Introduction
CCR9 is a member of the family of G-protein-coupled
receptors (GPCRs) and serves a role in T-cell differentiation and
tissue-specific homing once bound to its cognate ligand chemokine
(C-C motif) ligand 25 (CCL25) (1).
CCR9 has also been implicated in numerous types of cancer including
breast, lung, pancreas, prostate and colon cancer, in addition to
adult T-cell leukemia; it is also reported that CCR9 mediates
organ-selective metastasis and drug resistance (2–7). The
functions of CCR9 in metastasis and drug resistance depend on the
activation of the phosphoinositide 3-kinase (PI3K)/protein kinase B
(Akt) signaling pathway (8).
Furthermore, the differences in the cell-surface profile between
cancer cells and their normal counterparts may be used as a
molecular signature for targeted therapy and drug delivery. Thus,
CCR9 is a promising candidate for a target molecule based on its
rare cell-surface expression in normal tissues and increased
cell-surface expression in malignancies.
An immunotoxin CCL25-PE38 has previously been
developed that is able to specifically kill high CCR9-expressing
MOLT4 cells (9). However, several
issues remain unresolved, including uptake and expression of
CCL25-PE38. Peptides exhibit a short plasma half-life, increased
tissue penetration ability, decreased immunogenicity, and are
easily synthesized in comparison with macromolecular proteins
(10).
Phage display is a molecular technology that permits
the presentation of a number of peptides with high specificity and
affinity for a target on the surface of a bacteriophage. For
instance, the biopanning of phage display libraries on cellular
receptors has been successful in selecting peptides that exhibit
high affinity and specificity for the receptors (11–13).
Therefore, peptides screened from phage display may be useful for
the diagnosis and targeted therapy of tumors.
In the present study, the second extracellular loop
of CCR9 was used to screen a 12-mer phage display library and a
novel peptide (P1; VHWDFRQWWQPS) that was capable of specifically
binding to CCR9 was identified. Furthermore, this peptide promoted
the apoptosis of MOLT4 cells induced by doxorubicin (DOX) and
inhibited the migration of MOLT4 cells in the presence of CCL25.
The inhibitory effects may be associated with inhibition of the
activation of Akt in MOLT4 cells. In conclusion, the novel peptide
identified in the present study may inhibit the activity of CCR9
and is potentially useful in the research of novel treatments for
CCR9-overexpressed carcinoma.
Materials and methods
Affinity screening procedure
The Ph.D.-12 phage display library (version 1.1; New
England BioLabs, Ipswich, MA, USA) was used as the biopanning tool.
The panning procedure was performed according to the manufacturer's
protocol. Briefly, the second extracellular loop of CCR9
(AAADQWKFQTFMCKVVNSMYK; SBS Genetech Co., Ltd., Beijing, China) was
used to coat microtiter plates at 10 µg/ml. Phages that
specifically bound to the target were identified through three
rounds of in vitro selection. In each round, the bound
phages were rescued and amplified in ER2738 bacterial cells from
the phage display library (New England BioLabs, Inc., Ipswich, MA,
USA) for the subsequent panning, whereas the unbound phages were
removed by washing with Tris-buffered saline with Tween-20 (TBST).
The washing conditions for the second and third rounds were the
same as those for the first round, with the exception that the
Tween-20 concentration was increased from 0.1 to 0.5%,
respectively. Following the elution step in the third round, the
single clones of phage particles were harvested. The phages were
subjected to DNA extraction, sequencing and an ELISA.
DNA sequencing of the selected
phages
Following the third round of panning, 16 phage
clones were randomly selected, amplified and purified. The clones
were designated C-1 to C-16. Single-stranded DNA was extracted with
M13 DNA Kit (Beijing BLKW Biotechnology Co., Ltd., Beijing, China)
according to the manufacturer's protocol. DNA sequencing was
performed by Sangon Biotech Co., Ltd. (Shanghai, China) using the
−96gIII primer (5′-CCCTCATAGTTAGCGTAACG-3′). Sequence analysis was
performed and phages with the same sequence were classified.
