Introduction
Despite both diagnostic and therapeutic advances,
such as combining surgery, radiotherapy and chemotherapy, the
5-year survival rate of oral squamous cell carcinoma (OSCC) remains
between 70–80 % (1–3). This is primarily due to recurrences and
secondary metastases to cervical lymph nodes. Furthermore, it is
difficult to detect these recurrences and the metastases,
postoperatively, thus patients receive significant post-operative
monitoring. As early detection of recurrences and secondary
metastases in the cervical lymph node is key to good prognosis and
low morbidity in the follow-up period, reliable prognostic OSCC
biomarkers are needed. To date, there are no specific biomarkers
that can be used to post-operatively monitor OSCC patients.
The failure to reduce morbidity in OSCC patients
most likely results from the early dissemination of tumor cells
into bone marrow or peripheral blood (4–7), which is
usually missed by conventional staging procedures at the time of
surgery and leads to recurrences and metastases. Adjuvant therapy
can eradicate occult disseminated tumor cells (DTCs) before
recurrences or metastases become clinically evident; thus, there is
an urgent need to develop strategies to detect DTCs and enable
clinicians to identify patients who would benefit from systemic
treatments (8–12). A few studies have used RT-PCR-based
techniques to detect epithelial cell-specific mRNAs (13–16) and
have revealed the presence of circulating epithelial cells,
supposedly derived from the primary tumor, in the peripheral blood
samples from OSCC patients.
Cytokeratins (CKs) are intermediate filaments of the
cytoskeleton that are overexpressed in OSCC compared with normal
mucosa (17), and thus, are candidate
prognostic markers for OSCC. Recently, CK17 was identified as a
diagnostic marker for primary OSCC (18,19) and as
a specific immunohistochemical marker in SCC of the larynx as well
as breast and cervical carcinomas (20–22). The
presence of CK19 mRNA in tissues by real-time RT-PCR may be a
prognostic marker for OSCC (23).
Furthermore, CK19 mRNA and CK20 mRNA have been extensively
investigated as means of detecting DTCs in the peripheral blood and
bone marrow of breast, colorectal and pancreatic carcinoma patients
(8,24–26).
Moreover, CK20 expression in primary OSCC tissues was associated
with the occurrence of metastases to neck lymph nodes (19,27), and
in poorly differentiated adenocarcinoma, CK20 expression is closely
associated with invasive histological features and has prognostic
value (28).
Although several studies have examined CKs as OSCC
diagnostic markers, few studies have investigated them as
prognostic markers for OSCC. During the follow-up period, it is
easy to harvest peripheral blood from OSCC patients, and our
hypothesis is that CKs in the peripheral blood may predict
recurrences and metastases. The aim of this study was to determine
the relative expression of CK17 mRNA, CK19 mRNA and CK20 mRNA in
the pre- and post-operative PBMC of OSCC patients using real-time
RT-PCR to ascertain whether these CKs mRNA could be suitable
prognostic markers.
Materials and methods
Patients and sample collection
This study comprised PBMC samples from 19 OSCC
patients of different TNM classification and stages and from five
otherwise healthy volunteers acting as controls for the real-time
RT-PCR. The study was approved by the local Ethical Committee of
Kyushu University Hospital and patients gave informed consent to
Kyushu University Hospital prior to inclusion.
Peripheral blood was obtained pre- and
post-operatively. Pre-operative samples were obtained before any
treatments. And post-operative samples were obtained 1 month after
surgery, because surgical invasion has calmed down. And recurrence
and metastasis are often found a few months after surgery, so it is
important to predict in 1 month after surgery. After discarding the
first 5 ml of blood to avoid contamination with epidermal cells, 16
ml of blood was collected with heparin and diluted with 20 ml of
phosphate-buffered saline. Blood constituents were separated by
density centrifugation with Ficoll-Paque Plus (Amersham; GE
Healthcare, Chicago, IL, USA) and stored at −80°C in TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
All patients were clinically examined and staged
according to the TNM and UICC classifications (29,30).
