Introduction
Tricellular tight junctions (tTJs) form at the
convergence of bicellular tight junctions (bTJs) where three
epithelial cells meet in polarized epithelia (1). Lipolysis-stimulated lipoprotein receptor
(LSR) was identified as a novel molecular component of tricellular
contacts that is localized on the majority of epithelial tissues
(2). LSR is required for normal tTJ
formation and provides a high barrier function for the cellular
sheet. LSR recruits tricellulin (TRIC), which is a molecular
component of tTJs (1). A previous
study showed that LSR and TRIC are colocalized with the bTJ protein
claudin (CLDN)-based tight junction (TJ) strands reconstituted in
CLDN-1-overexpressing mouse L fibroblasts (2).
TJs are involved in signal transduction mechanisms
that regulate epithelial cell proliferation, gene expression,
differentiation and morphogenesis (3). Loss of TJs compromises cellular polarity
and stimulates dedifferentiation (4,5).
Furthermore, loss of several TJ proteins enhances tumor progression
(6), and downregulation of CLDN-7
promotes cell invasion in endometrial cancer (7).
The overexpression of certain TJ proteins, including
CLDNs, is associated with tumor growth and metastasis (8). In addition, the overexpression of CLDN-1
enhances cell invasion via the activation of matrix
metalloproteinases (MMPs) in certain cancer types (9,10). TRIC
expression is reduced in hepatic fibrolamellar carcinoma and
tonsillar squamous cell carcinoma as compared with normal tissues
(11,12), and also demonstrates a significant
negative correlation with the degree of differentiation in
pancreatic cancer (13). Furthermore,
TRIC expression in gastric carcinoma cells is negatively regulated
by Snail-induced epithelial-mesenchymal transition (EMT) (14).
Knockdown of LSR has been demonstrated to increase
cell motility and invasion in bladder cancer cells (15). LSR signaling also promotes
aggressive/tumor-initiating cell behaviors in breast cancer
(16). In addition, our recent study
revealed that small interfering RNA (siRNA)-mediated knockdown of
LSR promoted cell invasion in human endometrial cancer cells.
Although LSR expression level is associated with tumor progression
(15), it remains unclear how the
knockdown of LSR enhances cancer cell invasion.
In the present study, knockdown of LSR with siRNA in
human endometrial cancer Sawano cells induced cell invasion. In
LSR-knockdown Sawano cells, upregulation of CLDN-1 and MMPs, as
well as increased Sp1 transcription factor activity in the CLDN-1
promoter region, were observed. Knockdown of CLDN-1 with siRNA
prevented the induction of cell invasion by the downregulation of
LSR in Sawano cells. The aim of this study was to analyze the
function of LSR in cell invasion via CLDN-1-mediated MMPs in human
endometrial cancer.
Materials and methods
Antibodies
Rabbit polyclonal antibodies against TRIC (cat. no.
48-8400), occludin (OCLN; cat. no. 71-1500) and CLDNs-1 (cat. no.
71-7800), −3 (cat. no. 34-1700), −4 (cat. no. 36-4800) and −7 (cat.
no. 34-9100) were obtained from Zymed Laboratories (Thermo Fisher
Scientific, Inc., Waltham, MA, USA). A rabbit polyclonal anti-LSR
antibody was obtained from Novus Biological USA (Littleton, CO,
USA; cat. no. NBP1-89631). A mouse monoclonal anti-LSR antibody was
obtained from Abnova (Heidelberg, Germany; cat. no. H00051599-K).
Rabbit monoclonal antibodies against membrane-type 1 (MT1)-MMP
(cat. no. 13130), MMP-2 (cat. no. 4022) and MMP-9 (cat. no. 13667)
were obtained from Cell Signaling Technology, Inc. (Tokyo, Japan).
A rabbit polyclonal anti-MMP-10 antibody was obtained from Abcam
(Cambridge, UK; cat. no. ab199688). A rabbit polyclonal anti-actin
antibody was obtained from Sigma-Aldrich (Merck Millipore, St.
Louis, MO, USA; cat. no. A2066). Alexa Fluor 488 (green)-conjugated
anti-rabbit IgG and Alexa Fluor 594 (red)-conjugated anti-mouse IgG
antibodies were purchased from Molecular Probes, Inc. (Thermo
Fisher Scientific, Inc.). Horseradish peroxidase-conjugated
anti-mouse and anti-rabbit IgG antibodies were from Cell Signaling
Technology, Inc. (cat. nos. 7074 and 7076).
Cell line culture and treatment
The human endometrial cancer cell line Sawano
(RCB1152) was purchased from RIKEN BioResource Center (Tsukuba,
Japan). Sawano cells were maintained with minimal essential medium
(MEM; Sigma-Aldrich; Merck Millipore) supplemented with 10%
dialyzed fetal bovine serum (FBS; Thermo Fisher Scientific, Inc.).