Phage capture ELISA
A 10-µg/ml sample of the second extracellular loop
of CCR9 in 0.1 M NaHCO3, pH 8.6, was coated on ELISA
plates. The ELISA plates were incubated overnight at 4°C in an
air-tight humidified box. The excess solution was removed and the
plates were blocked with a blocking buffer (0.1 M NaHCO3
(pH 8.6), 5 mg/ml BSA) for 1 h at 4°C. Subsequently, the plates
were washed and phages including control phage, C-4, C-6, C-9 and
C-10 [at 1011 plaque-forming units (pfu)] were added to
the target-coated wells. The plates were incubated at room
temperature for 1 h with agitation. Finally, a horseradish
peroxidase-conjugated anti-M13 monoclonal antibody at 5 µg/ml (cat.
no. 111973-MM05; Sino Biological, Inc., Beijing, China) was used to
detect the bound phages. Data are reported from three independent
experiments.
Peptide synthesis and labeling
The candidate peptide P1 (VHWDFRQWWQPS) and
unrelated peptide (control P; WIRPPSGPMYSF) were synthesized by SBS
Genetech, Co., Ltd. and Sangon Biotech Co., Ltd., respectively,
using standard solid-phase chemistry. Fluorescein isothiocyanate
(FITC) was conjugated to the N-terminus of P1 (FITC-P1) or the
unrelated peptide (FITC-control P). The products were purified to a
minimum purity of 95% using high-performance liquid chromatography
and isolated by lyophilization. The sequence and structure of each
peptide were characterized by mass spectrometry.
Competitive inhibition assay
To investigate the inhibition effect of P1 on C-4, a
competitive inhibition ELISA was conducted. Briefly, wells were
incubated with 10 µg/ml of the second extracellular loop of CCR9.
P1 at various concentrations (0, 0.01, 0.1, 0.5, 1.0, 2.0 and 10
nmol/ml) was added to the cells. Subsequently, 1011 pfu
C-4 was added to the wells prior to incubation at 37°C for 1 h. The
subsequent procedure was similar to that of the ELISA performed for
the aforementioned phage capture. The wells were washed with 1× PBS
(pH 7.4) 3 times following incubation. Finally, a horseradish
peroxidase-conjugated anti-M13 monoclonal antibody at 10 µg/ml
(cat. no. 111973-MM05; Sino Biological, Inc.) was used to detect
the bound phages. The rate of inhibition was calculated using the
following formula: Rate of inhibition=[optical density (OD) of
control P-OD of P1]/OD of control P × 100%. An unrelated peptide
was used as the control P. Data are reported from three independent
experiments.
Confocal microscopy analysis
MOLT4 cells were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA). Cells were maintained
in RPMI-1640 medium (GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (GE Healthcare Life
Sciences), 100 U/ml penicillin and 100 mg/ml streptomycin. The
cells were cultured at 37°C in a humidified atmosphere containing
5% CO2. The MOLT4 cells were washed three times with
PBS. One group was washed three times with PBS and blocked with 2%
bovine serum albumin (BSA; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) at 4°C for 30 min and FITC-P1 (0.1 nmol/ml) was incubated
with the cells for 1 h at 4°C. Meanwhile, another group was
incubated with anti-CCR9 monoclonal antibody at 0.1 nmol/ml
(anti-CCR9 mAb; cat. no. 112509; R&D Systems, Inc.,
Minneapolis, MN, USA) for 30 min at 37°C, blocked with 2% BSA and
subsequently incubated with FITC-P1 at 4°C as above. Following
three washes, the cells were placed on poly-L-lysine-coated slides
and observed using a laser-scanning confocal microscope with the
magnification, ×200 (LSM710; Zeiss GmbH, Jena, Germany).
FITC-control P was used as the control.
Apoptosis assay
MOLT4 cells (4×105/well) were transferred
to 24-well plates with each well containing 500 µl RPMI-1640 medium
without FBS. Each well was treated with a distinct concentration of
P1 (0, 0.1 and 1.0 nmol/ml) for 12 h, and then treated with DOX (1
µg/ml) for 12 h. The control groups were treated with various
concentrations of P1 (0, 0.1 and 1.0 nmol/ml) for 24 h. The cells
were washed twice, and subsequently detected using an Annexin
V-FITC and propidium iodide (PI) double staining kit (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 15 min at
25°C in the dark, according to the manufacturer's protocol.
Subsequently, the processed cells were rinsed with PBS and
subjected to fluorescence-activated cell sorting (FACS) analysis.
