumbed to local recurrence, 2 of which were complicated by
radiation pneumonitis and 6 succumbed to distant metastases,
including brain, liver, renal and bone metastases.
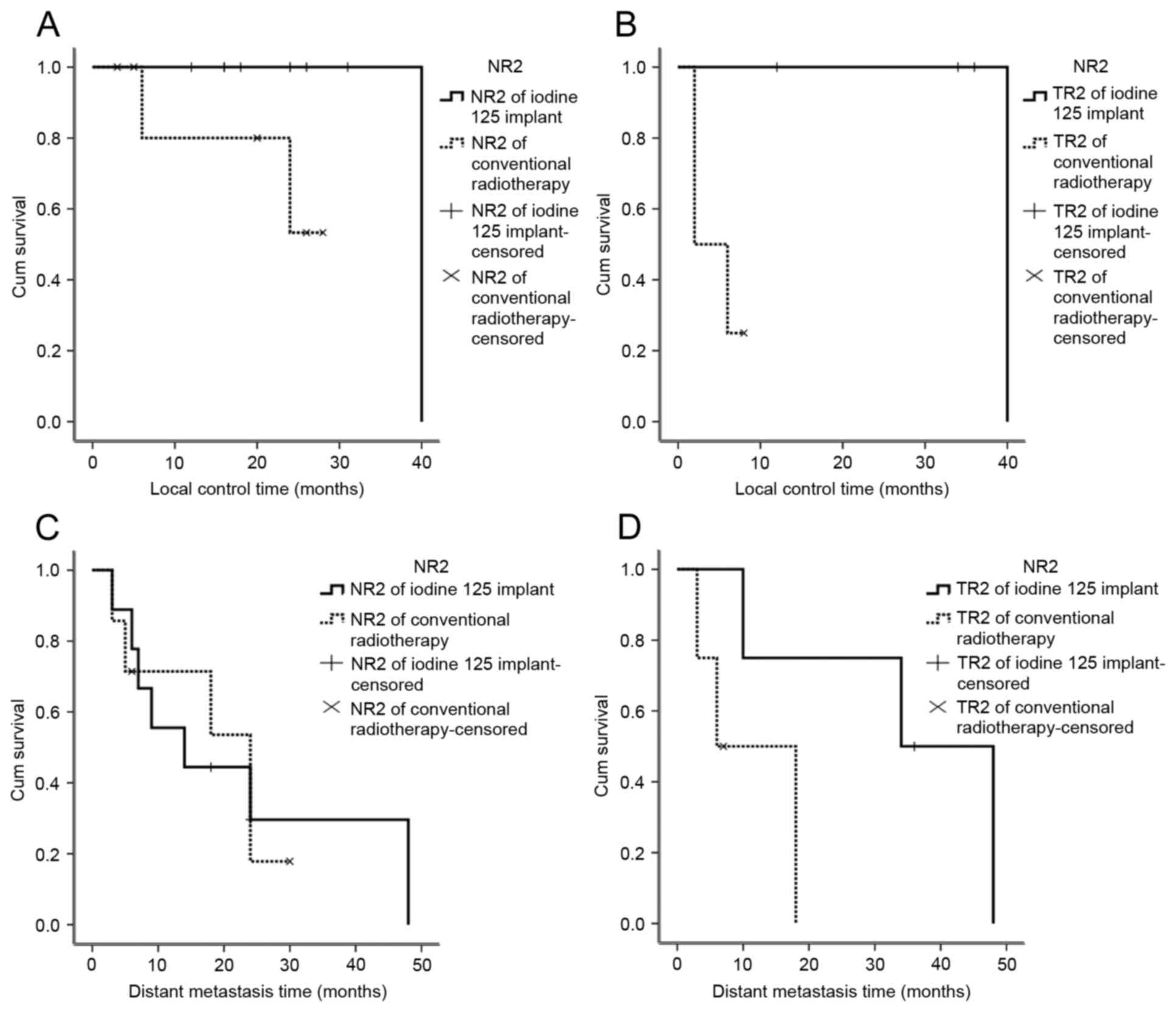
Control rate of local and distant
metastasis
All 23 patients underwent surgical resection, and
the site of particle implantation was confirmed asR2 resection. A
total of 121 125I particles were implanted in patients,
including 4 patients with incomplete tumor resection with
T-residual disease. In total, 8 patients exhibited lymph node
metastases, with enlarged lymph nodes and invasion of the pulmonary
artery, superior vena cava and the main bronchus. Of the patients
undergoing conventional treatment, T-residual disease was observed
in 4 patients and N-residual disease was revealed in 7 patients. No
tumor recurrence at the implantation site was observed 2 years
following seed implantation in the 12 patients, who underwent
125I particle implantation. The local control rate was
100%, and no patient succumbed to recurrence at the implantation
site (Fig. 2).