The medium contained 100 U/ml penicillin, 100 µg/ml streptomycin
and 50 µg/ml amphotericin-B. Sawano cells were plated on 35 and
60-mm culture dishes, which were coated with rat tail collagen (500
µg dried tendon/ml in 0.1% acetic acid) and incubated in a
humidified incubator with 5% CO2 at 37°C. The Sawano
cells were transfected with siRNAs against LSR and CLDN-1 for 48 h
prior to further observations being made. A scrambled siRNA
sequence (BLOCK-iT Alexa Fluor fluorescent; Invitrogen; Thermo
Fisher Scientific, Inc.) was used as control siRNA.
RNA interference and transfection
siRNA duplex oligonucleotides against LSR and CLDN-1
were synthesized by Thermo Fisher Scientific, Inc. The following
siRNAs against LSR were used: siRNA-A forward,
5′-CCCACGCAACCCAUCGUCAUCUGGA-3′; siRNA-A reverse,
5′-UCCAGAUGACGAUGGGUUGCGUGGG-3′; siRNA-B forward,
5′-GGCCGGAGGAUUACCAUCAUCACCGGA-3′; siRNA-B reverse,
5′-UUCCGGUGAUGGUAAUCCUCCGGCC-3′; siRNA-C forward,
5′-CACGGACAGCAGUGCGGCCUCUGAA-3′; and siRNA-C reverse
5′-UUCAGAGGCCACACUGCUGUCCGUG-3′. The siRNA sequences against CLDN-1
were as follows: Forward, 5′-GACUCCUUGCUGAAUCUG-3′; and reverse,
5′-UCAGAUUCAGCAAGGAGU-3′.
At 24 h after plating, the Sawano cells were
transfected with the three pairs of LSR siRNAs and the pair of
CLDN-1 siRNAs (100 nM of each) using Lipofectamine™ RNAiMAX Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.).
Immunostaining
The cultured cells in Iwaki 35-mm glass coated wells
(Asahi Techno Glass, Chiba, Japan) were fixed with cold acetone and
ethanol (1:1) at −20°C for 10 min. Following rinsing with PBS, the
cells were incubated with mouse monoclonal anti-LSR (1:100) and
rabbit polyclonal anti-CLDN-1 (1:100) antibodies at room
temperature for 1 h. Alexa Fluor 488-conjugated anti-rabbit IgG and
Alexa Fluor 594-conjugated anti-mouse IgG (1:200) were used as
secondary antibodies. The specimens were examined using an
epifluorescence microscope (Olympus Corporation, Tokyo, Japan) and
a confocal laser scanning microscope (LSM5; Carl Zeiss, Jena,
Germany).
Western blot analysis
The cultured cells were scraped from a 60-mm dish
containing 400 or 600 µl buffer (1 mM NaHCO3 and 2 mM
phenylmethylsulfonyl fluoride), transferred to microcentrifuge
tubes, and then sonicated for 10 sec. The protein concentrations of
the samples were determined using a Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). Aliquots of 15 µl of protein for
each sample were separated by electrophoresis on 5–20% SDS
polyacrylamide gels (Wako Pure Chemical Industries, Ltd., Osaka,
Japan), and electrophoretic transfer to a nitrocellulose membrane
(Immobilon; Merck Millipore) was performed. The membrane was
saturated for >30 min at room temperature with blocking buffer
(25 mM Tris, pH 8.0, 125 mM NaCl, 0.1% Tween-20, and 4% skimmed
milk) and incubated with polyclonal rabbit anti-LSR (1:1,000),
anti-TRIC (1:1,000), anti-OCLN (1:1,000), anti-CLDN-1, −3, −4 and
−7 (1:1,000) and anti-actin (1:1,000) antibodies at room
temperature for >1 h. Then the membrane was incubated with
horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG
antibodies (1:2,000) at room temperature for 1 h. The
immunoreactive bands were detected using an ECL western blotting
system (RPN2232; GE Healthcare, Chicago, IL, USA). The
quantification of the bands was performed using ImageJ software
1.48 (National Institutes of Health, Bethesda, MD, USA).
Matrigel invasion assay
For the invasion assay, Matrigel (BD Biosciences,
Bedford, MA, USA) and Cell Culture Inserts (pore size 8 µm; BD
Biosciences) were used. Sawano cells were plated using MEM without
FBS onto the upper Matrigel-coated chamber (354480; Corning
Incorporated, Corning, NY, USA), and the lower chamber of the
Transwell was filled with human fibroblast-conditioned medium
containing 10 nM epidermal growth factor as the chemoattractant.
The cells were then incubated for 36 h. Subsequent to this, the
medium was removed, and the Matrigel-coated upper chamber was fixed
with 100% methanol for 10 min and stained with Giemsa for 20 min.
The areas of invading cells were measured in 1,00,0000
µm2 area by automatic binarization using a microscope
imaging system (Olympus Corporation).