RBL-2H3 cells (ATCC, Manassas, VA, USA), were incubated with
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.)
at 4×105 cells/well. The subsequent procedure was the
same as that for the MOLT4 cells. Results were presented as the
percentage of Annexin V-FITC-positive cells, Annexin V-FITC- and
PI-double-positive cells. Data are reported from three independent
experiments.
Chemotaxis assay
The chemotaxis assay was performed in 24-well
Transwell chambers (Corning Life Sciences, Cambridge, MA, USA) as
previously described (14). MOLT4
cells (0.5×104 cells/well) were treated with P1 (0, 0.1
and 1.0 nmol/ml) and control P (0, 0.1 and 1.0 nmol/ml) for 1 h,
prior to being added to the upper chamber. CCL25 (0.1 nmol/ml) was
placed in the lower chamber (500 µl). Following incubation at 37°C
in 5% CO2 for 2 h, cells were collected from the lower
wells. The number of cells was counted using light microscopy.
Spontaneous baseline migration (BM) was used as a control. The
experiments were performed in triplicate.
Western blotting
To investigate the expression levels of Akt and
phospho-Akt (p-Akt) in MOLT4 cells in the presence of P1, MOLT4
cells incubated in 24-well plates were serum-starved for 12 h and
then treated with P1 at various concentrations (0, 0.1 and 1.0
nmol/ml) for 24 h. The protein extraction procedure was performed
as described previously (15). The
nitrocellulose membranes were incubated overnight at 4°C with
anti-p-Akt (Ser473) at 1:100 (cat. no. 9271; Cell
Signaling Technology, Danvers, MA, USA) or anti-Akt monoclonal
antibody at 1:100 (cat. no. 9272, Cell Signaling Technology) and
incubated with horseradish peroxidase-conjugated secondary antibody
at 1:2,000 (cat. no. D110058-0100; Sangon Biotech Co., Ltd.).
β-actin was used as the control. Finally, the membrane-bound
proteins were detected using enhanced chemiluminescence substrate
(Pierce ECL; cat. no. 32209, Thermo Fisher Scientific, Inc.). The
blots were incubated with the ECL working solution for 1 min at
room temperature, and then removed and placed in a film cassette
with the protein side facing up in the dark for X-ray film
exposure. The film was developed using the developing solution
(cat. no., AR0132; BOSTER Biological Technology Co., Ltd, Wuhan,
China) for 2 min and fixative solution (cat. no. AR0132; BOSTER
Biological Technology Co., Ltd, Wuhan, China) for 1 min at room
temperature following exposure of 60 sec. The experiments were
performed in triplicate.
Statistical analysis
The data are expressed as the mean ± standard
deviation or a percentage. Data analysis was performed using
GraphPad Prism (Version 5; GraphPad Software, Inc., La Jolla, CA,
USA). One-way analysis of variance and Student's t-test were used
to perform statistical comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Screening of the Ph.D.-12 phage
display library against CCR9
To obtain a specific peptide binding to the CCR9, a
Ph.D.-12 phage display library was screened for binding to the
second extracellular loop of CCR9. Following three rounds of
biopanning, the titers of phages increased ~79-fold compared with
the titer in the first round. The output/input ratio of phages
following each round of panning was used to determine the
enrichment efficiency, which increased from 5.2×10−6 to
2.4×10−4 (Table I). This
increase indicated that phages capable of specifically binding to
the second extracellular loop of CCR9 were significantly enriched
following the biopanning.
 | Table I.Enrichment of phages screened from the
Ph.D.-12 library. |
Table I.
Enrichment of phages screened from the
Ph.D.-12 library.
|
|
| Titer, pfu |
|---|
|
|
|
|
|---|
| Round | Second extracellular
loop of CCR9, µg | Input | Output | Recovery |
|---|
| First | 10 |
1.0×1011 |
5.2×105 |
5.2×10−6 |
| Second | 5 |
1.0×1011 |
3.6×106 |
3.3×10−5 |
| Third | 2 |
1.2×109 |
4.9×105 |
4.1×10−4 |
DNA sequencing of the selected phage
clones
The results demonstrated that 8 phage clones lacked
an exogenous sequence and the remaining 8 clones were confirmed to
be positive using DNA sequencing. A total of 8 phage clones and
corresponding peptide sequences were obtained (Table II). Sequence alignment analysis did
not identify homology among the peptide sequences.
 | Table II.DNA sequences of the selected phage
clones. |
Table II.
