Introduction
Salivary gland cancer is rare, with an overall
incidence of between 2.5 and 3.0 cases per 100,000 individuals
globally, accounting for <3.0–5.0% of all head and neck cancer
(1). Almost 80% of salivary gland
tumor occurs in the parotid gland, which are challenging to
differentially diagnose (2).
Mucoepidermoid carcinoma is the most common type of parotid gland
cancer, which is associated with favorable prognosis, with a 5-year
overall survival of 79% depending on clinical stage and grade
(3). Other pathological types of
salivary gland cancer include adenoid cystic carcinoma, acinic cell
carcinoma and salivary ductal carcinoma (4). Surgery coupled with potential use of
adjuvant radiation therapy and chemotherapy are performed for the
treatment of salivary gland malignancies (5). The clinical manifestations of parotid
tumors are variable and their pathological features are complex.
The selection of the optimal surgical procedure presents its own
challenges due to difficulties differentiating between malignant
and benign tumors through general clinical examination (6). A lack of pathological examination prior
to or during surgery, or misdiagnosis by preoperative punch biopsy
or intraoperative frozen section, may lead to the selection of an
inappropriate surgical procedure (7,8).
Reoperation, with caution, is required in cases where a diagnosis
of malignant parotid tumor is confirmed subsequent to surgery by
pathological examination (9).
The present study reviewed the cases of 30 patients
with malignant parotid tumor who underwent reoperation subsequent
to the use of a non-standardized procedure. The study aimed to
systematically evaluate the use of reoperation, explore the
preoperative diagnosis, selection of primary procedure and
necessity of reoperation subsequent to a non-standardized primary
procedure, and provide a reference for the clinical diagnosis and
treatment of patients with malignant parotid tumors.
Patients and methods
Ethical statement
The Ethics Committee of the Tumor Hospital of
Ganzhou Review Board (Ganzhou, China) approved the present study
protocol (no. 20080203). The present study was conducted in
accordance with the Declaration of Helsinki with regard to research
involving human subjects, and all patients provided written
informed consent to participate following explanation of the nature
of the study.
Inclusion criteria
The inclusion criteria were as follows: i) The
patient had a malignant parotid tumor and was recommended by their
primary surgeon for transfer to a higher tier hospital for
reoperation; ii) the patient underwent a primary surgery comprising
partial tumor resection, tumor enucleation and partial superficial
lobe parotidectomy; iii) the patient underwent physical examination
and enhanced computed tomography (CT) scans that revealed residual
tumor, or prompted suspicion of residual tumor, at the primary
tumor site or showed cervical lymph node enlargement; iv) the
patient was aged between 18 and 70 years; v) the patient had a
Karnofsky performance score of >80 (10); vi) the expected survival time of the
patient was >1 year; and vii) the patient voluntarily signed
informed consent forms.
Exclusion criteria
The exclusion criteria were as follows: i) >3
months had elapsed since the primary surgery; ii) the patient had
any other malignant tumors of the head and neck; iii) the patient
had a history of head and neck radiotherapy; and iv) the patient
had severe heart, lung, liver or kidney disease.
Clinical data
Between January 2008 and December 2014, 30
consecutive patients [17 male and 13 female; median age, 47 (range,
18–69) years] with malignant parotid tumors, who underwent
reoperation at the Tumor Hospital of Ganzhou and who met the
inclusion criteria, were included in the present study. Patients
had undergone either 1 or 2 prior procedures at other hospitals,
with the time elapsed between the primary surgery and the
reoperation ranging from 7 to 83 days (Table I).
 | Table I.General clinical data of the patients
(n=30). |
Table I.
General clinical data of the patients
(n=30).
| Parameter | Number of patients,
n (%) |
|---|
| Age, years |
|
|
18–39 | 9 (30.0) |
|
40–49 | 7 (23.3) |
|
50–59 | 8 (26.7) |
|
60–70 | 6 (20.0) |
| Sex |
|
|
Male | 17 (56.7) |
|
Female | 13 (43.3) |
| Karnofsky
performance score |
|
|
80–89 | 9 (30.0) |
|
≥90 | 21 (70.0) |
| Pathological
type |
|
|
Mucoepidermoid carcinoma | 12 (40.0) |
| Adenoid
cystic carcinoma | 7 (23.3) |
| Acinic
cell carcinoma | 4 (13.3) |
|
Nonspecific poorly
differentiated adenocarcinoma | 1 (3.3) |
|
Salivary duct carcinoma | 1 (3.3) |
|
Malignancy of pleomorphic
adenoma | 1 (3.3) |
|
Lymphoepithelial
carcinoma | 1 (3.3) |
|
Epithelial-myoepithelial
carcinoma | 1 (3.3) |
|
Carcinoma ex pleomorphic
adenoma | 1 (3.3) |
| Basal
cell carcinoma | 1 (3.3) |
| Previous
procedure |
|
| Partial
tumor resection | 5 (16.7) |
| Tumor
enucleation | 11 (36.7) |
| Partial
superficial lobe parotidectomy | 13 (43.3) |
|
Selective deep lobe
parotidectomy | 1 (3.3) |
| Previous surgical
complications |
|
| Facial
nerve branch injury | 2 (6.7) |
| Parotid
gland leakage | 2 (6.7) |
| Number of
procedures performed in other hospitals |
|
| 1 | 26 (86.7) |
| 2 | 4 (13.3) |
Reoperation procedures
All procedures were performed by two experienced,
qualified surgeons. Total parotidectomy was performed on all
patients for management of the primary tumor. With regard to lymph
node management, prophylactic neck dissection was not required for
patients with low-grade malignant parotid tumors of preoperative
cN0 stage if no metastasis-positive level II lymph nodes were
observed intraoperatively; while selective neck dissection of
levels I–III or I–IV was required if lymph nodes were revealed to
be positive for metastasis by intraoperative pathological
examination of frozen sections (11,12):
Freezing for 2 min at between −15 and −20°C and prepared to a
section thickness of 4.0–5.0 µm on a freezing microtome (Leica
CM1950; Leica Microsystems GmbH, Wetzlar, Germany). Tissue was
fixed with AF fixation fluid (75 ml 95% ethanol with 10 ml 40%
formaldehyde) for 30 sec at room temperature. Sections were stained
with hematoxylin-eosin for 15 min at 60°C and evaluated under
magnification, ×40 (Olympus BX-53; Olympus Corporation, Tokyo,
Japan). Selective or functional neck dissection was performed in
patients with undifferentiated carcinoma, poorly differentiated
mucoepidermoid carcinoma, squamous cell carcinoma, adenocarcinoma
or cystadenocarcinoma at cN0 stage. For patients identified to have
cervical lymph node enlargement preoperatively by physical
examination or enhanced CT scan, a functional or radical neck
dissection of the level I–IV nodes was performed according to lymph
node status, as described above. For patients with tumor invasion
of the skin in the parotid region, flap repair surgery was
performed according to the individual skin defect conditions
(13–15). All of the reoperation specimens were
confirmed by conventional pathological paraffin sections and
immunohistochemistry examination.