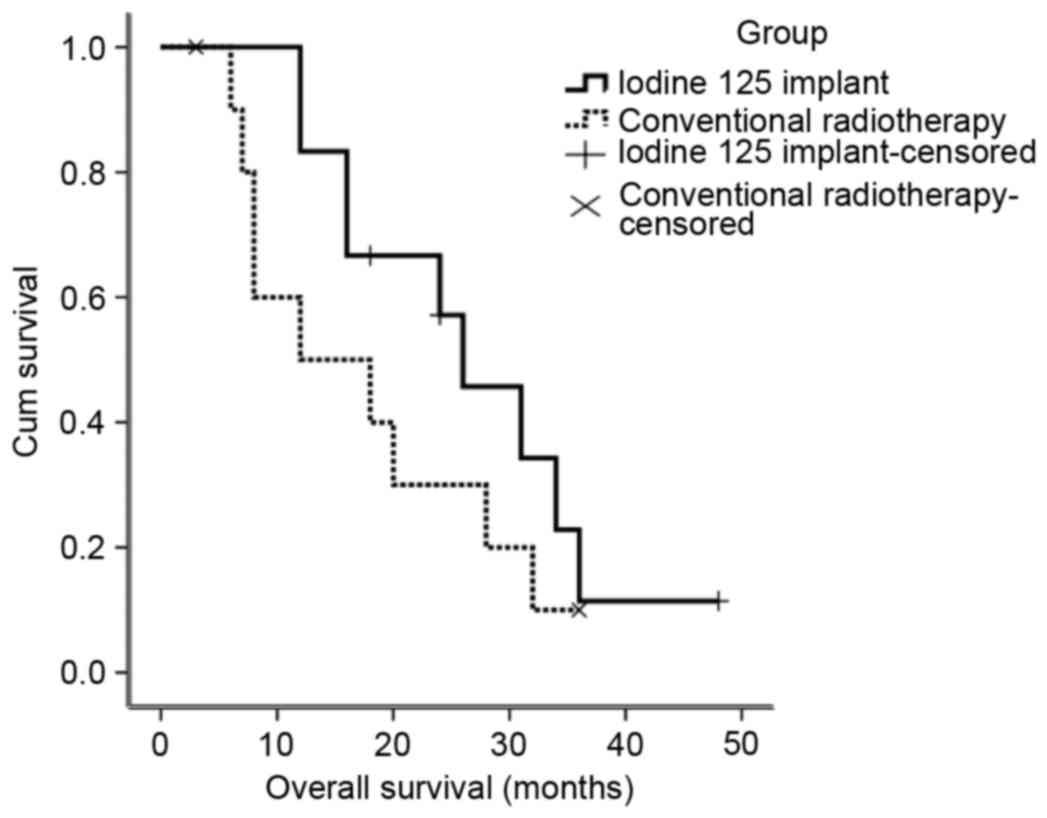
Of the 11 patients who underwent conventional
treatment, local recurrence was observed in 5 patients (Table II). The median survival time of the
patients in the conventional radiotherapy group was 12 months, and
the local recurrence rate was 45.4%. The survival rates of patients
in the conventional radiotherapy group and the radioactive seed
implant group are indicated in Fig.
3. Details of the complications of the treatments for the
patients in the two treatment groups are listed in Table III.
 | Table III.Comparison of the characteristics and
outcomes of the radioactive seed implant group and the conventional
radiotherapy group. |
Table III.