DNA sequences of the selected phage
clones.
| Phage clone | DNA sequence | Peptide sequence | Frequency |
|---|
| C-1/−2/−4/13/−14 |
GTGCATTGGGATTTTCGG | VHWDFRQWWQPS | 5 |
|
|
CAGTGGTGGCAGCCTTCT |
|
|
| C-6 |
GGGGATGGTAATTCGGTG | GDGNSVLKPGNW | 1 |
|
|
CTGAAGCCGGGGAATTGG |
|
|
| C-9 |
CATCTTAGTCTTCCGCTTTG | HLSLPLWKWEKS | 1 |
|
| GAAGTGGGAGAAGTCG |
|
|
| C-10 |
GTGTTTGCTTATCATCTT | VFAYHLRIPSGD | 1 |
|
|
AGGATTCCTTCTGGGGAT |
|
|
Phage-binding assay
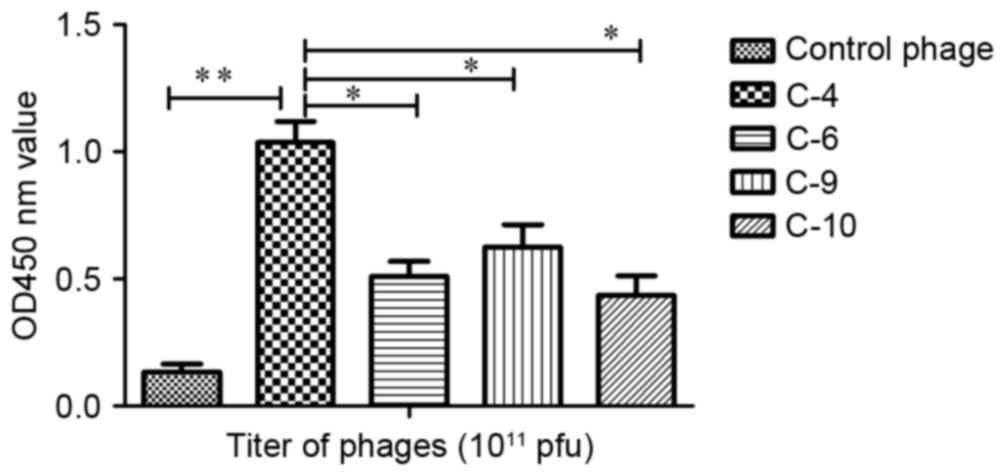
An ELISA was performed to determine the affinity and
specificity of positive phage clones. The results revealed that the
phage C-4 appeared to exhibit increased affinity for the target
molecule in comparison with the other phages (Fig. 1). The affinity of C-4 increased
>8-fold compared with the control phage, a statistically
significant increase (P<0.01). It also identified a significant
difference between group C-4 and group C-6 (P<0.05), C-9
(P<0.05) and C-10 (P<0.05). Therefore, the displaying peptide
(VHWDFRQWWQPS) derived from C-4 was designated P1 and was
investigated further.
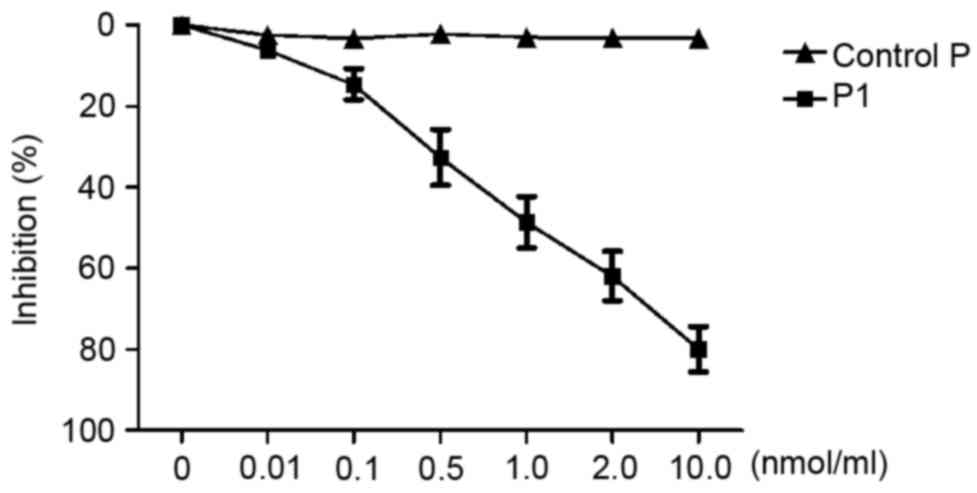
Competitive inhibition assay
To investigate whether the synthetic peptide P1 was
able to inhibit the corresponding phage binding to the target
molecule, competitive inhibition ELISA was performed. As presented
in Fig. 2, C-4 binding to the target
decreased following an increase in peptide P1. The peptide P1
appeared to inhibit C-4 binding to the second extracellular loop of
CCR9 in a dose-dependent manner. When the concentration of P1
reached 1.0 nmol/ml, C-4 binding to the second extracellular loop
of CCR9 was significantly inhibited (inhibition of 50.33%).