Facial nerve procedures
En bloc resection of the tumor with the facial nerve
was performed if facial paralysis was present prior to surgery. If
the tumor was attached to the nerve, the nerve was preserved if
separation was possible, postoperative adjuvant radiotherapy (60 Gy
in 30 fractions for 6 weeks) was administered. The facial nerve was
excised in the event that separation from the tumor mass was
difficult, or if the facial nerve was confirmed to pass through the
tumor (16–18). Following partial or complete excision
of the facial nerve, facial nerve reconstruction was performed if
possible (19,20). The House-Brackmann facial nerve
grading system was used to assess facial nerve function following
its reconstruction (21).
Radiation therapy
All patients who underwent reoperation required
supplementary postoperative radiation therapy (22). Conventional radiotherapy or
intensity-modulated radiation therapy were chosen according to the
patient's own economic conditions following reoperation.
Conventional radiation therapy was administered as follows
(23–25): The anterior boundary of the parotid
region was defined as the anterior edge of the masseter muscle, the
posterior boundary was defined as the posterior edge of the mastoid
process, the superior boundary was defined as the upper edge of the
zygomatic arch and the inferior boundary was defined at the level
1.0 cm beneath the mandible. Neck lymphatic drainage area radiation
was complementarily performed in patients confirmed with poorly
differentiated tumors, late-stage tumors or neck lymph node
metastases as previously described (26). The dosage of conventional
external-beam radiation therapy was 60 Gy in 30 fractions for 6
weeks following reoperation; the maximal local dose was increased
to 66–70 Gy in patients with positive surgical margins, and a dose
of ≥66 Gy was administered to patients with adenoid cystic
carcinoma.
Intensity-modulated radiation therapy was
administered as follows (27,28): The primary tumor site was defined as
the gross tumor volume (GTV) of the tumor bed (GTVtb); the residual
tumor was defined as the GTV; the subclinical stage and high-risk
lymphatic drainage region was defined as clinical target volume
(CTV) 1; and the prophylactic irradiation region of lymphatic
drainage was defined as CTV2. The doses of the intensity-modulated
radiation therapy following reoperation were as follows: 66–70 GY
in 30–33 fractions (6–6.5 weeks) for GTV, 60–66 GY in 30–33
fractions (6–6.5 weeks) for GTVtb, 56–60 GY in 30–33 fractions
(6–6.5 weeks) for CTV1, and 54 GY in 30–33 fractions (6–6.5 weeks)
for CTV2.
Observation indexes
The surgical wound healing process and facial nerve
function recovery were monitored following surgery according to
outpatient investigations, and any complications were noted.
Patients were also monitored for recurrences at the primary tumor
site and neck lymph nodes. Patients were followed up at an interval
of every 3 months for the first 2 years following treatment, every
6 months between years 2 and 5, and every 12 months thereafter.
Follow-ups were performed from the day immediately following the
completion of radiotherapy until December 31, 2015. The total
follow-up durations ranged between 12 and 87 months.
Statistical analysis
All data analyses were performed using SPSS (version
22.0; IBM Corp., Armonk, NY, USA). The Kaplan-Meier estimator
method was used for survival analysis.
Results
Patient treatments
A total of 30 patients underwent reoperation, and
the incidence of residual tumor was 63.3% (19/30), as confirmed by
conventional pathological paraffin sections and
immunohistochemistry examination. The intact facial nerve
preservation rate was 83.3% (25/30); 1 patient with complete facial
nerve excision did not undergo a facial nerve graft procedure as
complete tumor excision was considered to be the immediate
priority, 1 patient with buccal branch excision did not undergo
repair surgery for it did not affect important facial function, and
the remaining 3 patients with facial nerve branch excision or
partial excision underwent facial nerve reconstruction utilizing
the great auricular nerve. Facial nerve function was recovered to
House-Brackmann (27) grade II in 1
patient and grade III in 2 patients after a 3-month follow-up. A
single patient experienced surgical field hemorrhage following
surgery and underwent secondary debridement; 1 patient experienced
local flap necrosis and an opened incision, and underwent secondary
surgery with pectoralis major muscle flap transposition repair; and
2 patients experienced temporary facial paralysis and recovered
within 3 months through nutritional support and acupuncture
(Table II).
 | Table II.Procedures undergone and
complications experienced by the patients as part of reoperation
(n=30). |
Table II.
Procedures undergone and
complications experienced by the patients as part of reoperation
(n=30).
