Introduction
Gastric cancer (GC) is the fourth most common human
malignant disease and the second leading cause of cancer-associated
mortality worldwide (1–4). Although considerable advances have been
made in early diagnosis, surgical techniques and medical treatment,
the 5-year survival rate remains poor, particularly in China
(5,6).
Recurrence and metastasis are the biggest obstacles to the
treatment of GC. Therefore, novel prognostic and predictive markers
are necessary to improve the assessment of patient outcome and to
guide more targeted and individualized therapeutic decisions.
MDM2 binding protein (MTBP), a 104 kDa protein with
no known functional motifs, was originally identified as a protein
that interacts with the oncoprotein, murine double minute (MDM2),
using a yeast two-hybrid screen to bind to the E3 ubiquitin ligase
MDM2 (7). MTBP expression can be
detected in a wide variety of tissues, with the highest levels of
expression in the thymus, testis and ovary. Furthermore, the same
tissues exhibited the highest levels of expression of MDM2
(7). A previous study demonstrated
that decreased MTBP expression level was associated with tumor
metastasis (8). Clinically, loss of
MTBP expression is associated with reduced survival of patients
with head and neck carcinoma (9).
Overexpression of MTBP protects MDM2 from self-ubiquitination,
which induces MDM2 stabilization and p53 degradation (10). MDM2 is a major negative regulator of
the tumor suppressor p53 (11,12). p53,
a potent growth suppressive and proapoptotic molecule, may serve a
key role in cell-cycle regulation and be central to protecting
cells from uncontrolled growth (13,14). p53
is also the most frequently altered protein in human cancer
(15). In total, ~50% of all human
malignancies harbor mutations or deletions in the p53 gene that
disable the tumor suppressor function of the encoded protein
(16,17). A subsequent study revealed that MTBP
regulated p53 via modulation of MDM2 ubiquitin ligase activity
(10). Boyd et al (7) reported that overexpression of MTBP
induced G1 arrest of the cell cycle independent of p53.
MTBP contributed to p53/MDM2 homeostasis and generated
MTBP-reactive antisera, and MTBP phenotypes have been identified to
be compatible with potentially oncogenic and tumor suppressive
activities for this gene (7,10). In particular, overexpression of MTBP
in tumor cell lines increased MDM2 ubiquitin ligase activity, which
induced p53 degradation, whereas suppression of MTBP expression had
the opposite effect (7,10). MTBP has been involved in tumor
progression of patients with head and neck cancer; it may predict
disease progression for patients with squamous cell carcinoma of
the head and neck (9).
The present study evaluated MTBP expression level in
gastric tissue microarrays (TMA) by immunohistochemistry (IHC) and
its clinical significances. The results demonstrated the crucial
role of MTBP as a prognostic and metastatic marker as well as a
novel potential therapeutic target in GC.
Materials and methods
Patient selection and tissue
specimens
Tissue samples were obtained from 352 patients with
GC who underwent surgical resection at the Department of Gastric
Cancer and Soft Tissue Sarcoma, Fudan University Shanghai Cancer
Center from April 2010 to April 2011. No preoperative chemotherapy
or radiotherapy had been performed in any of these cases. In the
present study, there were 275 men and 77 women with a mean age of
58 years (range, 32–88 years). The clinical data of patients were
obtained from the medical record of Fudan University Shanghai
Cancer Center (Shanghai, China). Ethical approval for human
subjects was obtained from the Research Ethics Committee of Fudan
University Shanghai Cancer Center, and written informed consent was
obtained from all patients prior to enrollment in the present
study. One pair of tissue samples was obtained from each of the 352
patients. All patients were regularly followed-up by telephone. The
duration of follow-up was defined as the interval from the date of
the treatment to the date of mortality or the final follow-up, with
the final follow-up being the 15th of December 2014. The
clinicopathological characteristics of patients in the TMA are
summarized in Table I. Tumor size was
defined as the maximum diameter of the tumor. Tumor-node-metastasis
(TNM) classification of gastric carcinoma was based on the 7th
edition of the American Joint Committee on Cancer staging system
(18). Borrman type was classified as
previously described (19).
Histological grade was classified according to the Japanese
Classification of Gastric Carcinoma as well, moderately and poorly
differentiated (20). Preoperative
carcinoembryonic antigen (CEA) levels were obtained using an ELISA
kit according to the manufacturer's protocol (cat no. ab99992;
Abcam, Cambridge, UK). Briefly, the assay employed anti-human CEA
antibody coated onto a 96-well plate. Standard or serum samples
were pipetted into the wells and CEA present in samples was bound
to the wells by the immobilized antibody. The wells were washed and
biotinylated anti-human CEA antibody was added. Following the
washing of unbound biotinylated antibody, horseradish
peroxidase-conjugated streptavidin was pipetted into the wells. The
wells were washed again, tetramethylbenzidine substrate solution
was added and color developed in proportion to the amount of bound
CEA. Absorbance value was measured at 450 nm. CEA concentration in
serum was achieved according to standard curves.
 | Table I.Association between MTBP expression
levels and clinicopathological characteristics of gastric
cancer. |
Table I.