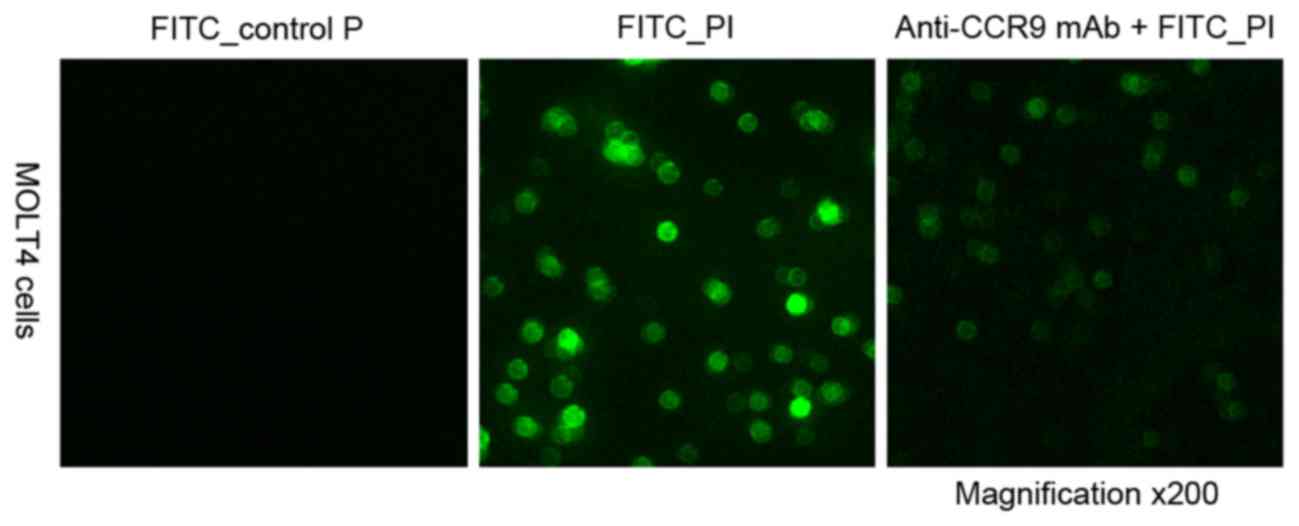
Identification of P1 binding
specifically to CCR9 high-expressing cells
To investigate the peptide specifically binding to
CCR9, confocal microscopy analysis was performed to evaluate the
binding specificity of the peptide P1. It was revealed that FITC-P1
bound directly to MOLT4 cells, and the anti-CCR9 mAb was able to
completely block the binding of P1. When a control peptide was
incubated with MOLT4 cells, no cell binding was observed (Fig. 3). This suggests that P1 is able to
specifically bind to CCR9 and is stable under physiological
conditions.
P1 increases apoptosis of MOLT4 cells
induced by DOX
To investigate the effect of P1 on cell apoptosis,
the number of apoptotic cells was measured using FACS following P1
and DOX treatments of MOLT4 cells and RBL-2H3 cells. As presented
in Table III, the number of
apoptotic MOLT4 cells induced by DOX increased to 64.7% following
pre-treatment with 0.1 nmol/ml P1 (P<0.05); apoptotic MOLT4
cells further increased to 79.5% following pre-treatment with 1.0
nmol/ml P1 (P<0.01). On the other hand, the number of apoptotic
RBL-2H3 cells induced by DOX revealed no significant difference
between cells treated with P1 and those receiving no treatment.