|
Procedure/complication type | Number of patients,
n (%) |
|---|
| Primary tumor |
|
| Total
parotidectomy | 30 (100.0) |
| Cervical lymph
nodes |
|
|
Selective neck dissection | 10 (33.3) |
|
Functional neck
dissection | 6 (20.0) |
| Radical
neck dissection | 4 (13.3) |
| Repair of skin
defects |
|
|
Adjacent flap for
transposition repair | 3 (10.0) |
|
Pectoralis major muscle flap
for transposition repair | 3 (10.0) |
| Facial nerve |
|
|
Resection of branch of the
facial nerve | 2 (6.7) |
| Partial
resection of the facial nerve | 2 (6.7) |
| Facial
nerve resection | 1 (3.3) |
| Repair
and reconstruction of great auricular nerve | 3 (10.0) |
| Complications |
|
|
Postoperative secondary
bleeding | 1 (3.3) |
| Partial
necrosis of adjacent flap opened incision | 1 (3.3) |
|
Temporary facial
paralysis | 2 (6.7) |
Follow-up
All 30 patients were followed-up. The follow-up rate
was 100%, with durations varying from 12 to 87 months. A total of 3
patients experienced local recurrence and 5 mortalities were
reported, of which 2 patients with adenoid cystic carcinoma
succumbed to lung metastasis, 1 patient with atypical poorly
differentiated adenocarcinoma succumbed to brain metastases, and 2
patients with poorly differentiated mucoepidermoid carcinoma
succumbed to local recurrence. As analyzed using Kaplan-Meier
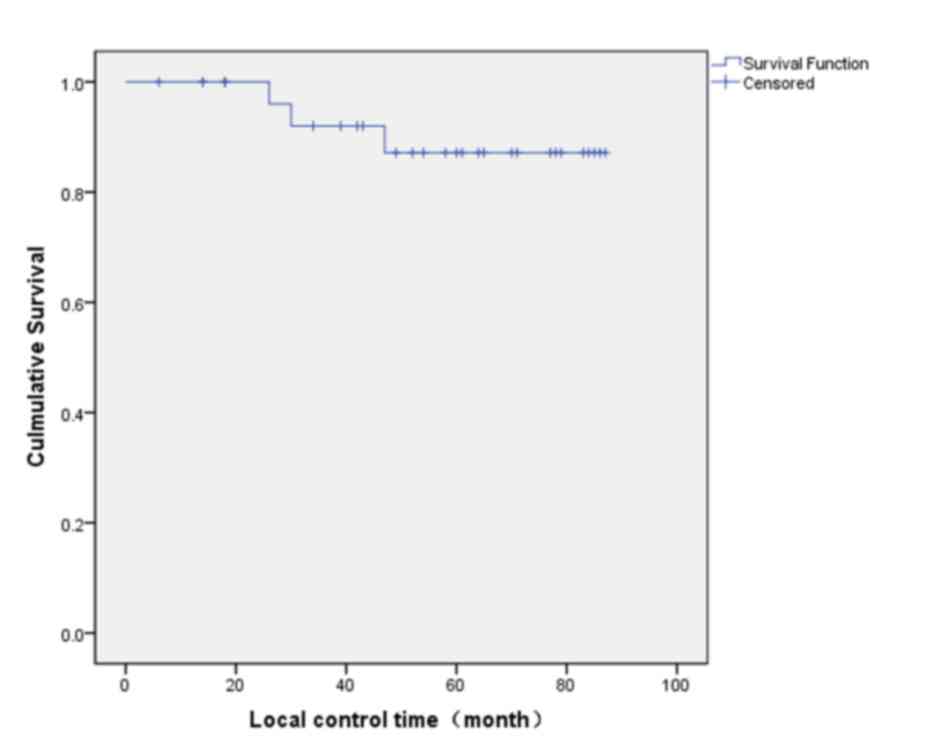
estimator curves, the 5-year local control rate was 87.2% (Fig. 1), the 5-year recurrence-free survival
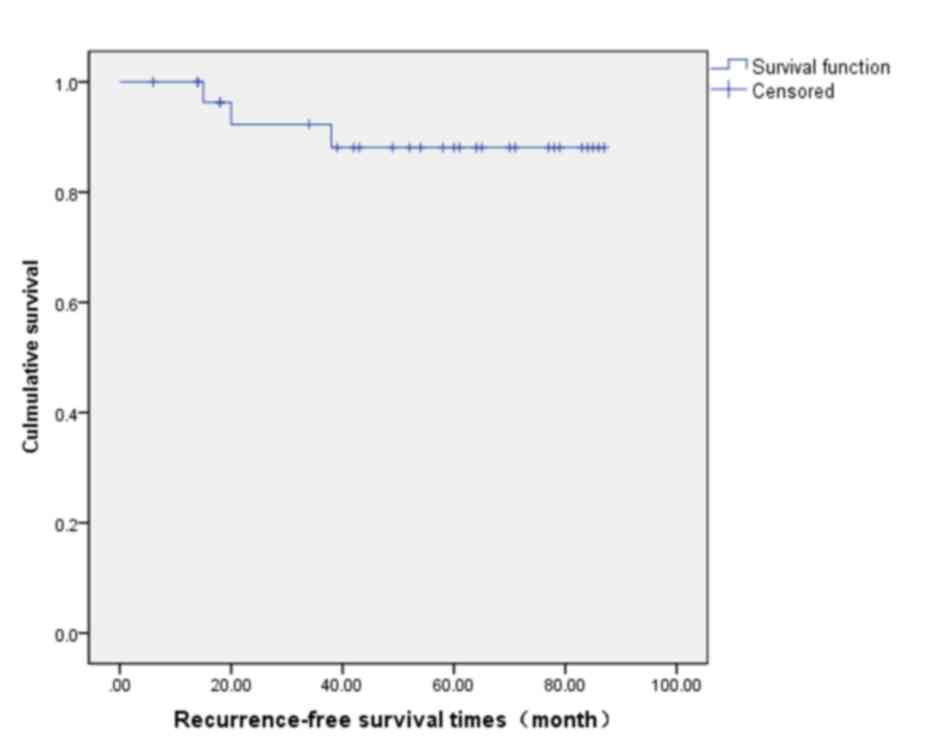
rate was 88.1% (Fig. 2) and the
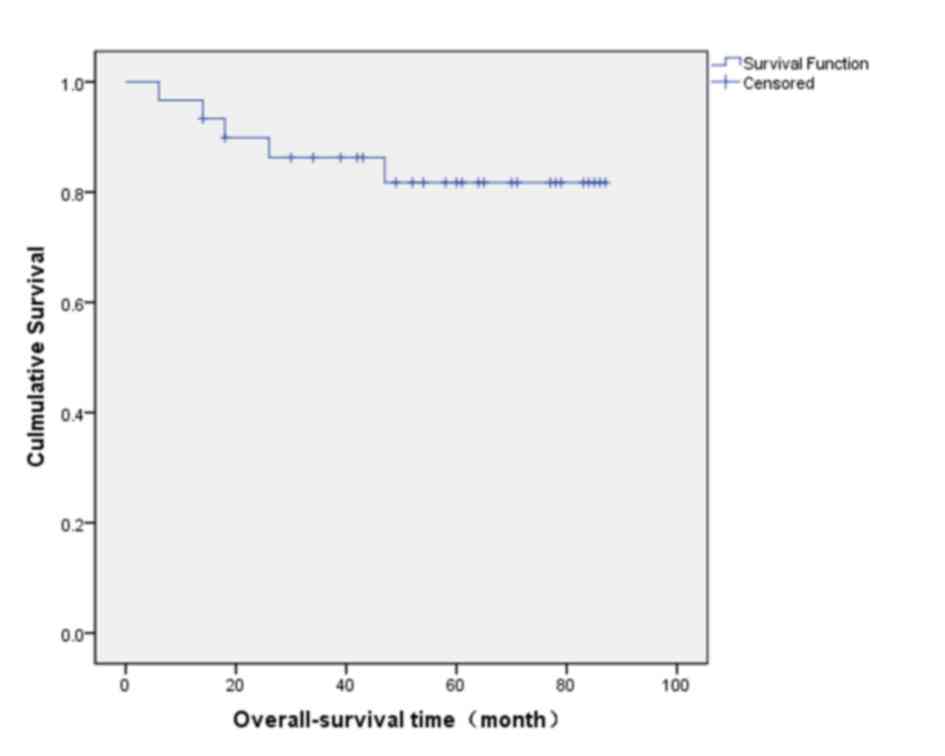
5-year overall survival rate was 81.7% (Fig. 3).