Association between MTBP expression
levels and clinicopathological characteristics of gastric
cancer.
|
|
| MTBP expression
level, n (%) |
|
|---|
|
|
|
|
|
|---|
| Variable | Patients, n (%) | Low | High | P-value |
|---|
| All cases | 352 (100) | 271 (77.0) | 81 (23.0) |
|
| Gender |
|
|
| 0.026 |
| Male | 275 (78.1) | 219 (79.6) | 56 (20.4) |
|
|
Female | 77
(21.9) | 52
(67.5) | 25 (32.5) |
|
| Age, years |
|
|
| 0.234 |
| ≤60 | 155 (44.0) | 124 (80.0) | 31 (20.0) |
|
|
>60 | 197 (56.0) | 147 (74.6) | 50 (25.4) |
|
| Tumor size, cm |
|
|
| 0.077 |
|
<5 | 187 (53.1) | 137 (73.3) | 50 (26.7) |
|
| ≥5 | 165 (46.9) | 134 (81.2) | 31 (18.8) |
|
| Borrmann type |
|
|
| 0.075 |
| I | 20
(5.70) | 14
(70.0) | 6
(30.0) |
|
| II | 17
(4.80) | 9
(52.9) | 8
(47.1) |
|
|
III | 302 (85.8) | 237 (78.5) | 65 (21.5) |
|
| IV | 13
(3.70) | 11
(84.6) | 2
(15.4) |
|
| Depth of
invasion |
|
|
| 0.171 |
| T1 | 12
(3.40) | 8
(66.7) | 4
(33.3) |
|
| T2 | 16
(4.50) | 10
(62.5) | 6
(37.5) |
|
| T3 | 27
(7.70) | 18
(66.7) | 9
(33.3) |
|
| T4 | 297 (84.4) | 235 (79.1) | 62 (20.9) |
|
| Lymph node
metastasis |
|
|
| <0.001 |
| N0 | 79
(22.4) | 45
(57.0) | 34 (43.0) |
|
| N1 | 69
(19.6) | 54
(78.3) | 15 (21.7) |
|
| N2 | 65
(18.5) | 55
(84.6) | 10 (15.4) |
|
| N3 | 139 (39.5) | 117 (84.2) | 22 (15.8) |
|
| Distant
metastasis |
|
|
| 0.026 |
| M0 | 304 (86.4) | 228 (75.0) | 76 (25.0) |
|
| M1 | 48
(13.6) | 43
(89.6) | 5
(10.4) |
|
| pTNM stage |
|
|
| <0.001 |
| I | 11
(3.10) | 7
(63.6) | 4
(36.4) |
|
| II | 72
(20.5) | 38
(52.8) | 34 (47.2) |
|
|
III | 221 (62.8) | 183 (82.8) | 38 (17.2) |
|
| IV | 48
(13.6) | 43
(89.6) | 5
(10.4) |
|
| Histological
grade |
|
|
| 0.441 |
|
Well | 18
(5.10) | 12
(66.7) | 6
(33.3) |
|
|
Moderately | 159 (45.2) | 126 (79.2) | 33 (20.8) |
|
|
Poorly | 175 (49.7) | 133 (76.0) | 42 (24.0) |
|
| Preoperative
CEA |
|
|
| 0.113 |
|
Elevated | 84
(24.1) | 70
(83.3) | 14 (16.7) |
|
|
Normal | 268 (75.9) | 201 (75.0) | 67 (25.0) |
|
TMA construction
TMAs were constructed as described previously
(21). In brief, identical 1.5 mm
diameter cylinders from the center of the tumor were included from
each case. Cylinders from the donor blocks were transferred to the
recipient paraffin block at defined array positions. Consecutive 4
mm sections were placed on 3-aminopropyltriethoxysilane coated
slides (in collaboration with Shanghai Biochip Company, Shanghai,
China). Hematoxylin and eosin-stained slides from each tissue block
were reviewed by a senior consultant pathologist, and performed
according to a previously described protocol (22).
Immunohistochemical staining
IHC was performed as previously described (23). Sections (4 µm) of primary gastric
adenocarcinoma and the adjacent normal gastric tissues were
subjected to immunohistochemical staining as follows:
Formalin-fixed and paraffin-embedded tissue sections were
deparaffinized in xylene, rehydrated in a graded alcohol series and
washed with PBS. Subsequently, the tissue sections were immersed in
10 mmol/l citrate buffer (pH 6.0) and heated in a microwave for 30
min. Following cooling to room temperature, endogenous peroxidase
was blocked by incubation with 3% H2O2 in
methanol (36°C, 60 min). Nonspecific binding was blocked by
incubating the tissue sections with 1% bovine serum albumin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in a humid chamber
for 60 min at 4°C. Incubation with the primary antibodies was
subsequently performed overnight at 4°C using mouse anti-human MTBP
(dilution, 1:200; cat no. sc-99047; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The negative controls were treated identically
but with the primary antibodies omitted. Subsequently, incubation
with suitable secondary antibodies (cat no. pk-6100; Vector
Laboratories, Burlingame, CA, USA) at room temperature. Finally,
diaminobenzidine (Vector Laboratories) was used for signal
development, and the sections were counterstained with 20%
hematoxylin for 8 min at room temperature. Detection was performed
using a Dako EnVision system (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA).
Immunohistochemical evaluation
The expression level of MTBP in tumor and normal
adjacent tissues was assessed by two investigators blinded to the
clinical data and to the other investigator's score. The scores
were determined by the proportion of positive tumor cells and the
intensity of the coloring for MTBP, as described previously
(9). MTBP cytoplasmic expression
level was initially assigned an intensity grade: 0, no staining; 1,
weak staining; 2, moderate staining; and 3, strong staining.