These results suggest that P1 may elicit its effect on MOLT4 cells
by promoting an apoptotic pathway.
 | Table III.Effect of P1 on apoptosis induced by
DOX. |
Table III.
Effect of P1 on apoptosis induced by
DOX.
| A, MOLT4 cells |
|---|
|
|---|
|
| P1, nmol/ml |
|---|
|
|
|
|---|
| Treatment | 0 | 0.1 | 1.0 |
|---|
| Control | 5.3±0.6 | 4.8±0.5 | 6.1±1.0 |
| DOX | 52.4±2.5 | 64.7±3.1a | 79.5±4.2b |
|
| B, RBL-2H3
cells |
|
|
| P1,
nmol/ml |
|
|
|
| Treatment | 0 | 0.1 | 1.0 |
|
| Control | 4.3±0.5 | 6.6±0.8 | 5.9±0.4 |
| DOX | 50.1±3.0 | 52.3±2.6 | 51.5±2.9 |
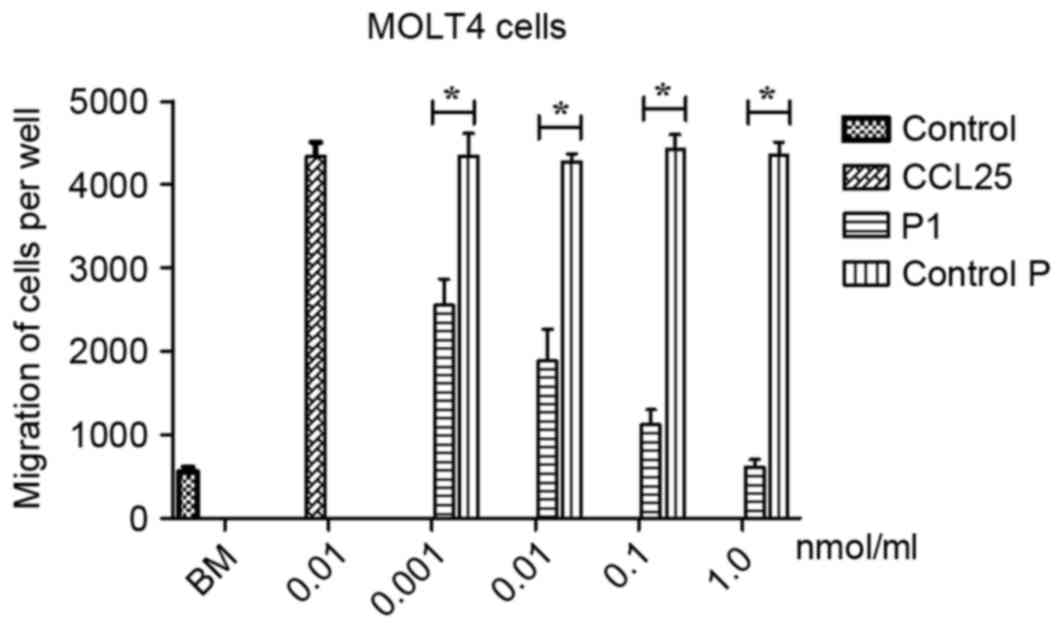
P1 inhibits migration of MOLT4
cells
To evaluate the effects of P1 on migration of MOLT4
cells, a Boyden chamber migration assay was performed. As presented
in Fig. 4, P1 significantly inhibited
the migration of CCR9-expressing MOLT4 cells induced by CCL25 in a
dose-dependent manner. No statistical comparison between the
different doses was performed. In contrast, the control P did not
affect the migration of MOLT4 cells induced by CCL25. A
statistically significant difference between P1 and group control P
was identified (P<0.05), revealing that P1 may antagonize the
effect of CCL25.