Dual-luciferase reporter assay
Sawano cells were placed in 96-well plates and
cultured overnight. An Sp1 luciferase reporter gene construct or
negative control (CCS-6027L; SABiosciences; Qiagen Inc.,
Germantown, MD, USA) and siRNA specific for LSR were cotransfected
into the cells for 48 h. Whole protein extracts were prepared and
luciferase activity was measured using a Dual-Luciferase Assay Kit
(Promega Corporation, Madison, WI, USA) according to the
manufacturer's protocol.
GeneChip analysis
Microarray analyses were performed using a 3D-Gene
Human Oligo chip 25k (Toray, Tokyo, Japan) and microarray images
were automatically analyzed using AROS™, version 4.0 (Operon
Biotechnologies, Tokyo, Japan).
Immunohistochemical analysis
This study was approved by the ethics committee of
the Sapporo Medical University School of Medicine (Sapporo, Japan).
Human endometrial carcinoma tissues were obtained from 15 patients
with endometrial adenocarcinoma who underwent hysterectomy between
January 2012 and December 2013 at Sapporo Medical University
Hospital. Hematoxylin and eosin-stained slides from each case were
reviewed, and the diagnosis and grades of the tumors were
determined according to the guidelines of the World Health
Organization classification (17).
Gynecologists and pathologists at the hospital established the
diagnosis of endometrial adenocarcinoma. All endometrial
adenocarcinoma were classic endometrial type I.
Human endometrial cancer tissues were embedded in
paraffin following fixation with 10% formalin in PBS. Briefly,
5-µm-thick sections were dewaxed in xylene, rehydrated in ethanol,
and heated with Vision BioSystems Bond Max using ER2 solution
(Leica Biosystems Nussloch GmBH, Nussloch, Germany) in an autoclave
for antigen retrieval. Endogenous peroxidase was blocked by
incubation with 3% hydrogen peroxide in methanol for 10 min. The
tissue sections were then washed twice with Tris-buffered saline
(TBS) and preblocked with Block Ace (DS Pharma Biomedical Co.,
Ltd., Suita, Japan) for 1 h. Subsequent to washing with TBS, the
sections were incubated with rabbit polyclonal anti-LSR (1:100) and
rabbit polyclonal anti-CLDN-1 (1:100) antibodies for 1 h. The
sections were then washed three times in TBS and incubated with
Vision BioSystems Bond Polymer Refine Detection kit (DS9800; Leica
Biosystems Nussloch GmBH). Following three washes in TBS, a
diaminobenzidine tetrahydrochloride solution was applied. Finally,
the sections were counterstained with hematoxylin. Immunoreactivity
for LSR and CLDN-1 on the invasive front of human endometrial
carcinoma was assessed.
Statistical analysis
Each set of results represents at least three
separate experiments. Results are presented as the mean ± standard
error of the mean. Differences between groups were tested by ANOVA
followed by a post hoc test. P<0.01 was considered to indicate a
statistically significant difference.
Results
Knockdown of LSR enhances cellular
invasion in Sawano cells
To investigate whether LSR expression contributed to
the cellular invasion of endometrial cancer, knockdown of LSR was
performed using siRNA in Sawano cells. In Sawano cells transfected