Typical case report
A 61-year-old male noticed a facial tumor below his
right ear in March 2000. The tumor size was 2.5×2.0 cm. No other
symptoms of discomfort were reported. Enlargement of the tumor
under his right ear was noted, along with pruritus, in June 2010.
Partial tumor excision was performed in Longkou Town Health Center
(Ganzhou, China); however, the procedure details and postoperative
pathological diagnosis were unknown. The residual tumor under the
patient's right ear was determined to be progressively growing in
May 2011. A reoperation was performed in the same health center in
January 2013, partially excising the tumor. Following surgery, the
surgical incision opened, with ulceration, pus, local redness,
swelling and pain. The patient was admitted to the Tumor Hospital
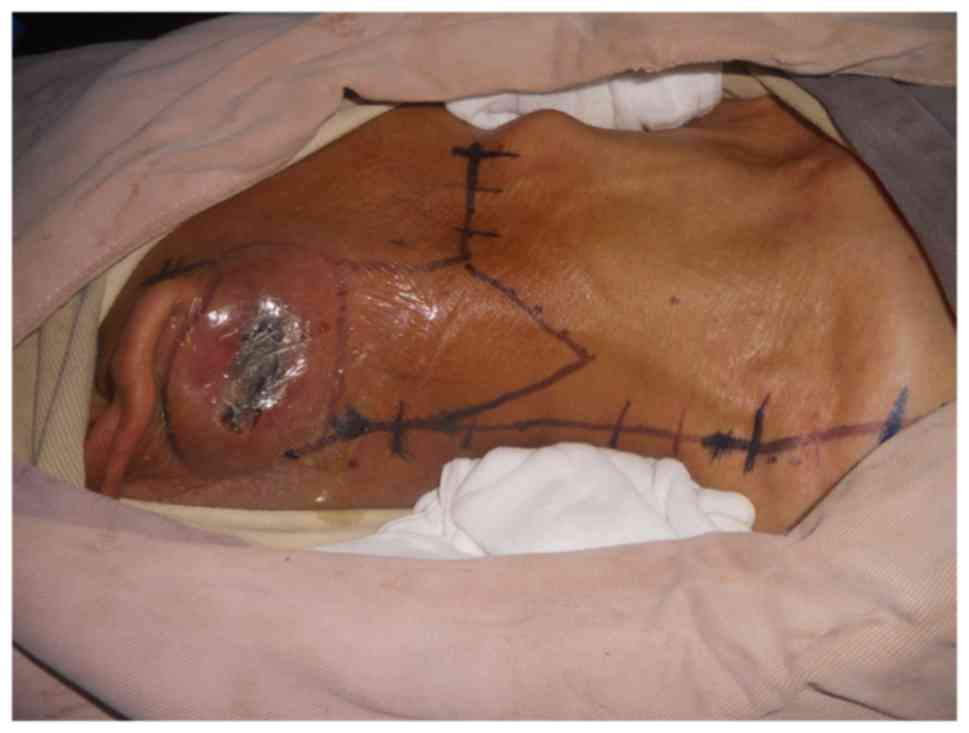
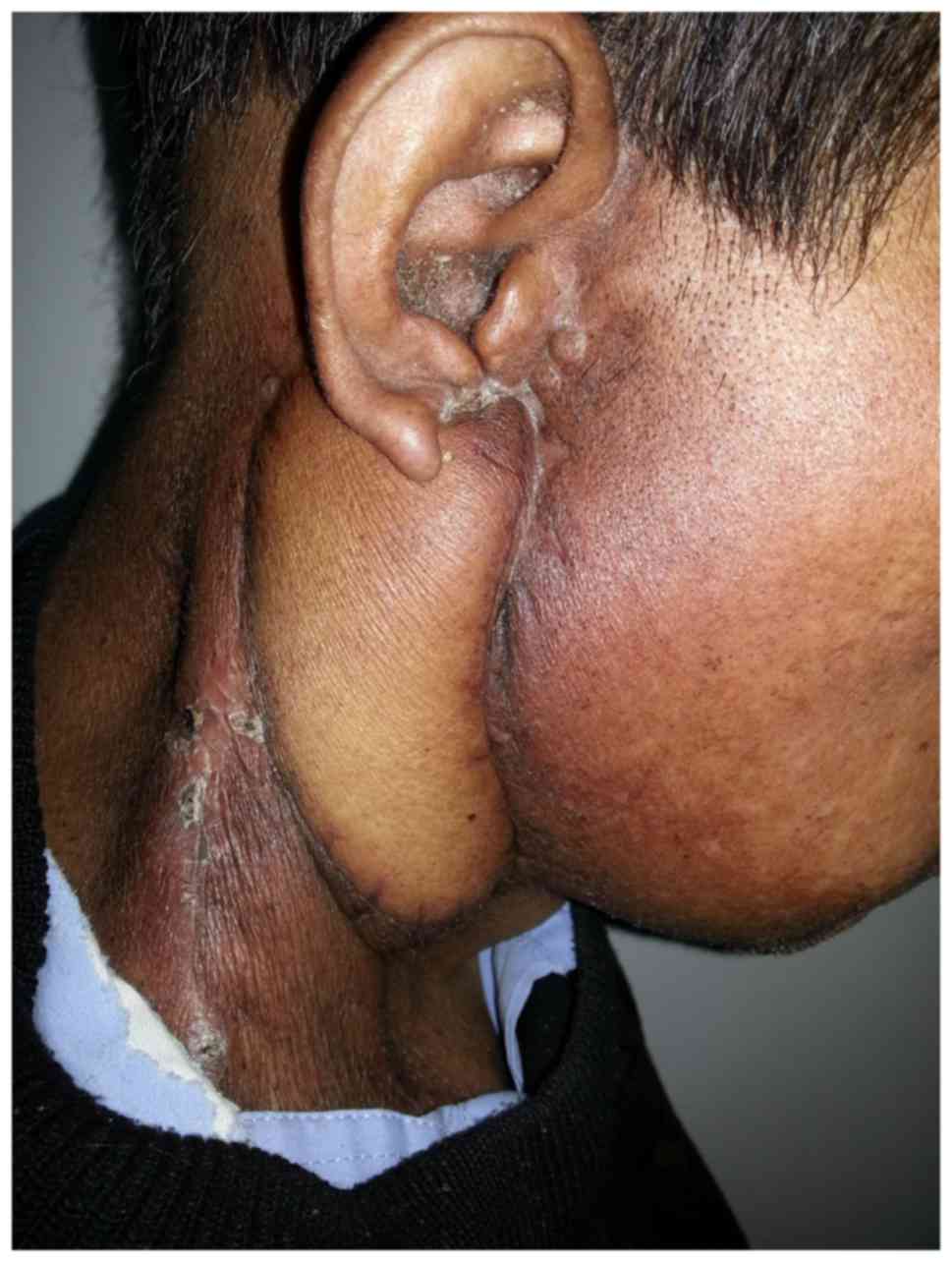
of Ganzhou in February 2013. On physical examination, a tumor
measuring 7.0×5.0 cm was observed inferior to his right ear with a
5-cm-long oblique wound visible on the top of the tumor. Purulent
discharge was noted in the wound, old scar formation was observed
at the edge of the wound, and the tumor was firm, non-tender,
ill-defined and fixed (Fig. 4).