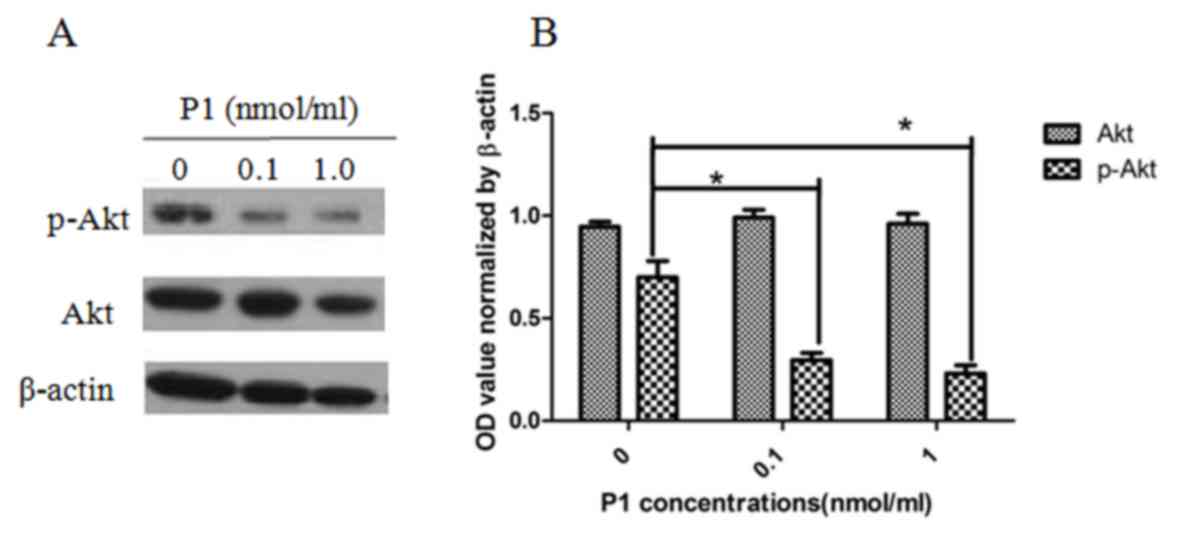
P1 inhibits the phosphorylation of Akt
in MOLT4 cells
As presented in Fig.
5, P1 caused a detectable inhibitory effect on the expression
of p-Akt in MOLT4 cells. The level of p-Akt was significantly
different when compared with Akt as a control. Each dose of P1 was
also significantly different from the subsequent dose, indicating
that the effect of P1 is dose-dependent (P<0.05). This result
implies that P1 may block the downstream CCR9 signaling
pathway.
Discussion
Phage display has been proved to be an effective
approach for screening the interaction of molecules, and to develop
novel agents for the diagnosis and treatment of numerous types of
cancer (16). In the present study,
the second loop of CCR9 was used as the target molecule to screen
the Ph.D.-12 phage display library for the first time, to the best
of our knowledge. Following three rounds of biopanning, 8 positive
phage clones expressing exogenous sequences were obtained. Of these
positive clones, C-4 appeared five times and exhibited increased
affinity compared with other phages detected by ELISA. Furthermore,
the results revealed that P1 (VHWDFRQWWQPS) derived from C-4
exhibits specificity towards and affinity for the second
extracellular loop of CCR9.
To investigate the ability of P1 binding to cells
under physiological conditions, a fluorescently labeled peptide
(FITC-P1) was synthesized and confocal microscopy analysis was
performed. It was demonstrated that FITC-P1 may bind specifically
to MOLT4 cells through CCR9. These results suggest that P1 may
specifically bind to the second loop of CCR9.
CCR9 may serve a role in the drug resistance and
metastasis of tumors. Furthermore, CCR9 may increase migration and
invasion of tumor cells, and activate of PI3K/Akt signaling pathway
in order to inhibit the apoptosis of tumor cells as induced by
chemotherapeutic agents (5,8). Experiments were performed to determine
the effect of P1 on CCR9-overexpressing cells and the
phosphorylation of Akt. As expected, the results indicate that P1
may increase the apoptosis of MOLT4 cells as induced by
chemotherapeutic agents. Furthermore, P1 may inhibit the migration
of CCR9-expressing MOLT4 cells induced by CCL25. Furthermore, it
was revealed that P1 may significantly decrease phosphorylation of
Akt. The results of the present study identified that P1 may have
an effect on CCR9-overexpressing cells, increasing their
susceptibility to chemotherapy and decreasing chemotaxis.
In conclusion, a novel peptide, P1 (VHWDFRQWWQPS),
was developed which may inhibit the activity of CCR9. P1 may
possess potential as a novel treatment for types of cancer which
overexpress CCR9. However, further investigation into the use of P1
as an inhibitor for carcinoma, including the plasma half-life,
tissue penetration ability, immunogenicity and in vivo
antitumor effect is required.