Nuclear MTBP staining was scored positive or negative. The scores
were categorized into 2 groups: Low expression (0/1 cytoplasmic
staining) with no nuclear staining and high expression (2/3
cytoplasmic staining) and/or nuclear positivity.
Western blotting
The reagents and protocols used were described in
detail previously (24). Paired tumor
tissues and surrounding non-tumor tissues were ground to powder in
liquid nitrogen and lysed with SDS-PAGE sample buffer. Primary
antibodies against MTBP were purchased from Abcam (1:500; cat no.
ab115529) and used according to the manufacturer's instructions. A
monoclonal antibody against GAPDH (1:1,000; cat no. sc-69778; Santa
Cruz Biotechnology, Inc.) was used as an internal control. GAPDH
was used as a control. The primary antibodies were detected with
horseradish peroxidase-conjugated secondary antibodies (1:1,000;
cat no. sc-2351; Santa Cruz Biotechnology, Inc.); Immunoreactive
bands were visualized using SuperSignal West Pico Chemiluminescent
Substrate (cat no. NCI5080; Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). The gray value of each band was measured and
data are presented as a ratio to GAPDH (ImageJ 1.46; National
Institutes of Health, Bethesda, MD, USA). Each experiment was
repeated ≥3 times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
As described previously (25), total RNA was extracted from tumor and
normal adjacent tissues using TRIzol® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). RT-qPCR analysis was
performed using an ABI PRISM 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Each well (20 µl
reaction volume) contained 10 µl Power SYBR-Green PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.), 1 µl of each
primer (5 µmol/l) and 1 µl template. The following primers were
used: MTBP forward, 5′-TCCTGTAGTTTCGTCAGATCCT-3′ and reverse,
5′-CCGTTTCAATCGGGATACTTCA-3′. GAPDH forward,
5′-ACAGCCTCAAGATCATCAGCA-3′ and reverse,
5′-ATGAGTCCTTCCACGATACCA-3′. GAPDH was used as internal control.
Quantification was performed as described previously (25).
Statistical analyses
All statistical analyses were performed using SPSS
version 19.0 for Windows (IBM Corp., Armonk, NY, USA). The
associations between MTBP expression level and clinicopathological
characteristics were evaluated using the χ2 test.