Differentiation grades were classified according to the WHO
(31). The mode of invasion was
determined on hematoxylin and eosin-stained specimens according to
the Yamamoto-Kohama criteria, as follows: Grade 1, well-defined
borderline; grade 2, cords, less-marked borderline; grade 3, group
of cells, no distinct borderline; and grade 4, diffuse invasion
with 4C: Cord-like type or 4D: Widespread type (32). Clinical information including age,
gender, TNM classification, clinical stage, differentiation grade
and disease-free endpoints were reviewed from the records of the
Department of Oral and Maxillofacial Surgery, Kyushu University
Hospital. Tumor recurrence was excluded by chest X-ray, bone scan
and abdominal and pelvic ultrasound. Patient survival was evaluated
within a maximum period of 50 months (range, 14–50 months; median,
38.6 months). The good-prognosis group comprised patients
completely cancer-free after surgery, while the poor-prognosis
group comprised patients with local recurrences or regional lymph
node metastases.
Detecting CK mRNAs by real-time
RT-PCR
Total PBMC RNA was extracted using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After 1-h DNase treatment at 37°C,
isolated total RNA was cleaned with the RNeasy kit (Qiagen GmbH,
Hilden, Germany), and cDNAs were synthesized using the High
Capacity cDNA Archive kit (4322171; Applied Biosystems; Thermo
Fisher Scientific, Inc.). Real-time RT-PCR analyses for CK17 mRNA,
CK19 mRNA, CK20 mRNA and GAPDH mRNA (normalization) were performed
using QuantiTect Primer Assay 200 (Hs KRT17 1 SG; QT00001680,
QT00081137, QT00014784 and QT00079247, respectively; Qiagen GmbH).
The relative quantification (RQ) of mRNA was performed with the ABI
Prism 7300 Sequence Detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). QuantiTect SYBR-Green PCR kit (204143;
Qiagen GmbH) was used for PCR amplification. In total, 40 ng of
cDNA was used for each PCR reaction in a total volume of 20 µl.
Each PCR reaction included a 15-min activation time at 95°C and a
three-step cycle including 94°C for 15 sec, 55°C for 30 sec and
72°C for 34 sec. The formation of undesired byproducts was assessed
by melting curve analysis after PCR. CK17 mRNA, CK19 mRNA and CK20
mRNA quantities were analyzed in duplicate, normalized against
GAPDH mRNA as an internal control and expressed in relation to mRNA
isolated from PBMCs from a healthy volunteer as a means of
calibrating the data. Relative gene expression was determined using
the ΔΔCt method (33). mRNA isolated
from the head and neck squamous cell carcinoma cell line HSC-2 was
used as a positive control.
Statistical analyses
All statistical analyses were performed using JMP 11
(SAS Institute, Inc., Cary, NC, USA). The mean value of duplicate
CK mRNA RQs was defined as positive if it was higher than 0.5-fold.
Chi-square tests or log-rank tests were used to assess correlations
between CKs mRNA expression and clinicopathological parameters. Cox
proportional hazard models were used to examine between survival
rates and clinicopathological parameters. Hazard ratios (HRs) and
95% confidence intervals (95% CIs) were estimated. Kaplan-Meier
analysis was used to evaluate the follow-up data. P-values <0.05
were considered significant.
Results
Among the included patients, there were 13 at stage
T2, one at T3 and five at T4, and 12 at N0 and seven at N1 or N2
according to TNM classification. According to the UICC for the
clinical staging of oral cancer, nine patients were at stage II,
two at stage III and eight at stage IV; furthermore, 12 patients
had well differentiated OSCC and seven had moderately
differentiated OSCC (Table I).
 | Table I.Association between CK17, CK19, CK20
mRNA detection in PBMCs of patients before surgery and
clinicopathological parameters. |
Table I.