Acknowledgements
This study was supported, in part, by the Natural
Science Foundation of Fujian Province, China (grant no. 2013D012)
and National Science Foundation of China (grant no. 81371902). The
authors thank Mr. Fuyi Huang (Institute of Urban Environment,
Chinese Academy of Sciences, Xiamen, China) for support with
technology.
References
|
1
|
Zlotnik A and Yoshie O: Chemokines: A new
classification system and their role in immunity. Immunity.
12:121–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nagakubo D, Jin Z, Hieshima K, Nakayama T,
Shirakawa AK, Tanaka Y, Hasegawa H, Hayashi T, Tsukasaki K, Yamada
Y, et al: Expression of CCR9 in HTLV-1+T cells and ATL cells
expressing tax. Int J Cancer. 120:1591–1597. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heinrich EL, Arrington AK, Ko ME, Luu C,
Lee W, Lu J and Kim J: Paracrine activation of chemokine receptor
CCR9 enhances the invasiveness of pancreatic cancer cells. Cancer
Microenviron. 6:241–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta P, Sharma PK, Mir H, Singh R, Singh
N, Kloecker GH, Lillard JW Jr and Singh S: CCR9/CCL25 expression in
non-small cell lung cancer correlates with aggressive disease and
mediates key steps of metastasis. Oncotarget. 5:10170–10179. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma PK, Singh R, Novakovic KR, Eaton
JW, Grizzle WE and Singh S: CCR9 mediates PI3K/AKT-dependent
antiapoptotic signals in prostate cancer cells and inhibition of
CCR9-CCL25 interaction enhances the cytotoxic effects of etoposide.
Int J Cancer. 127:2020–2030. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Singh S, Singh UP, Stiles JK, Grizzle WE
and Lillard JW Jr: Expression and functional role of CCR9 in
prostate cancer cell migration and invasion. Clin Cancer Res.
10:8743–8750. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen HJ, Edwards R, Tucci S, Bu P, Milsom
J, Lee S, Edelmann W, Gumus ZH, Shen X and Lipkin S: Chemokine
25-induced signaling suppresses colon cancer invasion and
metastasis. J Clin Invest. 122:3184–3196. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tu Z, Xiao R, Xiong J, Tembo KM, Deng X,
Xiong M, Liu P, Wang M and Zhang Q: CCR9 in cancer: Oncogenic role
and therapeutic targeting. J Hematol Oncol. 9:102016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Y, Zhang L, Wu R, Han R, Jia Y, Jiang
Z, Cheng M, Gan J, Tao X and Zhang Q: Specific killing of CCR9
high-expressing acute T lymphocytic leukemia cells by CCL25 fused
with PE38 toxin. Leuk Res. 35:1254–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brasnjevic I, Steinbusch HW, Schmitz C and
Martinez-Martinez P: European NanoBioPharmaceutics research
initiative: Delivery of peptide and protein drugs over the
blood-brain barrier. Prog Neurobiol. 87:212–251. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang FY, Zhang TY, Luo JX, He GA, Gu QL
and Xiao F: Selection of CC chemokine receptor 5-binding peptide
from a phage display peptide library. Biosci Biotechnol Biochem.
70:2035–2041. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Skelton NJ, Chen YM, Dubree N, Quan C,
Jackson DY, Cochran A, Zobel K, Deshayes K, Baca M, Pisabarro MT,
et al: Structure-function analysis of a phage display-derived
peptide that binds to insulin-like growth factor binding protein 1.
Biochemistry. 40:8487–8498. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang B, Li Z, Huang D, Zheng L and Li Q:
Screening of a specific peptide binding to VPAC1 receptor from a
phage display peptide library. PLoS One. 8:e542642013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Houimel M and Mazzucchelli L:
Identification of biologically active peptides that inhibit binding
of human CXCL8 to its receptors from a random phage-epitope
library. J Leukoc Biol. 85:728–738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Wang Z, Zhong Y, Lan J, Li X and Lin
H: CCR9-CCL25 interaction suppresses apoptosis of lung cancer cells
by activating the PI3K/Akt pathway. Med Oncol. 32:662015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Yin G, Yan D, Wei Y, Ma C, Huang
Z, Liao X, Yao Y, Chen X and Hao B: In vitro screening of ovarian
tumor specific peptides from a phage display peptide library.
Biotechnol Lett. 33:1729–1735. 2011. View Article : Google Scholar : PubMed/NCBI
|