Cumulative survival time was determined by the Kaplan-Meier method
using GraphPad Prism software (version 5.0; GraphPad Software,
Inc., La Jolla, CA, USA), and differences in survival curves were
analyzed using the log-rank test. Multivariate logistic regressions
were used to assess the association between MTBP expression level
and metastasis. Multivariate analysis was performed using the Cox's
proportional hazards regression model on all significant
characteristics evaluated for univariate analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
MTBP mRNA and protein expression
levels in GC tissues and normal adjacent tissues
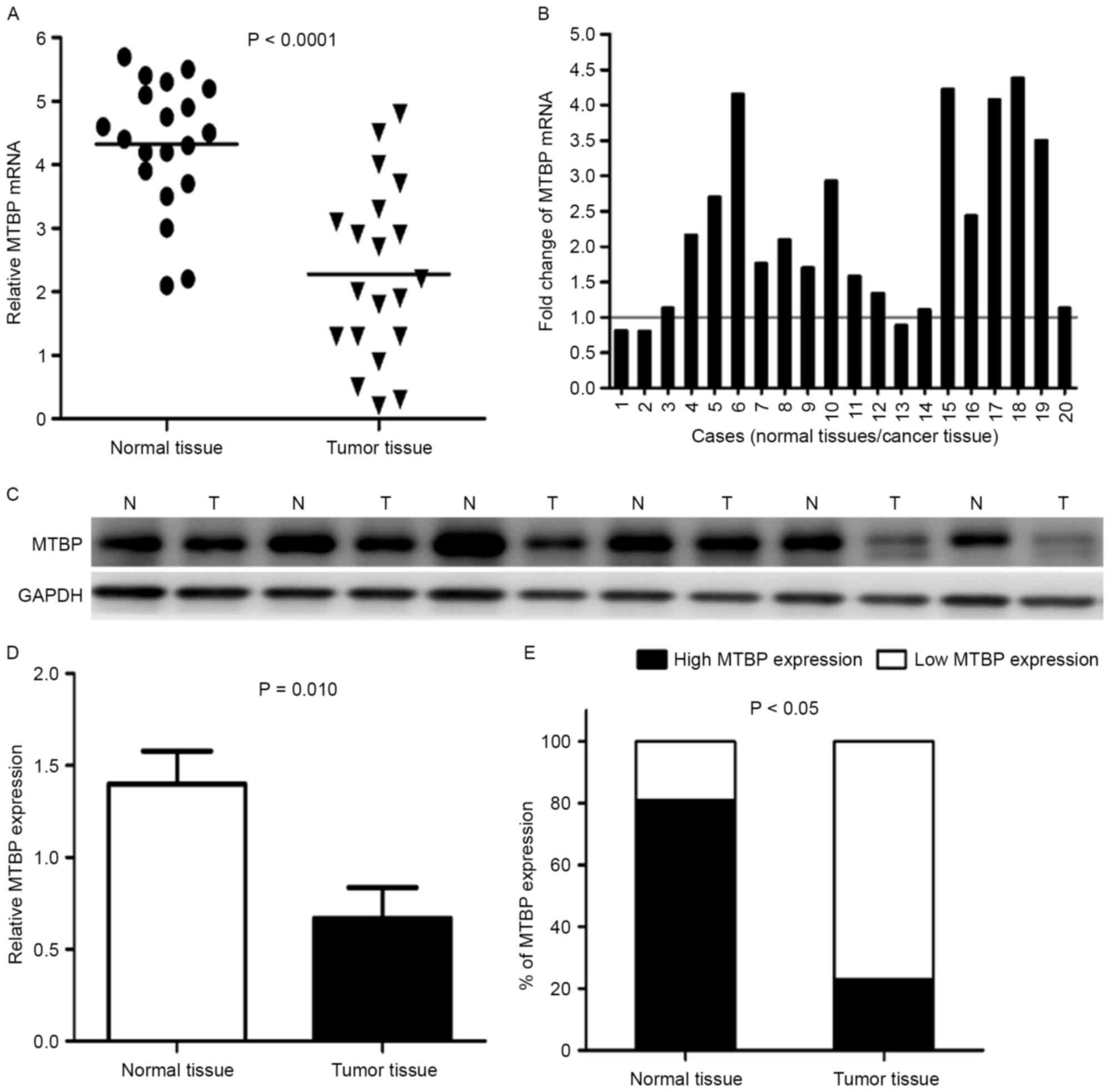
Representative data are presented in Fig. 1. RT-qPCR and western blot analysis
were used to confirm mRNA and protein expression levels. The mean
expression level of MTBP mRNA in normal tissues was significantly
higher compared with in tumor tissues (P<0.0001; Fig. 1A). Furthermore, the MTBP mRNA
expression level was significantly lower in 17/20 (85%) GC tissues
compared with in the matched adjacent normal gastric mucosa tissues
(Fig. 1B).
The difference in MTBP expression levels between
tumors and normal tissues revealed at the protein level was
investigated by western blotting. The representative western
blotting results in six cases are presented in (Fig. 1C). The relative level of MTBP
expression was normalized to the GAPDH of the same samples. Western
blot analysis demonstrated that the expression level of MTBP
protein was markedly decreased in 10/14 (71.4%) gastric tumor
tissues compared with in the corresponding adjacent normal tissues,
which was consistent with that of the RT-qPCR results. The average
MTBP protein expression level in 14 GC tissues was significantly
lower compared with in the matched adjacent normal gastric mucosa
tissues (P<0.05) (Fig. 1D).
Fig. 1E revealed that normal tissues
demonstrated higher expression levels of MTBP (81%), statistical
analysis revealed a lower expression level of MTBP in gastric
tumors (19%) compared with in adjacent normal tissues
(P<0.05).
Association between MTBP expression
level and clinicopathological characteristics in GC
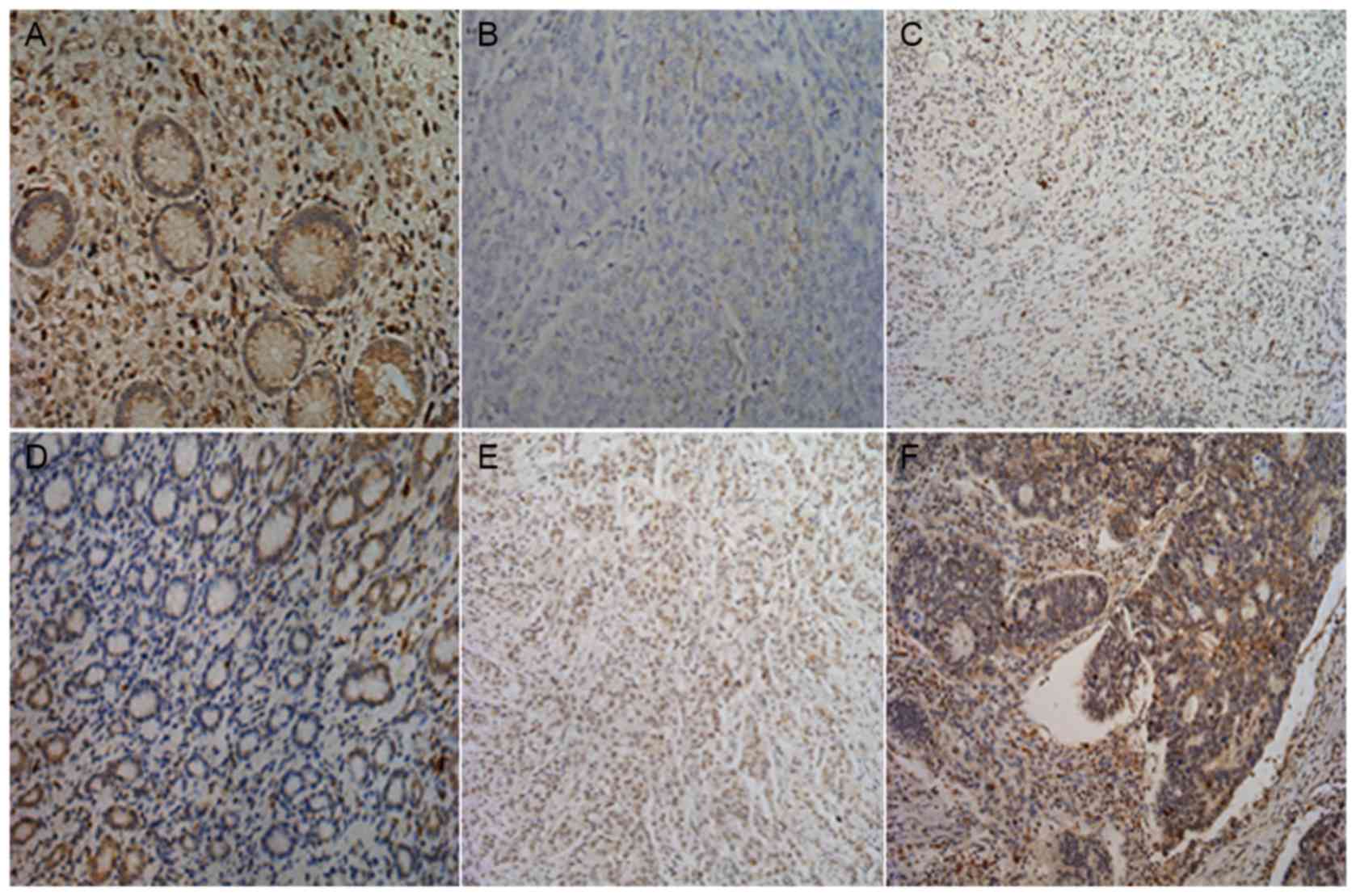
To elucidate the biological significance of MTBP in
GC, the present study examined the immunohistochemical expression
levels of MTBP in GC tissues (Fig.