Comparison of the characteristics and
outcomes of the radioactive seed implant group and the conventional
radiotherapy group.
| Complications | Radioactive seed
implant group (n=12) | Conventional
radiotherapy group (n=11) | P-value |
|---|
| Bronchopleural
fistula | 0 (0) | 0 (0) | – |
| Great vessel
rupture | 0 (0) | 0 (0) | – |
| Radiation
pneumonitis | 0 (0) | 3 (27.3) | 0.093 |
| Dislocation of
125I particle | 1 (8.3) | 0 (0) | 0.522 |
| Bone marrow
suppression | 1 (8.3) | 3 (27.3) | 0.317 |
Details of an example case
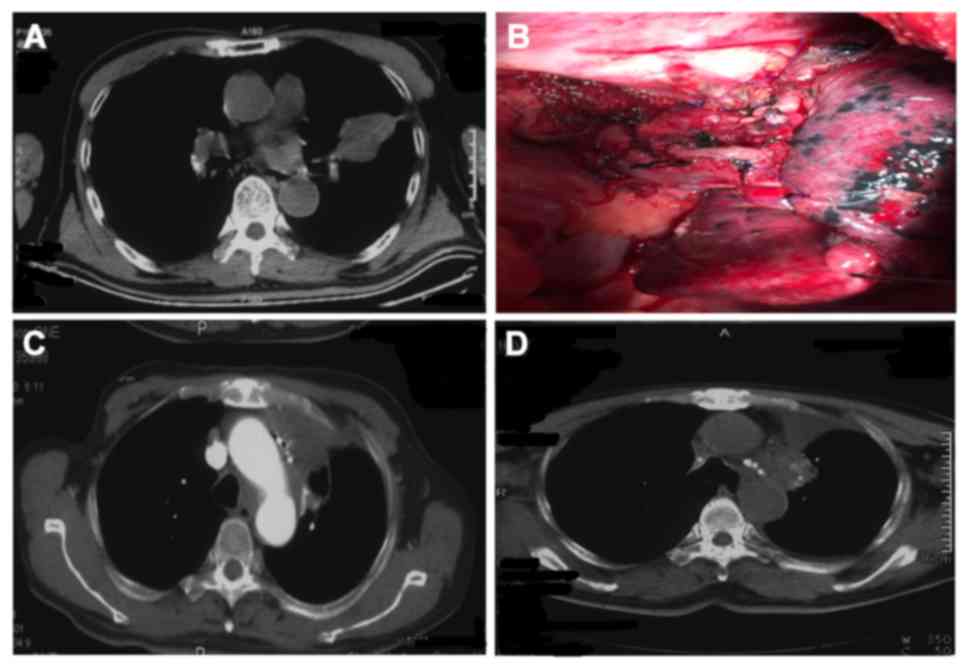
Case 1 (Fig. 4) was a
72-year-old male patient. Macroscopic residual disease was
observedin the left upper lobe. Intraoperative 125I
particle implantation was performed at the bronchial stump and the
peri-pulmonary artery. The patient underwent intraoperative
125I particle implantation for squamous carcinoma of the
left upper lobe, with residual diseasein the tissues adjacent to
the left main bronchus. The left upper lobe was resected in October
2010, and a total of 12 125I particles
(29.6MBq/particle) were implanted at the site of the residual
disease. No metastasis was identifiedat the implantation sites at
follow-up 2 weeks following treatment, and the local control rate
was satisfactory (Fig. 4).
Discussion
The incidence of macroscopic residual disease is ~4%
in NSCLC (3). Conventional
radiochemotherapy is generally performed following incomplete
resection of tumor tissue to prevent local recurrence and
metastasis. However, for limited residual disease in vital organs,
the benefit/risk ratio from external irradiation is relatively
low.