Multiple swollen lymph nodes, which ranged in diameter from 0.8 to
1 cm and were firm, non-tender and movable, were palpable in the
right neck and supraclavicular fossa. A CT scan revealed a tumor
with multiple swollen lymph nodes in the right parotid region,
which indicated a diagnosis of parotid cancer. The chest radiograph
revealed the presence of chronic bronchitis and emphysema, and
pulmonary function tests identified severe mixed pulmonary
ventilation dysfunction. Staphylococcus aureus was
identified in the bacterial secretion culture, and was sensitive to
cefotaxime, ceftriaxone, ampicillin and levofloxacin. On February
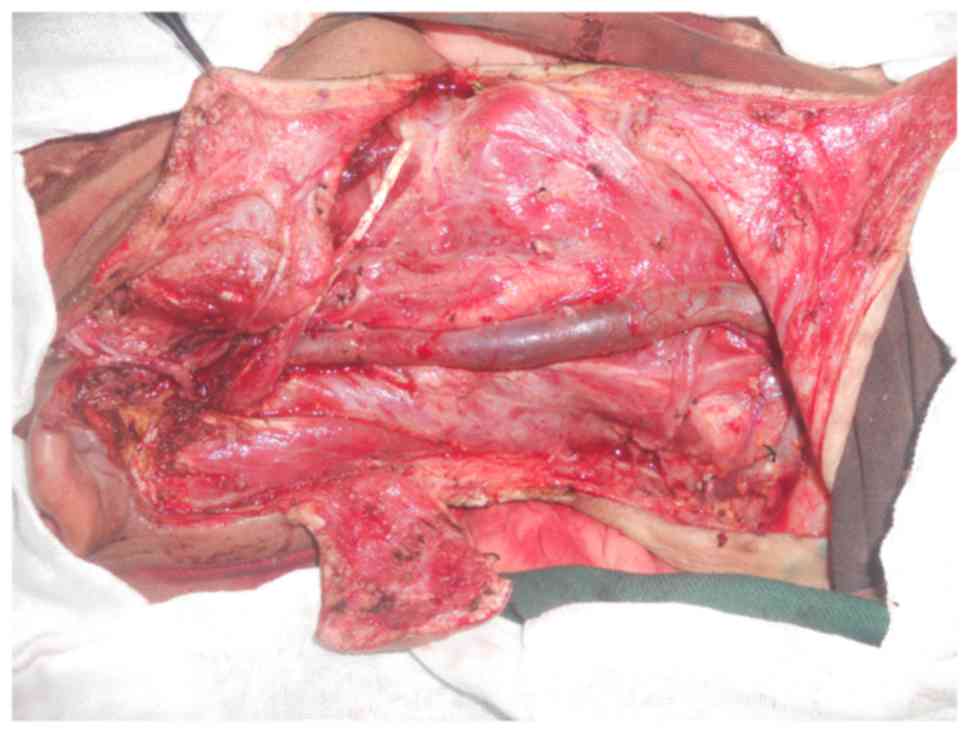
26th, 2013, right total parotidectomy, facial nerve buccal branch
resection, right modified radical neck dissection and local flap
transposition repair were performed under general anesthesia
(Figs. 5–7). The postoperative hispathological
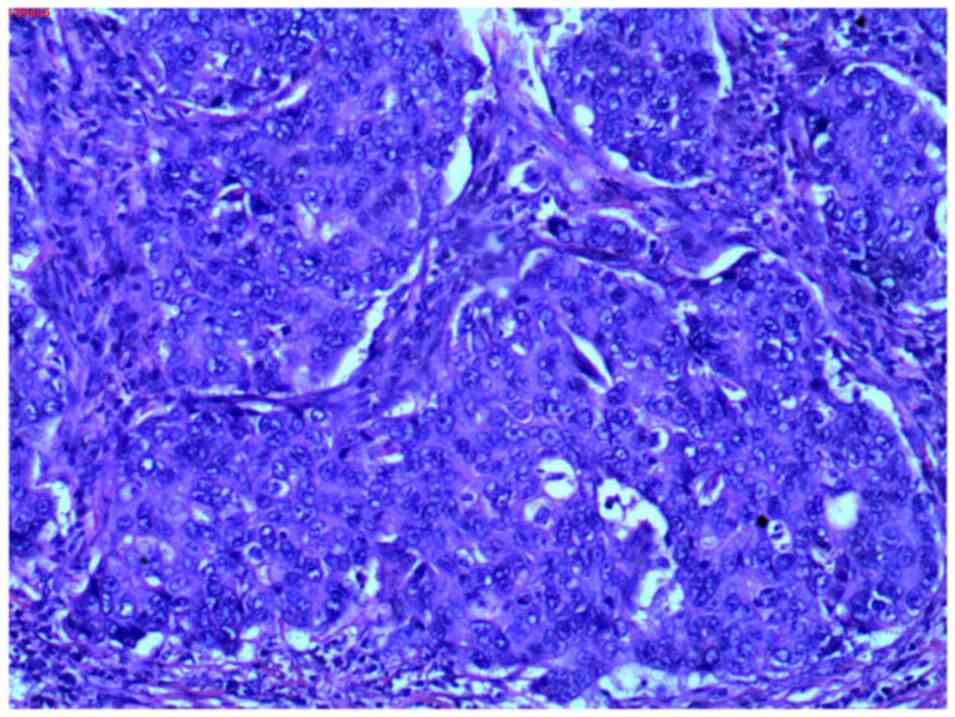
examination (pathological report no. 130435) revealed parotid
ductal carcinoma (Fig. 8) and
cervical lymph node metastases (93/95). Carcinoma cells were
observed with an increased volume compared with adjacent
noncarcinoma cells, with round-to-oval-shaped nuclei, coarse
chromatin and distinct nucleoli, abundant cytoplasm and sieve-like
or solid-nested structures were present. In addition, malignant
cells also partially invaded the surrounding tissue, including
muscle, nerve, neovascularization and the dermis. Partial necrosis
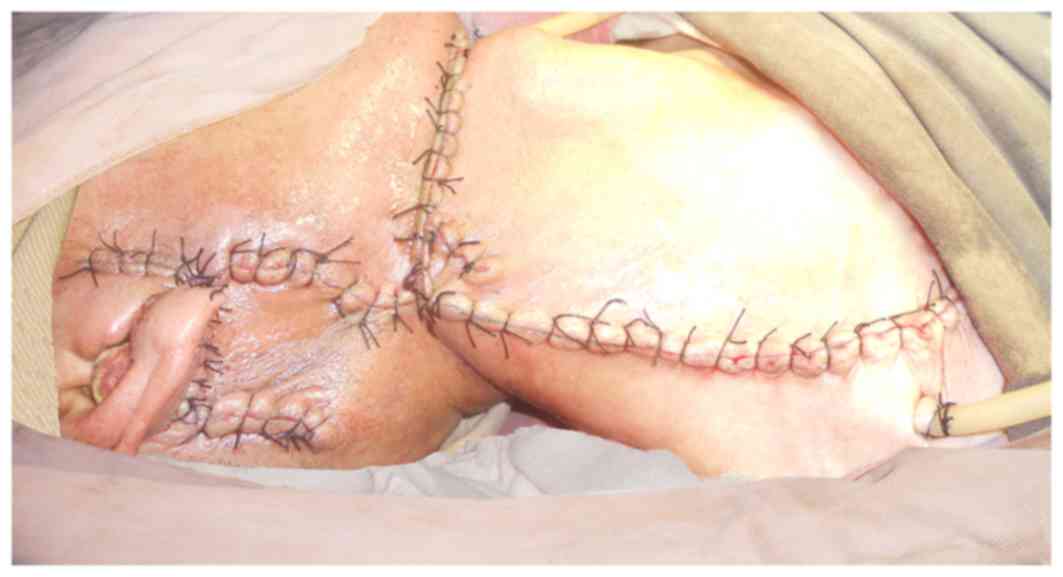
of the trans-positioned flap was noted on March 3rd, 2013. The
surgical incision was partially opened and vessels were exposed on
March 5th, and pectoralis major muscle flap transposition repair
was performed under general anesthesia on March 6th, 2013.
Conventional radiotherapy was initiated on March 25th, 2013. The
radiation dose for the primary tumor site was 60 Gy in 30 fractions
for 6 weeks and the dose for the neck lymphatic drainage region was
60 Gy in 30 fractions for 6 weeks. Follow-up was continued until
December 2015, where it was observed that the repair flap was
maintaining well (Fig. 9), and no
recurrence or metastasis of the tumor were reported during this
time.
Discussion
Malignant parotid tumors are the most common type of
malignant salivary gland tumor, with the highest incidence rates
(29). It is difficult to confirm the
diagnosis of malignant parotid tumor prior to surgery. Malignant
tumors are typically irregularly shaped, firm, ill-defined and
poorly movable, and show fast growth; however, for certain
painless, slow-growing tumors of the parotid gland, the possibility
of malignancy cannot be easily ruled out (30,31). In
previous studies, the accuracy of fine-needle aspiration biopsy in
the diagnosis of parotid gland tumor prior to surgery ranged from
91 to 98% (32–35). There were some misdiagnoses in
pathological examination among the preoperative fine-needle
aspiration biopsy specimens, intraoperative frozen sections, and
paraffin-embedded sections (31,32,36,37).