with one of the three sets of siRNA against LSR, cellular invasion
measured using the Matrigel invasion assay appeared to be enhanced
compared to the control (Fig.
1A).
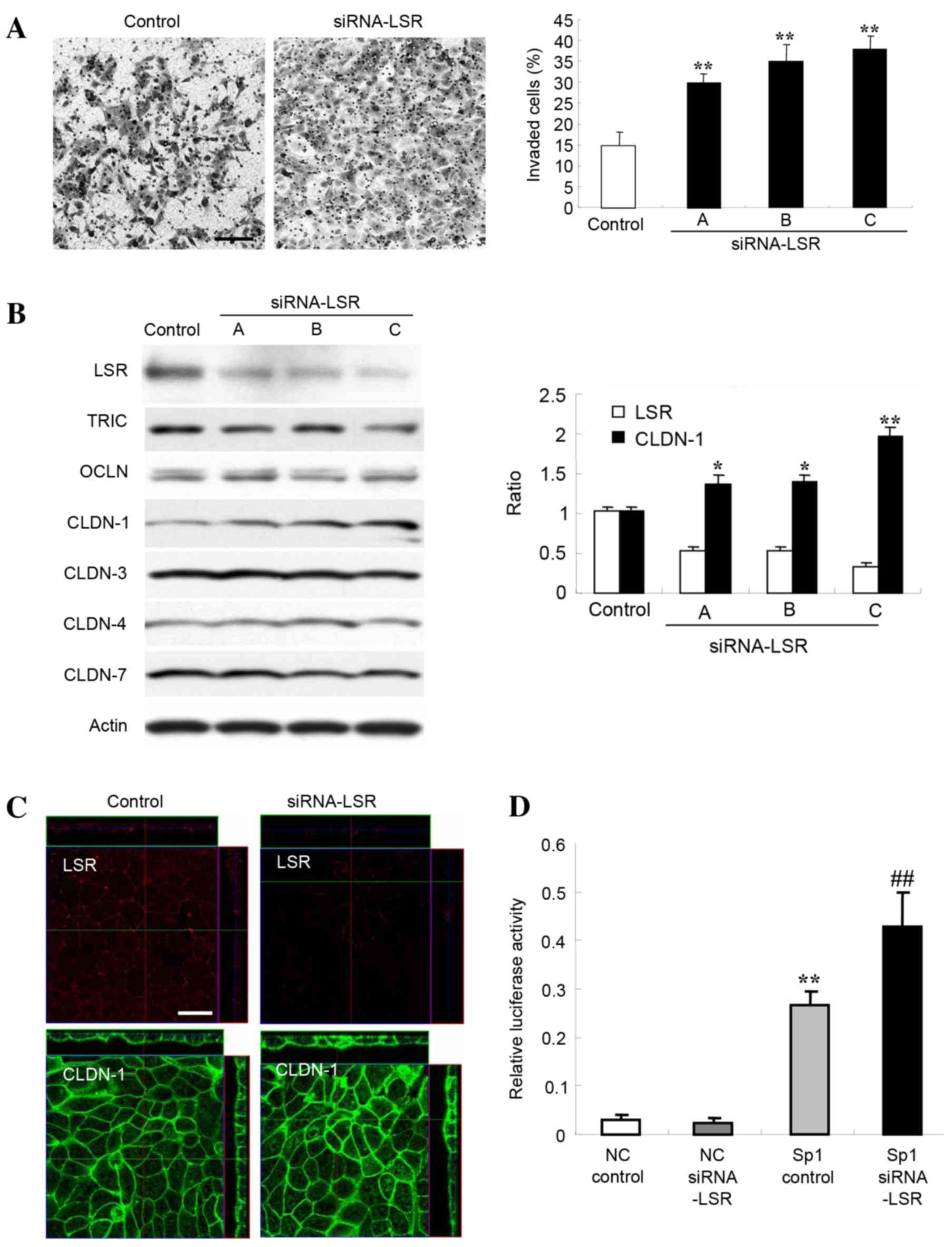 | Figure 1.(A) Matrigel invasion assay of Sawano
cells transfected with three sets of siRNA, A, B and C, LSR. Scale
bar, 100 µm. The results are presented below as a histogram.
Control vs. siRNA, **P<0.01. (B) Western blotting for LSR, TRIC,
OCLN, CLDN-1, −3, −4 and −7 in Sawano cells transfected with three
sets of siRNA against LSR. The results for the expression of LSR
and CLDN-1 are presented as a histogram. Control vs. siRNA,
*P<0.05, **P<0.01. (C) Immunostaining of LSR and CLDN-1 in
Sawano cells transfected with siRNA-C against LSR. Scale bar, 20
µm. (D) Sp1 transcription factor activity in Sawano cells
transfected with siRNA-C against LSR. NC vs. Sp1 control,
**P<0.01; Sp1 control vs. Sp1 siRNA, ##P<0.01.
siRNA, small interfering RNA; LSR, lipolysis-stimulated lipoprotein
receptor; TRIC, tricellulin; OCLN, occludin; CLDN, claudin; NC,
negative control. |
Knockdown of LSR increases CLDN-1
protein in Sawano cells
Alterations in the levels of other TJ proteins
subsequent to knockdown of LSR in Sawano cells were investigated.
As revealed by western blot analysis, 48 h after transfection with
three sets of siRNAs (A, B and C) against LSR, LSR protein
abundance was decreased and CLDN-1 protein significantly increased
compared with the control (P<0.01), whereas no changes in TRIC,
OCDN, CLDN-3, CLDN-4 or −7 abundance were observed (Fig. 1B). The increase in CLDN-1 protein
level enhanced by knockdown of LSR was inversely associated with
the value of LSR expression (Fig.
1B). Using immunostaining 48 h after transfection with the
siRNA-C against LSR, LSR protein could no longer be detected at the
subapical membranes and CLDN-1 protein abundance was increased at
the subapical and basolateral membranes of cells (Fig. 1C).
Knockdown of LSR induces Sp1 activity
in Sawano cells
The CLDN-1 promoter region contains an Sp1 binding
site, and a mutation in the region results in a loss of CLDN-1
transcription (18). Since knockdown
of LSR enhanced CLDN-1 protein in Sawano cells, the present study
investigated the change in Sp1 activity in Sawano cells transfected
with siRNA against LSR. Knockdown of LSR by siRNA-C significantly
induced Sp1 activity in the Sawano cells (Fig. 1D).
Knockdown of LSR increases the
expression of MMPs in Sawano cells
Overexpression of CLDN-1 enhances cell invasion of
certain types of cancer via activation of MMPs, including MT1-MMP
and MMP-2 (9,10). To investigate whether upregulation of
CLDN-1 by knockdown of LSR affected the expression of MMPs, DNA
microarray analysis of Sawano cells transfected with siRNA against
LSR was performed. In the DNA microarray, the mRNAs of MMP1, MMP2
and MMP10 were increased compared with the control (Table I). Next, using western blotting,
changes to MT1-MMP, MMP-2, MMP-9 and MMP-10 levels following the
knockdown of LSR were confirmed. The levels of MT1-MMP, MMP-2,
MMP-9 and MMP-10 proteins in Sawano cells transfected with siRNA
against LSR were increased compared with the control (Fig. 2A).