In the present study, 23 patients were included, of
whom 12 underwent 125I particle implantation. For the
patients who underwent 125I particle implantation, the
2-year local control rate was 100%, and the median survival time
was 24 months. Only one patient who underwent 125I
particle implantation exhibited recurrence near the implantation
region at 40 months following surgery, and the other 8 patients
were free from local recurrence after 2 years (the other 3 patients
succumbed <2 years). The local control ratein the groups who
underwent postoperative external irradiation group was 54.6%. The
local control rate in the 125I particle implantation
group was 100%, and no patient succumbed to recurrence at the
implantation site. The local control rate in the 125I
particle implantation group was significantly higher compared with
patients who underwent postoperative external irradiation,
irrespective of T- or N2- residual disease (P<0.05). In
addition, the 2-year survival rate was also higher in the
125I particle implantation group compared with the
postoperative external irradiation group, although this difference
was not statistically significant. Subgroup analysis revealed that
in patients with simple T-residual disease, the survival rate in
the 125I particle implantation group was markedly higher
compared with the postoperative external irradiation group, whereas
the incidence of distant metastasis was markedly lower. However,
for patients with N2-residual disease, the survival and metastasis
rates were not significantly different between the two groups. In
patients who underwent conventional treatment following
R2resection, the median survival time was 12 months and the local
control rate was 54.6% A meta-analysis of 9 studies revealed that
in the majority of patients who underwent incomplete resection
(R1+R2), radiochemotherapy resulted in a median survival time of
6.5–19.1 months (7), which was
consistent with the survival rate in the conventional treatment
group in the current study.
The T-residual sizes during R2 resection are
generally small. Therefore, intraoperative implantation with a
small number of 125I particles results in an effective
dose of 100–120 Gy to the target region (1). 125I particle implantation
provides sufficient radiation for the target regions, with minimal
toxicity to the surrounding tissues (1,8).
Therefore, intraoperative implantation results in relatively high
local control rates (4). In a
previous study performed by Heelan et al (9) surgical treatment and intraoperative
brachytherapy with 125I particles were used to treat patients with
stage-IIIa NSCLC cancer with mediastinal lymph node metastases. The
treatment resulted in an increase in the local control rate from 63
to 76%. In a study performed by Lee et al (10), where 33 patients with lung cancer who
were not candidates for lobectomy or pneumonectomy underwent
limited resection, 125I particles were implanted into
the tissues forinternal irradiation. The findings of the study
suggested that internal irradiation with 125I particles
reduces the recurrence rate in patients with lung cancer that are
undergoing limited resection (10). A
multicenter study revealed that, for selected NSCLC patients,
sub-lobar resection combined with 125I particle
implantation may result in similar local recurrence and survival
rates, compared with lobectomy (11).
Irradiation of the residual disease tissues with a low dose of
125I particles was effective, with a half-life of 4.5
years. For patients with low volume T-residuals 125I
particle implantation was comparable with R0 resection. In patients
with simple T4 disease contraindicated for extended radical
resection, 125I particle implantation during R2
resection for local control results in improved outcomes compared
with external irradiation. Control of the primary tumors by
125I particle implantation was able to reduce the risk
of distant metastases and increase the survival rate (12,13).
However, in patients with N2-residual disease, the
metastatic lymph nodes were generally enlarged and fixed, and
invasion of the pulmonary artery, superior vena cava and main
bronchus was also present. In addition, these patients were
generally diagnosed with advanced lung cancer, with the majority of
patients succumbing to distant metastases and even extensive
resection was associated with poor efficacy.
Therefore, effective control of the residual cancer
made no significant difference to the survival rate and distant
metastases when compared with patients without effective control.
For patients with N2-residual disease, local control was effective.
However, the extent of lymph node metastasis was generally wide.
Unfortunately, the external radiation dose cannot generally exceed
60 Gy due to the tolerance limits of normal lung tissue. However,
60 Gy is a dose that is not sufficient for tumor eradication
(14). The pulmonary volume of the
patients was reduced following the operation, resulting in poor
pulmonary function. The pulmonary V20 is generally 30% higher
compared with the normal pulmonary volume, with a high risk of
developing radiation pneumonitis (15).
Although increasing the dose of external irradiation
is difficult due to the dose-tolerance limits of the normal lung
tissue (4). 125I particle
implantation is able to reduce the total radiation and increase the
dosage in the irradiation region (1).