2). MTBP staining was mainly located in the cytoplasm and/or
the nucleus of tumor cells, as presented in Table II, consistent with that of a previous
study (9). As presented in Table I, there were no significant
associations between the expression level of MTBP protein and age,
tumor size, Borrmann type, depth of invasion, histological grade or
preoperative carcinoembryonic antigen expression levels in patients
with GC. However, the expression level of MTBP was significantly
associated with gender, lymph node metastasis, distant metastasis
and pathological tumor-node-metastasis (pTNM) stage in GC
tissues.
 | Table II.Immunohistochemical evaluation of
nuclear and cytoplasmic staining. |
Table II.
Immunohistochemical evaluation of
nuclear and cytoplasmic staining.
| Staining | Number | Intensity |
|---|
| Cytoplasmic |
|
|
| 0 | 122 | No staining |
| 1 | 149 | Weak staining |
| 2 | 47 | Moderate
staining |
| 3 | 34 | Strong
staining |
| Nuclear |
|
|
|
Positive | 81 | Nuclear
staining |
|
Negative | 271 | No nuclear
staining |
| Combined
scoring |
|
|
| Low
expression | 271 | 0/1 cytoplasmic
staining + no nuclear staining |
| High
expression | 81 | 2/3 cytoplasmic
staining + nuclear positivity |
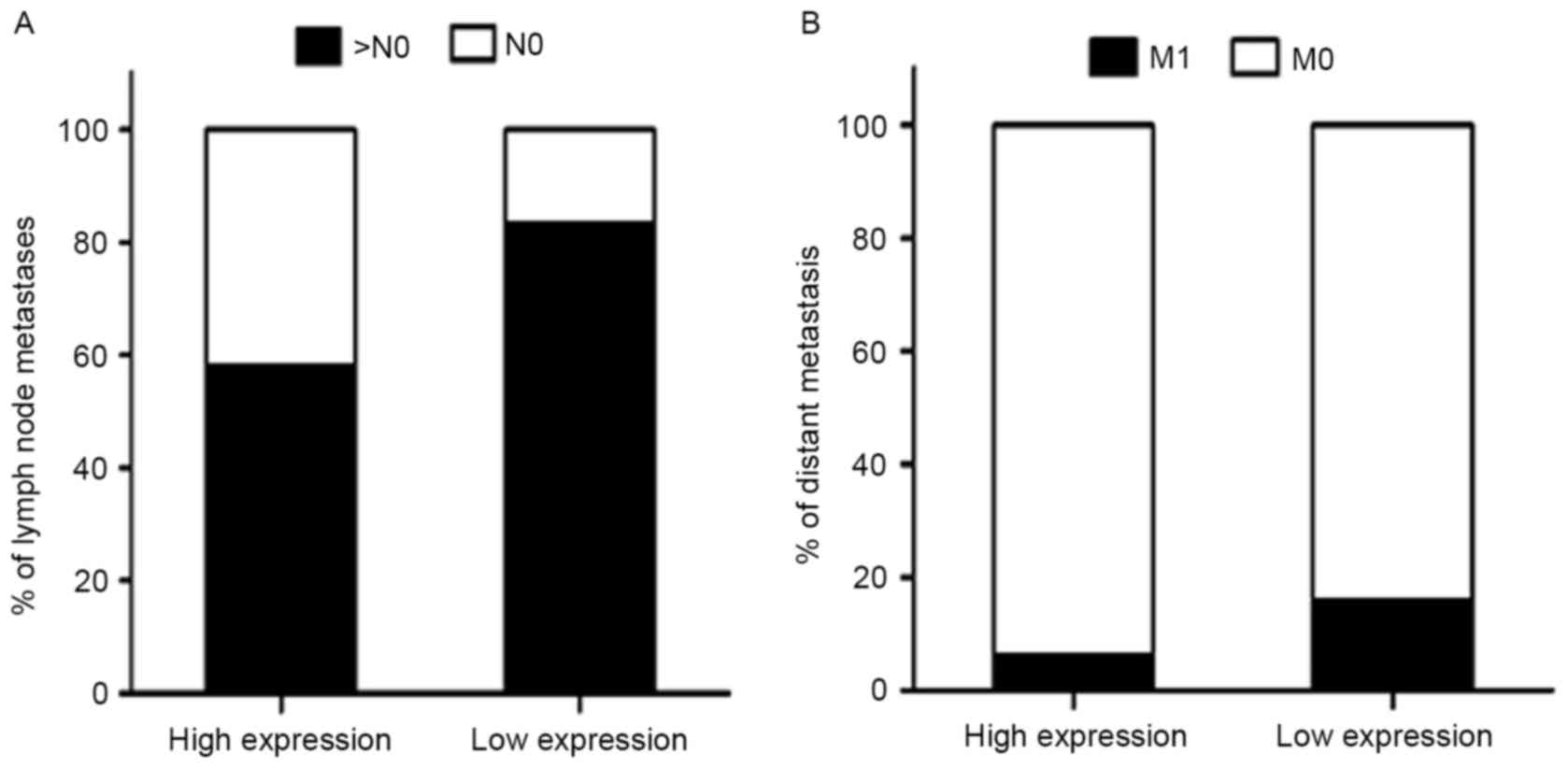
Predictive significance of MTBP
expression level in GC with distant and lymph node metastases
It was revealed that low MTBP expression level was
associated with higher distant metastasis and lymph node metastases
rate (Fig. 3). A multivariate
logistic regression analysis was performed to evaluate the
independently predictive significance of MTBP expression level for
distant and lymph node metastases (Table III). The results demonstrated that
the tumor size [OR, 1.942; 95% confidence interval (CI),
1.031–3.659; P=0.040] and MTBP expression level (OR, 0.365; 95% CI,
0.138–0.965; P=0.042) were independently associated with distant