Final diagnosis of the tumor must be confirmed by routine
pathological evaluation.
It is difficult to select the most suitable
procedure for the treatment of a malignant parotid tumor due to
difficulties in preoperative diagnosis and the complexity of
intraoperative pathological evaluation of frozen specimens
(38). Consequently, partial
superficial lobe parotidectomy may be initially performed for
superficial lobe parotid tumors that lack accurate preoperative
diagnosis; and complete superficial lobe parotidectomy may be
performed in the event of suspicion of malignancy in an
intraoperative frozen section. The intraoperative exploration of
level II lymph nodes is required for parotid tumors suspected of
being malignant based pathological examination of frozen sections.
Prophylactic neck dissection is unnecessary if no positive lymph
nodes are observed, while repeated frozen sectioning is required if
lymph nodes are revealed during surgery to be involved. These
management approaches may assist in avoiding reoperation for
parotid tumors confirmed to be malignant by pathological
examination of paraffin-embedded sections postoperatively (39–41).
Total parotidectomy may be performed at the time of
reoperation, and the decision on whether to perform neck dissection
depends on the pathological findings and extent of lymph node
metastasis (42). Local adjacent flap
transposition repair may be performed if there is a small area of
skin invasion by the malignant parotid tumor or a low-grade tumor,
and if the patient refuses free-flap or pedicle-flap repair. The
submental island flap may be selected in patients without removal
of sternocleidomastoid muscle in the setting of a large area of
skin invasion by the tumor or a high-grade tumor (43). If neck dissection plus
sternocleidomastoid excision are performed, the pectoralis major
flap may be the first-line selection for skin defect repair since
necrosis of the free flap, adjacent flap or submental island flap
may lead to vascular exposure, increasing the risk of surgery and
negatively impacting postoperative radiotherapy. The pectoralis
major muscle flap comprises a large bulk of tissue that may
completely repair neck tissue defects following neck dissection and
cover skin defects of the parotid region (44). The pectoralis major muscle flap also
has a rich blood supply, which leads to strong resistance to
infection and necrosis, as well as allowing for fast healing; its
application may prevent neck vascular exposure, which has been
observed following necrosis of other types of flap, and will
therefore not delay radiotherapy following surgery (14,15). In
addition, the use of the pectoralis major muscle flap allows for
more natural wound appearance following surgery, and the cosmetic
appearance is improved compared to those using the submental island
flap (45). In the present study, 1
patient undergoing adjacent flap repair experienced flap necrosis,
an opened incision site and vascular exposure, and was treated with
secondary pectoralis major muscle flap transposition repair.