When combined with external irradiation, 125I particle
implantation may aid management of regions where N2 lymph node
metastases are present. Future studies should therefore focus on
methods, which can effectively control N-residual disease.
The safety profile of intraoperative 125I
particle implantation, including the occurrence of bronchial stump
fistula and major blood vessel rupture, and the radiation dose, are
the most pressing surgical concerns. The pathological changes
induced by NSCLC include shrinkage necrosis, while liquefactive
necrosis and perforation are very rare (16). Other studies have reported small areas
of fibrosis in the tissues around the 125I particles.
However, the effects on pulmonary function were relatively low, and
the safety profiles were higher for intraoperative 125I
particle implantation compared with external radiation (17,18).
Therefore, 125I particle implantation is a reliable
treatment method for lung cancer. Trombetta et al (19) investigated 29 NSCLC patients with
tumors adjacent to the aorta, and a 125I particle mesh
was used to cover the surface of the aorta for the treatment,
suggesting that the treatment was safe and effective (6). The effective tumor irradiation time was
four half-value period (59 days). An average of 10 particles were
implanted during the operation. The radiation dose for the surgeons
was <200 µSv each time. This dose was demonstrated to be safe
(20), and the cost for
intraoperative 125I particle implantation and
postoperative radiotherapy was $1,000 and $6,000, respectively, in
China. Additionally, for postoperative radiotherapy, one more month
hospital stay is required, which is a marked improvement on
postoperative radiotherapy.
In the present retrospective study, the results
revealed a higher overall survival rate in the 125I
particle treatment group compared with the conventional treatment
group within 2 years following surgery, while the difference after
2 years was not evident between the two treatment groups. The
incidence of local control in the 125I particle
treatment group was significantly higher compared with the
conventional group (P<0.05). Particularly for patients with
simple T-residual disease, the intraoperative implantation of
125I particles was able to significantly (P<0.05)
reduce the tumor recurrence and increase the survival rate compared
with conventional postoperative radiotherapy. Therefore,
intraoperative 125I particle implantation is a promising
treatment option for NSCLC patients contraindicated for extended
radical treatment.
The limitations of the current study relate to the
relatively small number of patients and short follow-up time. A
multi-center clinical trial with a larger sample size is required
to confirm these data prior to routine clinical application. The
findings of the present study revealed that, in lung cancer
patients undergoing R2 resection, 125I particle
implantation at the regions of residual disease was able to improve
patient outcomes. However, for patients with N-residual disease,
the survival rate was not significantly different from those that
have undergone conventional treatment.
Acknowledgements
The present study was supported by grants from the
Sichuan Science and Technology Agency Science-Technology Support
Project (grant no. ZCD162). The authors would like to thank
Professor Shude Chai (Department of Thoracic Surgery, Tianjin
Medical University, Tianjin, China) for technical assistance.
References
|
1
|
Li W, Dan G, Jiang J, Zheng Y, Zheng X and
Deng D: Repeated iodine-125 seed implantations combined with
external beam radiotherapy for the treatment of locally recurrent
or metastatic stage III/IV non-small cell lung cancer: A
retrospective study. Radiat Oncol. 11:1192016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
LoCicero JI: General Thoracic
SurgeryShields T, LoCicero JI, Reed C and Feins R: 7th edition.
Lippincott Williams & Wilkins; Philadelphia, PA: pp. 1387–1425.
2009
|
|
3
|
Foucault C, Mordant P, Grand B, Achour K,
Arame A, Dujon A, Le Pimpec Barthes F and Riquet M: Unexpected
extensions of non-small-cell lung cancer diagnosed during surgery:
Revisiting exploratory thoracotomies and incomplete resections.