metastasis. As described above, the multivariate logistic
regression analysis revealed that MTBP expression level was
significantly associated with the presence of lymph node metastasis
(OR, 0.282; 95% CI, 0.161–0.494; P<0.001) (all Table III).
 | Table III.Associations of MTBP expression level
with distant and lymph node metastasis. |
Table III.
Associations of MTBP expression level
with distant and lymph node metastasis.
| Variable | B | SE | OR | 95% CI | P-value |
|---|
| Distant metastasis,
n=48 |
|
|
|
|
|
| Age,
years (≤60 vs. >60) | 0.025 | 0.320 | 1.025 | 0.547–1.921 | 0.938 |
| Tumor
size (<5 vs. ≥5 cm) | 0.664 | 0.323 | 1.942 | 1.031–3.659 | 0.040 |
|
Differentiation
(well/moderately vs. poorly) | −0.402 | 0.323 | 0.669 | 0.355–1.259 | 0.213 |
|
Preoperative CEA (elevated vs.
normal) | 0.051 | 0.362 | 1.052 | 0.512–2.163 | 0.890 |
| MTBP
expression (high vs. low) | −1.007 | 0.496 | 0.365 | 0.138–0.965 | 0.042 |
| Lymph node
metastasis, n=273 |
|
|
|
|
|
| Age,
years (≤60 vs. >60) | −0.369 | 0.276 | 0.691 | 0.402–1.188 | 0.182 |
| Tumor
size (<5 vs. ≥5 cm) | 0.500 | 0.275 | 1.649 | 0.962–2.826 | 0.690 |
|
Differentiation
(well/moderately vs. poorly)a | −0.344 | 0.271 | 0.709 | 0.417–1.206 | 0.204 |
|
Preoperative CEA (elevated vs.
normal) | −0.112 | 0.314 | 0.894 | 0.483–1.653 | 0.720 |
| MTBP
expression (high vs. low) | −1.265 | 0.285 | 0.282 | 0.161–0.494 | <0.001 |
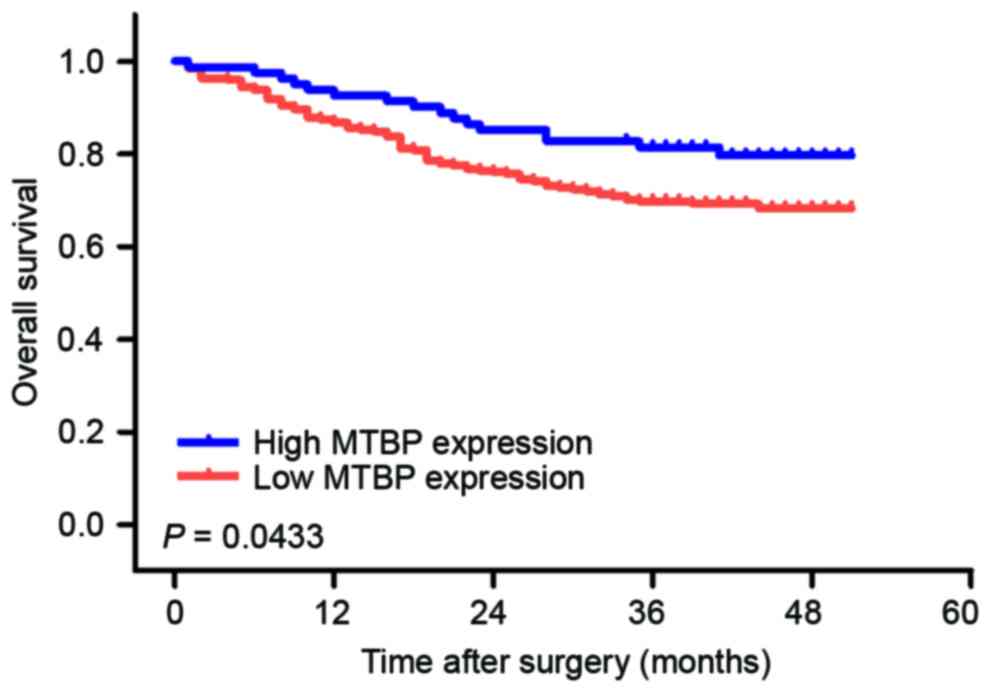
Prognostic significance of MTBP
expression level in GC tissues
To investigate the prognostic value of MTBP
expression level in patients with GC, overall survival (OS)
analysis was performed in 352 patients with GC. The 1- and 3-year
OS rates were 88.1 and 72.4%, respectively. The OS rate of the
high-level expression group was significantly longer compared with
that of the low-level expression group (Fig. 4).