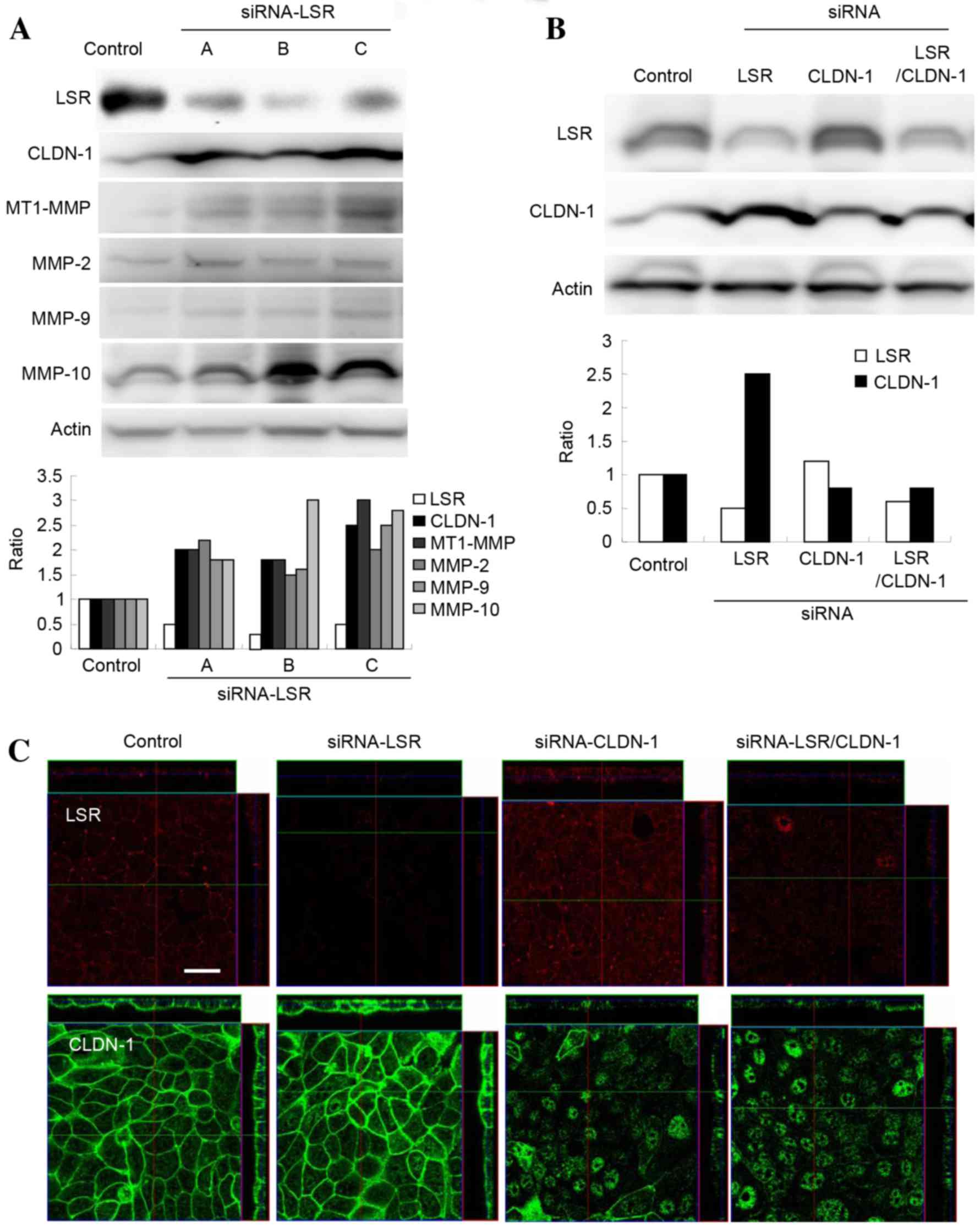 | Figure 2.(A) Western blotting for LSR, CLDN-1,
MT1-MMP, MMP-2, MMP-9 and MMP-10 in Sawano cells transfected with
three sets of siRNA against LSR. The results are presented below as
a histogram. (B) Western blotting for LSR and CLDN-1 in Sawano
cells transfected with or without siRNAs against LSR and CLDN-1.
The results are presented as a histogram. (C) Immunostaining for
LSR and CLDN-1 in Sawano cells transfected with or without siRNAs
against LSR and CLDN-1. Scale bar, 20 µm. LSR, lipolysis-stimulated
lipoprotein receptor; CLDN, claudin; MT1, membrane-type 1; MMP,
metalloproteinase; siRNA, small interfering RNA. |
 | Table I.List of probes for genes that were
upregulated in Sawano cells transfected with small interfering RNA
against lipolysis-stimulated lipoprotein receptor. |
Table I.