Association between CK17, CK19, CK20
mRNA detection in PBMCs of patients before surgery and
clinicopathological parameters.
|
|
| CK17a | CK19a | CK20a |
|---|
|
|
|
|
|
|
|---|
| Variables | Casesb (%) | + | − | P-values | + | − | P-values | + | − | P-values |
|---|
| Gender |
|
|
|
|
|
|
|
|
|
|
|
Male | 15 (78.9) | 15 | 0 |
| 11 | 4 |
| 12 | 3 |
|
|
Female | 4 (21.1) | 4 | 0 | N.S. | 4 | 0 | N.S. | 4 | 0 | N.S. |
| Age (years) |
|
|
|
|
|
|
|
|
|
|
|
65> | 10 (52.6) | 10 | 0 |
| 9 | 1 |
| 10 | 0 |
|
|
65< | 9 (47.4) | 9 | 0 | N.S. | 6 | 3 | N.S. | 6 | 3 | N.S. |
|
Differentiationc |
|
|
|
|
|
|
|
|
|
|
|
Well | 12 (63.2) | 12 | 0 |
| 10 | 2 |
| 10 | 2 |
|
|
Moderate | 7 (36.8) | 7 | 0 |
| 5 | 2 |
| 6 | 1 |
|
|
Poor | 0 (0) | 0 | 0 | N.S. | 0 | 0 | N.S. | 0 | 0 | N.S. |
| T |
|
|
|
|
|
|
|
|
|
|
| 1 | 0 (0) | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| 2 | 13 (68.4) | 13 | 0 |
| 11 | 2 |
| 12 | 1 |
|
| 3 | 1 (5.2) | 1 | 0 |
| 0 | 1 |
| 0 | 1 |
|
| 4 | 5 (26.4) | 5 | 0 | N.S. | 4 | 1 | N.S. | 4 | 1 | N.S. |
| N |
|
|
|
|
|
|
|
|
|
|
|
Positive | 7 (36.8) | 7 | 0 |
| 5 | 2 |
| 6 | 1 |
|
|
Negative | 12 (63.2) | 12 | 0 | N.S. | 10 | 2 | N.S. | 10 | 2 | N.S. |
| Mode of
invasiond |
|
|
|
|
|
|
|
|
|
|