It is difficult to confirm the diagnosis of
malignant parotid tumor prior to or during surgery. High incidence
rate of residual tumor is associated with non-standardized
procedures compared with standardized procedures (46,47). The
present study demonstrated a residual tumor rate of 63.3% (19/30)
for patients who underwent non-standardized procedures, which was
confirmed by pathological examination following reoperation. This
suggests that reoperation is required in patients with malignant
parotid tumor who have undergone non-standardized procedures. The
Kaplan-Meier method revealed that the 5-year local control rate was
87.2%, the 5-year recurrence-free survival rate was 88.1%, and the
5-year survival rate was 81.7% following reoperation. These results
were similar to those of patients undergoing primary surgery
(48). However, there are certain
limitations to the present study. The present study was a
single-center study, the sample size was small, the definitive T
stage was missing, the follow-up durations were short in certain
patients, and there may be a certain bias in the local control
rate, recurrence-free survival rate and overall survival rate due
to the sample size. Multi-center studies with large sample sizes
are required to support the results.
With the development of head and neck functional
surgery, parotid tumor surgery has evolved from the initial tumor
enucleation to superficial lobe parotidectomy or total
parotidectomy with facial nerve preservation, and further to the
current recommended method of partial parotidectomy for benign
parotid tumors (49,50). In addition, with extensive application
of frozen section pathological examination and the improvement of
diagnostic techniques, non-standardized procedures may decrease
under strict compliance with recommended surgical protocols.
Acknowledgements
The authors would like to thank the Health and
Family Planning Commission of Jiangxi for supporting the present
study (grant no. 20133218).
References
|
1
|
Namboodiripad PC: A review: Immunological
markers for malignant salivary gland tumors. J Oral Biol Craniofac
Res. 4:127–134. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stryjewska-Makuch G, Kolebacz B, Janik MA
and Wolnik A: Increase in the incidence of parotid gland tumors in
the years 2005–2014. Otolaryngol Pol. 71:29–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McHugh CH, Roberts DB, El-Naggar AK, Hanna
EY, Garden AS, Kies MS, Weber RS and Kupferman ME: Prognostic
factors in mucoepidermoid carcinoma of the salivary glands. Cancer.
118:3928–3936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ellington CL, Goodman M, Kono SA, Grist W,
Wadsworth T, Chen AY, Owonikoko T, Ramalingam S, Shin DM, Khuri FR,
et al: Adenoid cystic carcinoma of the head and neck: Incidence and
survival trends based on 1973–2007 Surveillance, Epidemiology, and
end results data. Cancer. 118:4444–4451. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deschler DG and Eisele DW: Surgery for
primary malignant parotid neoplasms. Adv Otorhinolaryngol.
78:83–94. 2016.PubMed/NCBI
|
|
6
|
Spiro RH, Huvos AG and Strong EW: Cancer
of the parotid gland. A clinicopathologic study of 288 primary
cases. Am J Surg. 130:452–459. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chakrabarti S, Bera M, Bhattacharya PK,
Chakrabarty D, Manna AK, Pathak S and Maiti K: Study of salivary
gland lesions with fine needle aspiration cytology and
histopothology along with immunohistochemistry. J Indian Med Assoc.
108:833–836. 2010.PubMed/NCBI
|
|
8
|
Mohammed F, Asaria J, Payne RJ and Freeman
JL: Retrospective review of 242 consecutive patients treated
surgically for parotid gland tumours. J Otolaryngol Head Neck Surg.
37:340–346. 2008.PubMed/NCBI
|
|
9
|
Kaya BV, Kılıç C, Özlügedik S, Tuncel Ü
and Cömert E: Long-term effects of parotidectomy. Eur Arch
Otorhinolaryngol. 273:4579–4583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chambless LB, Kistka HM, Parker SL,
Hassam-Malani L, McGirt MJ and Thompson RC: The relative value of
postoperative versus preoperative karnofsky performance scale
scores as a predictor of survival after surgical resection of
glioblastoma multiforme. J Neurooncol. 121:359–364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nobis CP, Rohleder NH, Wolff KD,
Wagenpfeil S, Scherer EQ and Kesting MR: Head and neck salivary
gland carcinomas-elective neck dissection, yes or no? J Oral
Maxillofac Surg. 72:205–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Min R, Siyi L, Wenjun Y, Ow A, Lizheng W,
Minjun D and Chenping Z: Salivary gland adenoid cystic carcinoma
with cervical lymph node metastasis: A preliminary study of 62
cases. Int J Oral Maxillofac Surg. 41:952–957. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Revenaugh PC, Knott PD, Scharpf J and
Fritz MA: Simultaneous anterolateral thigh flap and temporalis
tendon transfer to optimize facial form and function after radical
parotidectomy. Arch Facial Plast Surg. 14:104–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Liu F, Lan X, Huang J, Luo K and
Li S: Resection and reconstruction of giant cervical metastatic
cancer using a pectoralis major muscular flap transfer: A
prospective study of 16 patients. Oncol Lett. 10:372–378.
2015.PubMed/NCBI
|
|
15
|
Emerick KS, Herr MW, Lin DT, Santos F and
Deschler DG: Supraclavicular artery island flap for reconstruction
of complex parotidectomy, lateral skull base, and total
auriculectomy defects. JAMA Otolaryngol Head Neck Surg.
140:861–866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bendet E, Talmi YP and Kronenberg J:
Preoperative electroneurography (ENoG) in parotid surgery:
Assessment of facial nerve outcome and involvement by tumor-a
preliminary study. Head Neck. 20:124–131. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujita Y, Kubota A, Furukawa M, Yagi H and
Tsukuda M: Parotid gland cancer treatment with facial nerve
preservation. Nihon Jibiinkoka Gakkai Kaiho. 113:115–122. 2010.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Voss PJ, Leow AM, Schulze D, Metzger MC,
Liebehenschel N and Schmelzeisen R: Navigation-guided resection
with immediate functional reconstruction for high-grade malignant
parotid tumour at skull base. Int J Oral Maxillofac Surg.
38:886–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kimata Y, Sakuraba M, Hishinuma S, Ebihara
S, Hayashi R and Asakage T: Free vascularized nerve grafting for
immediate facial nerve reconstruction. Laryngoscope. 115:331–336.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iida T, Nakagawa M, Asano T, Fukushima C
and Tachi K: Free vascularized lateral femoral cutaneous nerve
graft with anterolateral thigh flap for reconstruction of facial
nerve defects. J Reconstr Microsurg. 22:343–348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
House JW and Brackmann DE: Facial nerve
grading system. Otolaryngol Head Neck Surg. 93:146–147. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sood S, McGurk M and Vaz F: Management of
Salivary Gland Tumours: United Kingdom national multidisciplinary
guidelines. J Laryngol Otol. 130:S142–S149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagliati M, Bolner A, Vanoni V, Tomio L,
Lay G, Murtas R, Deidda MA, Madeddu A, Delmastro E, Verna R, et al:
Surgery and radiotherapy in the treatment of malignant parotid
tumors: A retrospective multicenter study. Tumori. 95:442–448.