Univariate and multivariate analyses were performed
to determine the predictors for OS (Table IV). To further determine the effect
of MTBP expression on OS, the present study first performed
univariate analysis of traditional clinicopathological variables
for prognosis. In univariate analysis, low expression level of MTBP
(P=0.017), larger tumor size (P=0.001), lymph node metastasis
(P=0.001), elevated preoperative CEA (P=0.047) and TNM stage
(P<0.001) were revealed to be associated with a poor OS rate of
patients with GC. Furthermore, to evaluate the independent impact
of MTBP expression level on OS, a multivariate Cox's regression
model was performed. The results demonstrated that low MTBP
expression level (HR, 0.633; 95% CI, 0.417–0.961) was a poor
independent prognostic factor for OS in patients with GC. In
addition, tumor size (HR, 0.582; 95% CI, 0.389–0.871) and TNM stage
(HR, 2.720; 95% CI, 1.395–5.305) revealed independent prognostic
value in the multivariate analysis (Table IV).
 | Table IV.Univariate and multivariate survival
analyses in patients with gastric cancer. |
Table IV.
Univariate and multivariate survival
analyses in patients with gastric cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Independent
factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender (male vs.
female) | 1.099 | 0.629–1.619 | 0.970 |
|
|
|
| Age, years (≤60 vs.
>60) | 0.992 | 0.669–1.473 | 0.969 |
|
|
|
| Tumor size, cm
(<5 vs. ≥5) | 0.520 | 0.349–0.777 | 0.001 | 0.582 | 0.389–0.871 | 0.008 |
| Borrmann type (I/II
vs. III/IV) | 1.293 | 0.652–2.565 | 0.462 |
|
|
|
| pTNM stage (I/II
vs. III/IV) | 3.233 | 1.682–6.214 | <0.001 | 2.720 | 1.395–5.305 | 0.003 |
| Differentiation
(well/moderate vs. poor)a | 1.444 | 0.972–2.145 | 0.069 |
|
|
|
| Preoperative CEA
(elevated vs. normal) | 1.719 | 1.007–2.934 | 0.047 | 1.290 | 0.749–2.222 | 0.359 |
| MTBP expression
level High vs. low | 0.602 | 0.397–0.913 | 0.017 | 0.633 | 0.417–0.961 | 0.032 |
Discussion
MTBP is a protein that interacts with MDM2, a major
inhibitor of the tumor suppressor p53. p53 was identified as a
tumor suppressor protein and is the most commonly mutated gene in
human cancer (14,16). Alam et al (26) revealed that the impact of this
particular protein MTBP is to stabilize the steady state expression
level of MDM2.
MTBP is a cellular protein and the expression levels
have been found to be higher in cancer cells (8,9). It has
been established that MTBP serves a role in the suppression of
tumor progression, and MTBP has been considered to be a biomarker
for various types of cancer. However, the role of MTBP in GC
remains unknown. To the best of our knowledge, the present study is
the first attempt to elucidate the role of MTBP in GC. The present
study revealed that MTBP expression level was reduced in GC tissues
compared with in matched non-tumor tissues, which implied that MTBP
may be a candidate tumor suppressor molecule in GC. Furthermore,
the present study investigated the expression level of MTBP in 352
GC tissues by IHC. It was demonstrated that MTBP was significantly
associated with gender, lymph node metastasis, distant metastasis
and pTNM stage. Agarwal et al (27) revealed that overexpression of MTBP in
osteosarcoma cells suppressed metastasis with little effect on
primary tumor growth in an orthotopic mouse model, and that MTBP
inhibited cell migration and filopodia formation. The present study
demonstrated that MTBP expression level was significantly
associated with the OS rate of patients with GC. Kaplan-Meier
analysis of OS revealed that a unique cohort of patients with GC
with low MTBP expression level exhibited significantly poorer OS
rate, indicating that low MTBP expression level was a marker of
poor prognosis for patients with GC. Furthermore, Cox proportional
hazards model revealed that MTBP status was an independent
prognostic predictor for patients with GC. Vlatkovic et al
(9) demonstrated that loss of MTBP
expression was associated with reduced survival of patients with
head and neck squamous cell carcinoma and served as an independent
prognostic factor. These observations indicated that MTBP
expression levels may serve as a powerful novel prognosticator of
GC. The TMA immunohistochemical assay of the present study
supported this hypothesis, as the TMA results and clinical data
revealed that MTBP exhibited a positive impact on patient outcomes.