List of probes for genes that were
upregulated in Sawano cells transfected with small interfering RNA
against lipolysis-stimulated lipoprotein receptor.
| Gene name | ID | GenBank ID | Fold-change |
|---|
| MMP1 | H200007011 | NM_002421 | 1.90 |
| MMP2 | H200011747 | NM_004530 | 1.72 |
| MMP10 | H200000576 | NM_002425 | 2.14 |
Knockdown of CLDN-1 prevents cell
invasion induced by knockdown of LSR in Sawano cells
To investigate whether downregulation of CLDN-1
affected the upregulation of cell invasion induced by knockdown of
LSR in Sawano cells, the cells were transfected with siRNAs against
LSR and CLDN-1. In these cells, upregulation of CLDN-1 protein by
knockdown of LSR was inhibited, as revealed by western blotting and
immunostaining (Fig. 2B and C). In
addition, the upregulation of cell invasion induced by the
knockdown of LSR was also inhibited, whereas the knockdown of
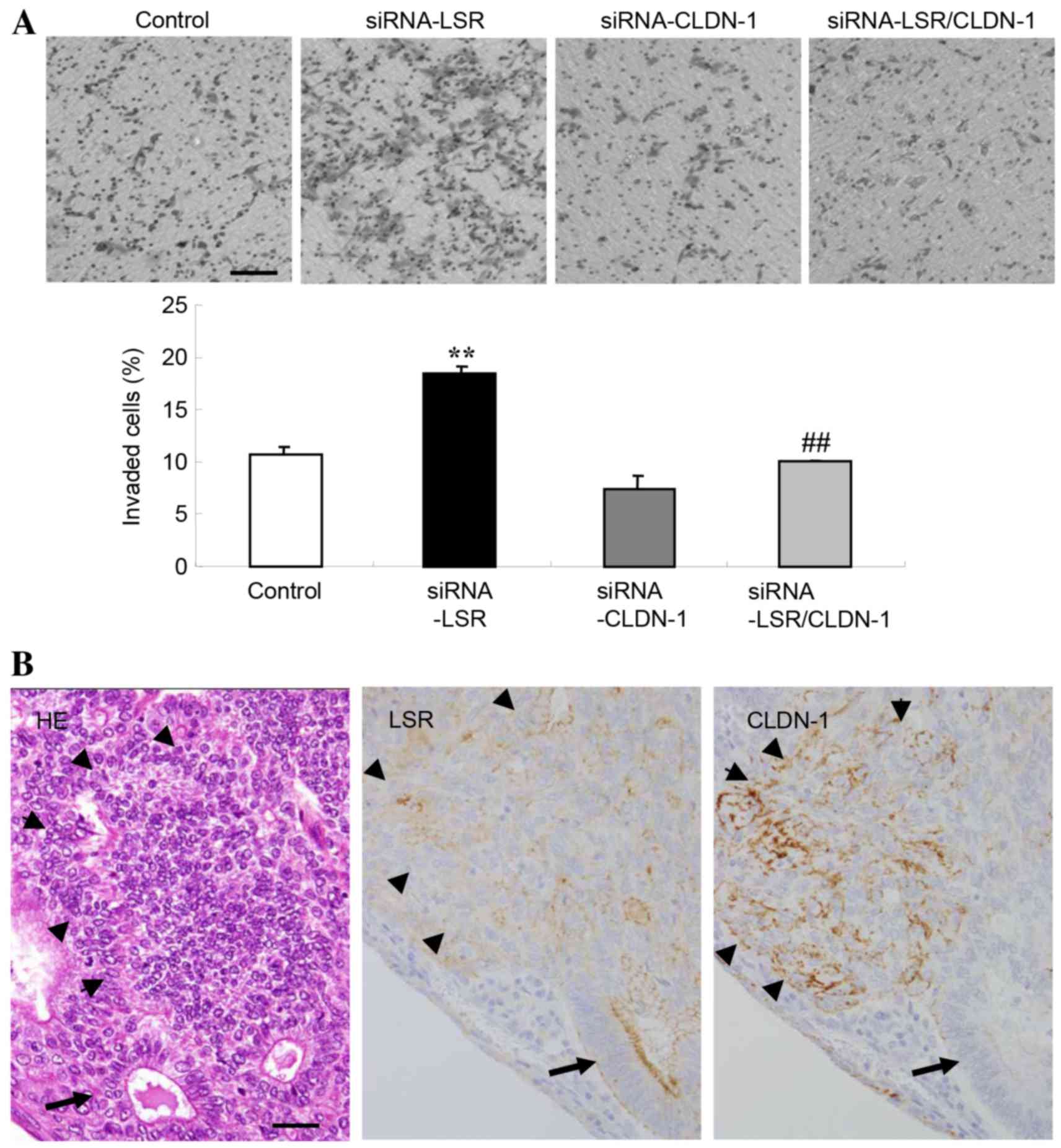
CLDN-1 alone did not affect cell invasion (Fig. 3A).