Interact Cardiovasc Thorac Surg. 16:667–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Guan J, Yang L, Zheng X, Yu Y and
Jiang J: Iodine-125 brachytherapy improved overall survival of
patients with inoperable stage III/IV non-small cell lung cancer
versus the conventional radiotherapy. Med Oncol. 32:3952015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsim S, O'Dowd CA, Milroy R and Davidson
S: Staging of non-small cell lung cancer (NSCLC): a review. Respir
Med. 104:1767–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson M, Colonias A, Parda D, Trombetta
M, Gayou O, Reitz B and Miften M: Dosimetric and technical aspects
of intraoperative I-125 brachytherapy for stage I non-small cell
lung cancer. Phys Med Biol. 52:1237–1245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dall K, Ford C, Fisher R and Dunning J: Is
there a survival advantage of incomplete resection of
non-small-cell lung cancer that is found to be unresectable at
thoracotomy? Interact Cardiovasc Thorac Surg. 16:529–532. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aye RW, Mate TP, Anderson HN, Johnson LP
and Hill L: Extending the limits of lung cancer resection. Am J
Surg. 165:572–576. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heelan RT, Hilaris BS, Anderson LL, Nori
D, Martini N, Watson RC, Caravelli JF and Linares LA: Lung tumors:
Percutaneousimplantation of I-125 sources with CT treatment
planning. Radiology. 164:735–740. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee W, Daly BD, DiPetrillo TA, Morelli DM,
Neuschatz AC, Morr J and Rivard MJ: Limited resection for non-small
cell lung cancer: Observed local control with implantation of I-125
brachytherapy seeds. Ann Thorac Surg. 75:237–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birdas TJ, Koehler RP, Colonias A,
Trombetta M, Maley RH Jr, Landreneau RJ and Keenan RJ: Sublobar
resection with brachytherapy versus lobectomy for stage Ib nonsmall
cell lung cancer. Ann Thorac Surg. 81:434–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cahlon O, Zelefsky MJ, Shippy A, Chan H,
Fuks Z, Yamada Y, Hunt M, Greenstein S and Amols H: Ultra-high dose
(86.4 Gy) IMRT for localized prostate cancer: toxicity and
biochemical outcomes. Int J Radiat Oncol Biol Phys. 71:330–337.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zelefsky MJ, Yamada Y, Fuks Z, Zhang Z,
Hunt M, Cahlon O, Park J and Shippy A: Long-term results of
conformal radiotherapy for prostate cancer: Impact of dose
escalation on biochemical tumor control and distant metastases-free
survival outcomes. Int J Radiat Oncol Biol Phys. 71:1028–1033.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Langer M, Kijewski P, Brown R and Ha C:
The effect on minimum tumor dose of restricting target-dose
inhomogeneity in optimized three-dimensional treatment of lung
cancer. Radiother Oncol. 21:245–256. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim TH, Cho KH, Pyo HR, Lee JS, Zo JI, Lee
DH, Lee JM, Kim HY, Hwangbo B, Park SY, et al: Dose-volumetric
parameters for predicting severe radiation pneumo-nitis after
three-dimensional conformal radiation therapy for lung cancer.
Radiology. 235:208–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Wang J, Liao A, Zhuang H and Zhao
Y: The direct biologic effects of radioactive 125I seeds on
pancreatic cancer cells PANC-1, at continuous low-dose rates.
Cancer Biother Radiopharm. 24:409–416. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnson M, Colonias A, Parda D, Trombetta
M, Gayou O, Reitz B and Miften M: Dosimetric and technical aspects
of intraoperative I-125 brachytherapy for stage I non-small cell
lung cancer. Phys Med Biol. 52:1237–1245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H, Bao Y, Yu L, Jia R, Cheng W and
Shao C: Comparison of cellular damage response to low-dose-rate
125I seed irradiation and high-dose-rate gamma irradiation in human
lung cancer cells. Brachytherapy. 11:149–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trombetta MG, Colonias A, Makishi D,
Keenan R, Werts ED, Landreneau R and Parda DS: Tolerance of the
aorta using intraoperative iodine-125 interstitial brachytherapy in
cancer of the lung. Brachytherapy. 7:50–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Anglesio S, Calamia E, Fiandra C, Giglioli
FR, Ragona R, Ricardi U and Ropolo R: Prostate brachytherapy with
iodine-125 seeds: Radiation protection issues. Tumori. 91:335–338.
2005.PubMed/NCBI
|