Thus, MTBP may constitute a molecular prognostic marker for
patients with GC, identifying the individuals who are more likely
to have a higher risk of mortality. To the best of our knowledge,
this is the first study to demonstrate the positive role of MTBP in
patients with GC. However, this present retrospective cohort study
was limited to relatively small case series. Therefore, further
validation is required.
In conclusion, the present study determined that
MTBP serves a critical role in the suppression of tumor progression
and metastasis formation of GC. MTBP expression levels demonstrated
significant associations with tumor metastasis. These findings
suggested that MTBP may be a potential prognostic marker for GC,
and possibly an individual therapeutic target.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant no. 81272726), the Specialized
Research Fund for the Doctoral Program of Higher Education (grant
no. 20110071120097) and Shanghai Municipal Health Bureau Research
Project (grant no. 20114174).
References
|
1
|
González CA, Sala N and Rokkas T: Gastric
cancer: Epidemiologic aspects. Helicobacter. 18 Suppl 1:S34–S38.
2013. View Article : Google Scholar
|
|
2
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peleteiro B, Bastos A, Ferro A and Lunet
N: Prevalence of Helicobacter pylori infection worldwide: A
systematic review of studies with national coverage. Dig Dis Sci.
59:1698–1709. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boyd MT, Vlatkovic N and Haines DS: A
novel cellular protein (MTBP) binds to MDM2 and induces a G1 arrest
that is suppressed by MDM2. J Biol Chem. 275:31883–31890. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iwakuma T, Tochigi Y, Van Pelt CS,
Caldwell LC, Terzian T, Parant JM, Chau GP, Koch JG, Eischen CM and
Lozano G: Mtbp haploinsufficiency in mice increases tumor
metastasis. Oncogene. 27:1813–1820. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vlatković N, El-Fert A, Devling T,
Ray-Sinha A, Gore DM, Rubbi CP, Dodson A, Jones AS, Helliwell TR,
Jones TM and Boyd MT: Loss of MTBP expression is associated with
reduced survival in a biomarker-defined subset of patients with
squamous cell carcinoma of the head and neck. Cancer.
117:2939–2950. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brady M, Vlatkovic N and Boyd MT:
Regulation of p53 and MDM2 activity by MTBP. Mol Cell Biol.
25:545–553. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iwakuma T and Lozano G: MDM2, an
introduction. Mol Cancer Res. 1:993–1000. 2003.PubMed/NCBI
|
|
12
|
Marine JC and Lozano G: Mdm2-mediated
ubiquitylation: p53 and beyond. Cell Death Differ. 17:93–102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carson DA and Lois A: Cancer progression
and p53. Lancet. 346:1009–1011. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lane DP: Cancer. p53, guardian of the
genome. Nature. 358:15–16. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vassilev LT: p53 Activation by small
molecules: Application in oncology. J Med Chem. 48:4491–4499. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hollstein M, Sidransky D, Vogelstein B and
Harris CC: p53 mutations in human cancers. Science. 253:49–53.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hainaut P and Hollstein M: p53 and human
cancer: The first ten thousand mutations. Adv Cancer Res.
77:81–137. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumors. 7th. Wiley-Liss; New
York, NY: 2009
|
|
19
|
Borrmann R: Geschwülste des Magens und
DuodenumsVerdauungsschlauch. Erster Teil Rachen und Tonsillen,
Speiseröhre Magen und Darm, Bauchfell. Borchardt H, Borrmann R,
Christeller E, et al: Vienna: Springer; pp. 812–1054. 1926
|
|
20
|
Sano T and Aiko T: New Japanese
classifications and treatment guidelines for gastric cancer:
Revision concepts and major revised points. Gastric Cancer.
14:97–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simon R, Mirlacher M and Sauter G: Tissue
microarrays. Biotechniques. 36:98–105. 2004.PubMed/NCBI
|
|
22
|
Zhou Z, Chen ZW, Yang XH, Shen L, Ai XH,
Lu S and Luo QQ: Establishment of a biomarker model for predicting
bone metastasis in resected stage III non-small cell lung cancer. J
Exp Clin Cancer Res. 31:342012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma Q, Li P, Xu M, Yin J, Su Z, Li W and
Zhang J: Ku80 is highly expressed in lung adenocarcinoma and
promotes cisplatin resistance. J Exp Clin Cancer Res. 31:992012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan C, Miao Y, Zhang X, Liu D, Jiang G,
Lin X, Han Q, Luan L, Xu Z and Wang E: Btbd7 contributes to reduced
E-cadherin expression and predicts poor prognosis in non-small cell
lung cancer. BMC Cancer. 14:7042014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ji K, Ye L, Ruge F, Hargest R, Mason MD
and Jiang WG: Implication of metastasis suppressor gene, Kiss-1 and
its receptor Kiss-1R in colorectal cancer. BMC Cancer. 14:7232014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alam MJ, Fatima N, Devi GR Ravins and
Singh RK: The enhancement of stability of p53 in MTBP induced
p53-MDM2 regulatory network. Biosystems. 110:74–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Agarwal N, Adhikari AS, Iyer SV,
Hekmatdoost K, Welch DR and Iwakuma T: MTBP suppresses cell
migration and filopodia formation by inhibiting ACTN4. Oncogene.
32:462–470. 2013. View Article : Google Scholar : PubMed/NCBI
|