Expression of LSR and CLDN-1 on the
invasive fronts of human endometrial carcinoma tissues
The expression of LSR and CLDN-1 on the invasive
front of human endometrial carcinoma was examined using hematoxylin
and eosin and immunohistochemical staining on paraffin sections. A
decrease of LSR protein and increase of CLDN-1 protein were
observed on the invasive front of endometrial carcinoma, whereas in
the gland-like structure, LSR expression was high (Fig. 3B).
Discussion
In the present study, it was demonstrated that the
downregulation of LSR enhanced cell invasion via CLDN-1-mediated
MMPs in human endometrial cancer. LSR expression in endometrial
cancer cells was reduced; this is assumed to be associated with
poor prognosis, as a low LSR protein level was observed in the
invasive front, and a high level in the gland-like structures
(Fig. 3B). However, little is yet
known about the detailed alterations of LSR expression during the
carcinogenesis of endometrial carcinoma. A low level of the bTJ
protein CLDN-7 is associated with a late tumor stage and low
histological grade in endometrial carcinoma (7). In intrahepatic cholangiocarcinoma,
expression of the tTJ protein TRIC decreases in parallel with
dedifferentiation (19). TRIC
expression is associated with Snail-induced EMT in gastric
carcinoma cells and shows a negative correlation with the degree of
differentiation in pancreatic ductal adenocarcinomas (13,14). The
expression of the tTJ protein LSR may be associated with tumor
progression and the degree of differentiation in addition to TRIC
(16).
In the present study, the knockdown of LSR by the
siRNA in Sawano cells was observed to induce cell invasion. Loss of
the bTJ proteins OCLN and CLDNs in certain types of cancer enhances
cell invasion (6,20). Downregulation of CLDN-7 promotes cell
invasion in endometrial cancer (7).
It is reported that knockdown of LSR increases cell motility and
invasion in bladder cancer cells (15). In the present study, the detailed
mechanisms of how loss of LSR promoted cell invasion in endometrial
cancer were investigated. Knockdown of LSR induced CLDN-1
expression and Sp1 activity in the CLDN-1 promoter region in Sawano
cells. Furthermore, in LSR-knockdown Sawano cells, the mRNA levels
of MMP-1, MMP-2 and MMP-10, and the protein levels of MT1-MMP,
MMP-2, MMP-9 and MMP-10 were increased. Knockdown of CLDN-1 by
siRNA prevented the upregulation of cell invasion induced by
knockdown of LSR in the cells. CLDNs are regulated via various
transcriptional factors (21) and the
CLDN-1 promoter region contains an Sp1 binding site (18). In oral squamous cell carcinoma, CLDN-1
upregulates cancer cell invasion activity through activation of
MT1-MMP and MMP-2, which results in enhanced cleavage of laminin-5
γ2 chains (9). CLDN-1 appears to
contribute to melanoma cell invasion via increased MMP-2 secretion
and activation (10). These findings
suggest that loss of LSR signaling enhances CLDN-1 expression via
Sp1 activity and upregulation of CLDN-1 subsequently promotes cell
invasion through activation of MMPs. In the present study, it
remained unclear how loss of LSR enhanced the Sp1 activity.
Recently, the involvement of the Hippo/YAP pathway in the
development and progression of endometrial cancer was reported
(22). It is possible that the loss
of LSR may induce cell invasion via the Hippo/YAP pathway as well
as angiomotin/merlin in endometrial cancer (23).
However, in the present study, on the invasive front
of human endometrial carcinoma in vivo, a decrease of LSR
and increase of CLDN-1 were observed. These findings suggest that
downregulation of LSR may induce malignancy, including by inducing
cell invasion via upregulation of CLDN-1 in human endometrial
carcinoma in vivo.
Taken together, the results of this study suggested
a novel mechanism in which downregulation of the tTJ protein LSR
promotes cell invasion in endometrial cancer. The mechanism is
important for studying the association of tTJs with the cellular
invasion of cancer.
Acknowledgements
This study was supported by the Ministry of
Education, Culture, Sports, Science, and Technology, and the
Ministry of Health, Labour and Welfare of Japan.
References
|
1
|
Ikenouchi J, Furuse M, Furuse K, Sasaki H
and Tsukita S and Tsukita S: Tricellulin constitutes a novel
barrier at tricellular contacts of epithelial cells. J Cell Biol.
171:939–945. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Masuda S, Oda Y, Sasaki H, Ikenouchi J,
Higashi T, Akashi M, Nishi E and Furuse M: LSR defines cell corners
for tricellular tight junction formation in epithelial cells. J
Cell Sci. 124:548–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matter K and Balda MS: Signaling to and
from tight junctions. Nat Rev Mol Cell Biol. 4:225–236. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukita S, Yamazaki Y, Katsuno T, Tamura A
and Tsukita S: Tight junction-based epithelial microenvironment and
cell proliferation. Oncogene. 27:6930–6938. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin TA and Jiang WG: Loss of tight
junction barrier function and its role in cancer metastasis.