2009.PubMed/NCBI
|
|
24
|
Bhide SA, Miah A, Barbachano Y, Harrington
KJ, Newbold K and Nutting CM: Radical radiotherapy for treatment of
malignant parotid tumours: A single centre experience 1995–2005. Br
J Oral Maxillofac Surg. 47:284–289. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuda S, Iguchi H, Tada T, Hosono M,
Osawa M, Kuwae Y, Morimoto H, Okazaki E, Amano K, Miki Y, et al:
Results of surgery plus postoperative radiotherapy for patients
with malignant parotid tumor. Jpn J Radiol. 33:533–537. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Herman MP, Amdur RJ, Werning JW,
Dziegielewski P, Morris CG and Mendenhall WM: Elective neck
management for squamous cell carcinoma metastatic to the parotid
area lymph nodes. Eur Arch Otorhinolaryngol. 273:3875–3879. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jensen AD, Nikoghosyan AV, Poulakis M,
Höss A, Haberer T, Jäkel O, Münter MW, Schulz-Ertner D, Huber PE
and Debus J: Combined intensity-modulated radiotherapy plus
raster-scanned carbon ion boost for advanced adenoid cystic
carcinoma of the head and neck results in superior locoregional
control and overall survival. Cancer. 121:3001–3009. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qi XS, Ruan D, Lee SP, Pham A, Kupelian P,
Low DA, Steinberg M and Demarco J: Dependence of achievable plan
quality on treatment technique and planning goal refinement: A
head-and-neck intensity modulated radiation therapy application.
Int J Radiat Oncol Biol Phys. 91:817–824. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stodulski D, Mikaszewski B and Stankiewicz
C: Are all prognostic factors in parotid gland carcinoma well
recognized? Eur Arch Otorhinolaryngol. 269:1019–1025. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishikawa S, Kawata R, Higashino M, Lee K,
Terada T, Kurisu Y and Tsuji M: Assessing the histological type and
grade of primary parotid carcinoma by fine-needle aspiration and
frozen section. Auris Nasus Larynx. 42:463–468. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fakhry N, Santini L, Lagier A, Dessi P and
Giovanni A: Fine needle aspiration cytology and frozen section in
the diagnosis of malignant parotid tumours. Int J Oral Maxillofac
Surg. 43:802–805. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Catania A, Falvo L, D'Andrea V,
Biancafarina A, De Stefano M and De Antoni E: Parotid gland
tumours. Our experience and a review of the literature. Chir Ital.
55:857–864. 2003.PubMed/NCBI
|
|
33
|
Jurczyk M, Peevey JF, Haar MA Vande and
Lin X: Pitfalls of fine-needle aspiration cytology of parotid
membranous basal cell adenoma-A review of pitfalls in FNA cytology
of salivary gland neoplasms with basaloid cell features. Diagn
Cytopathol. 43:432–437. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Iguchi H, Wada T, Matsushita N, Oishi M,
Teranishi Y and Yamane H: Evaluation of usefulness of fine-needle
aspiration cytology in the diagnosis of tumours of the accessory
parotid gland: A preliminary analysis of a case series in Japan.
Acta Otolaryngol. 134:768–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fundakowski C, Castaño J, Abouyared M, Lo
K, Rivera A, Ojo R, Gomez-Fernandez C, Messinger S and Sargi Z: The
role of indeterminate fine-needle biopsy in the diagnosis of
parotid malignancy. Laryngoscope. 124:678–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Longuet M, Nallet E, Guedon C, Depondt J,
Gehanno P and Barry B: Diagnostic value of needle biopsy and frozen
section histological examination in the surgery of primary parotid
tumors. Rev Laryngol Otol Rhinol (Bord). 122:51–55. 2001.(In
French). PubMed/NCBI
|
|
37
|
O'Brien CJ: Current management of benign
parotid tumors-the role of limited superficial parotidectomy. Head
Neck. 25:946–952. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ettl T, Schwarz-Furlan S, Gosau M and
Reichert TE: Salivary gland carcinomas. Oral Maxillofac Surg.
16:267–283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Swoboda H and Franz P: Salivary gland
tumors. Clinical aspects and therapy. Radiologe. 34:232–238.
1994.(In German).
|
|
40
|
Obaid MA and Yusuf A: Surgical management
of epithelial parotid tumours. J Coll Physicians Surg Pak.
14:394–399. 2004.PubMed/NCBI
|
|
41
|
Shah SA, Riaz U, Zubair M and Saaiq M:
Surgical presentation and outcome of parotid gland tumours. J Coll
Physicians Surg Pak. 23:625–628. 2013.PubMed/NCBI
|
|
42
|
Shinomiya H, Otsuki N, Yamashita D and
Nibu K: Patterns of lymph node metastasis of parotid cancer. Auris
Nasus Larynx. 43:446–450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sagayaraj A, Deo RP, Mohiyuddin SM Azeem
and Modayil G Oommen: Island pectoralis major myocutaneous flap: An
Indian perspective. Indian J Otolaryngol Head Neck Surg.
64:270–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ioannides C and Fossion E: Reconstruction
of extensive defects of the parotid region: Experience with the
pectoralis major and free latissimus dorsi flaps. J
Craniomaxillofac Surg. 25:57–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tripathi M, Parshad S, Karwasra RK and
Singh V: Pectoralis major myocutaneous flap in head and neck
reconstruction: An experience in 100 consecutive cases. Natl J
Maxillofac Surg. 6:37–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ezeanolue BC: Residual and recurrent
parotid gland neoplasm after surgical excision. West Afr J Med.
21:5–8. 2002.PubMed/NCBI
|
|
47
|
Becelli R, Perugini M, Mastellone P and
Frati R: Surgical treatment of recurrences of pleomorphic adenoma
of the parotid gland. J Exp Clin Cancer Res. 20:487–489.
2001.PubMed/NCBI
|
|
48
|
Lima RA, Tavares MR, Dias FL, Kligerman J,
Nascimento MF, Barbosa MM, Cernea CR, Soares JR, Santos IC and
Salviano S: Clinical prognostic factors in malignant parotid gland
tumors. Otolaryngol Head Neck Surg. 133:702–708. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kaur J, Goyal S, Muzumder S, Bhasker S,
Mohanti BK and Rath GK: Outcome of surgery and post-operative
radiotherapy for major salivary gland carcinoma: Ten year
experience from a single institute. Asian Pac J Cancer Prev.
15:8259–8263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Adebiyi KE and Emmanuel MM: Neoplastic
salivary gland lesions: A retrospective analysis of 135 Cases from
Lagos state university teaching hospital, Ikeja, Lagos, Nigeria.
West Afr J Med. 33:206–210. 2014.PubMed/NCBI
|