Biochim Biophys Acta. 1788:872–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin TA: The role of tight junctions in
cancer metastasis. Semin Cell Dev Biol. 36:224–231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Li Y, Qiu H and Wang Y:
Downregulation of claudin-7 potentiates cellular proliferation and
invasion in endometrial cancer. Oncol Lett. 6:101–105.
2013.PubMed/NCBI
|
|
8
|
Leech AO, Cruz RG, Hill AD and Hopkins AM:
Paradigms lost-an emerging role for over-expression of tight
junction adhesion proteins in cancer pathogenesis. Ann Transl Med.
3:1842015.PubMed/NCBI
|
|
9
|
Oku N, Sasabe E, Ueta E, Yamamoto T and
Osaki T: Tight junction protein claudin-1 enhances the invasive
activity of oral squamous cell carcinoma cells by promoting
cleavage of laminin-5 gamma2 chain via matrix metalloproteinase
(MMP)-2 and membrane-type MMP-1. Cancer Res. 66:5251–5257. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leotlela PD, Wade MS, Duray PH, Rhode MJ,
Brown HF, Rosenthal DT, Dissanayake SK, Earley R, Indig FE,
Nickoloff BJ, et al: Claudin-1 overexpression in melanoma is
regulated by PKC and contributes to melanoma cell motility.
Oncogene. 26:3846–3856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kondoh A, Takano K, Kojima T, Ohkuni T,
Kamekura R, Ogasawara N, Go M, Sawada N and Himi T: Altered
expression of claudin-1, claudin-7 and tricellulin regardless of
human papilloma virus infection in human tonsillar squamous cell
carcinoma. Acta Otolaryngol. 131:861–868. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patonai A, Erdélyi-Belle B, Korompay A,
Somorácz A, Straub BK, Schirmacher P, Kovalszky I, Lotz G, Kiss A
and Schaff Z: Claudins and tricellulin in fibrolamellar
hepatocellular carcinoma. Virchows Arch. 458:679–688. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Korompay A, Borka K, Lotz G, Somorácz A,
Törzsök P, Erdélyi-Belle B, Kenessey I, Baranyai Z, Zsoldos F,
Kupcsulik P, et al: Tricellulin expression in normal and neoplastic
human pancreas. Histopathology. 60:E76–E86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Masuda R, Semba S, Mizuuchi E, Yanagihara
K and Yokozaki H: Negative regulation of the tight junction protein
tricellulin by snail-induced epithelial-mesenchymal transition in
gastric carcinoma cells. Pathobiology. 77:106–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herbsleb M, Birkenkamp-Demtroder K,
Thykjaer T, Wiuf C, Hein AM, Orntoft TF and Dyrskjøt L: Increased
cell motility and invasion upon knockdown of lipolysis stimulated
lipoprotein receptor (LSR) in SW780 bladder cancer cells. BMC Med
Genomics. 1:312008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reaves DK, Fagan-Solis KD, Dunphy K,
Oliver SD, Scott DW and Fleming JM: The role of lipolysis
stimulated lipoprotein receptor in breast cancer and directing
breast cancer cell behavior. PLoS One. 9:e917472014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of tumours of Female Reproductive
Organs. 4th. Lyon, France: International Agency for Research on
Cancer; 2014
|
|
18
|
Wang HB, Wang PY, Wang X, Wan YL and Liu
YC: Butyrate enhances intestinal epithelial barrier function via
up-regulation of tight junction protein Claudin-1 transcription.
Dig Dis Sci. 57:3126–3135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Somorácz A, Korompay A, Törzsök P, Patonai
A, Erdélyi-Belle B, Lotz G, Schaff Z and Kiss A: Tricellulin
expression and its prognostic significance in primary liver
carcinomas. Pathol Oncol Res. 20:755–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Süren D, Yıldırım M, Kaya V, Alikanoğlu
AS, Bülbüller N, Yıldız M and Sezer C: Loss of tight junction
proteins (Claudin 1, 4 and 7) correlates with aggressive behavior
in colorectal carcinoma. Med Sci Monit. 20:1255–1262. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khan N and Asif AR: Transcriptional
regulators of claudins in epithelial tight junctions. Mediators
Inflamm. 2015:2198432015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Romero-Pérez L, Garcia-Sanz P, Mota A,
Leskelä S, Hergueta-Redondo M, Díaz-Martín J, López-García MA,
Castilla MA, Martínez-Ramírez A, Soslow RA, et al: A role for the
transducer of the Hippo pathway, TAZ, in the development of
aggressive types of endometrial cancer. Mod Pathol. 28:1492–1503.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moleirinho S, Guerrant W and Kissil JL:
The Angiomotins-from discovery to function. FEBS Lett.
588:2693–2703. 2014. View Article : Google Scholar : PubMed/NCBI
|