| 1 | 0 (0) | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| 2 | 1 (5.2) | 1 | 0 |
| 1 | 0 |
| 1 | 0 |
|
| 3 | 10 (52.6) | 10 | 0 |
| 8 | 2 |
| 3 | 1 |
|
| 4C | 4 (21.1) | 4 | 0 |
| 3 | 1 |
| 3 | 1 |
|
| 4D | 4 (21.1) | 4 | 0 | N.S. | 0 | 4 | N.S. | 0 | 4 | N.S. |
| Clinical
stagee |
|
|
|
|
|
|
|
|
|
|
| I | 0 (0) | 0 | 0 |
| 0 | 0 |
| 0 | 0 |
|
| II | 9 (47.4) | 9 | 0 |
| 7 | 2 |
| 7 | 2 |
|
|
III | 2 (10.5) | 2 | 0 |
| 2 | 0 |
| 2 | 0 |
|
| IV | 8 (42.1) | 8 | 0 | N.S. | 6 | 2 | N.S. | 7 | 1 | N.S. |
| Localization of the
lesion |
|
|
|
|
|
|
|
|
|
|
|
Tongue | 10 (52.6) | 10 | 0 |
| 7 | 3 |
| 8 | 2 |
|
|
Gingiva | 7 (36.8) | 7 | 0 |
| 6 | 1 |
| 6 | 1 |
|
|
Others | 2 (10.6) | 2 | 0 | N.S. | 2 | 0 | N.S. | 2 | 0 | N.S. |
Analyses of CK17 mRNA, CK19 mRNA and
CK20 mRNA expressions by real-time RT-PCR
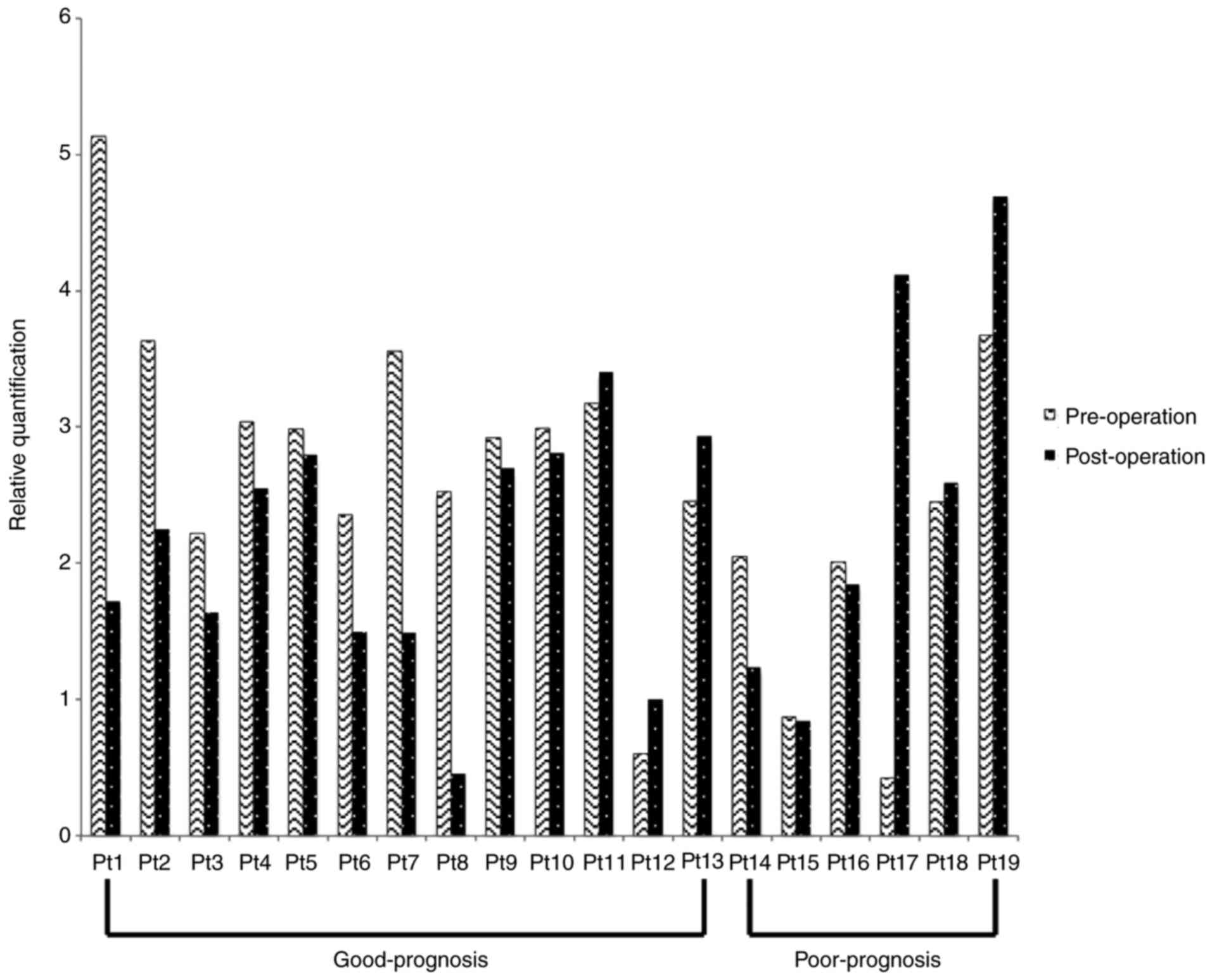
In both pre- and post-operative samples, CK17 mRNA
was overexpressed in all 19 patients (100%), while CK19 mRNA and
CK20 mRNA were overexpressed in 9 (47%) and 13 (68%) patients,
respectively. CK17 mRNA was expressed in the PBMC from all OSCC
patients regardless of clinicopathological parameters. There were
no significant differences between the expression of the different
CK mRNAs or clinicopathological parameters (Table I).
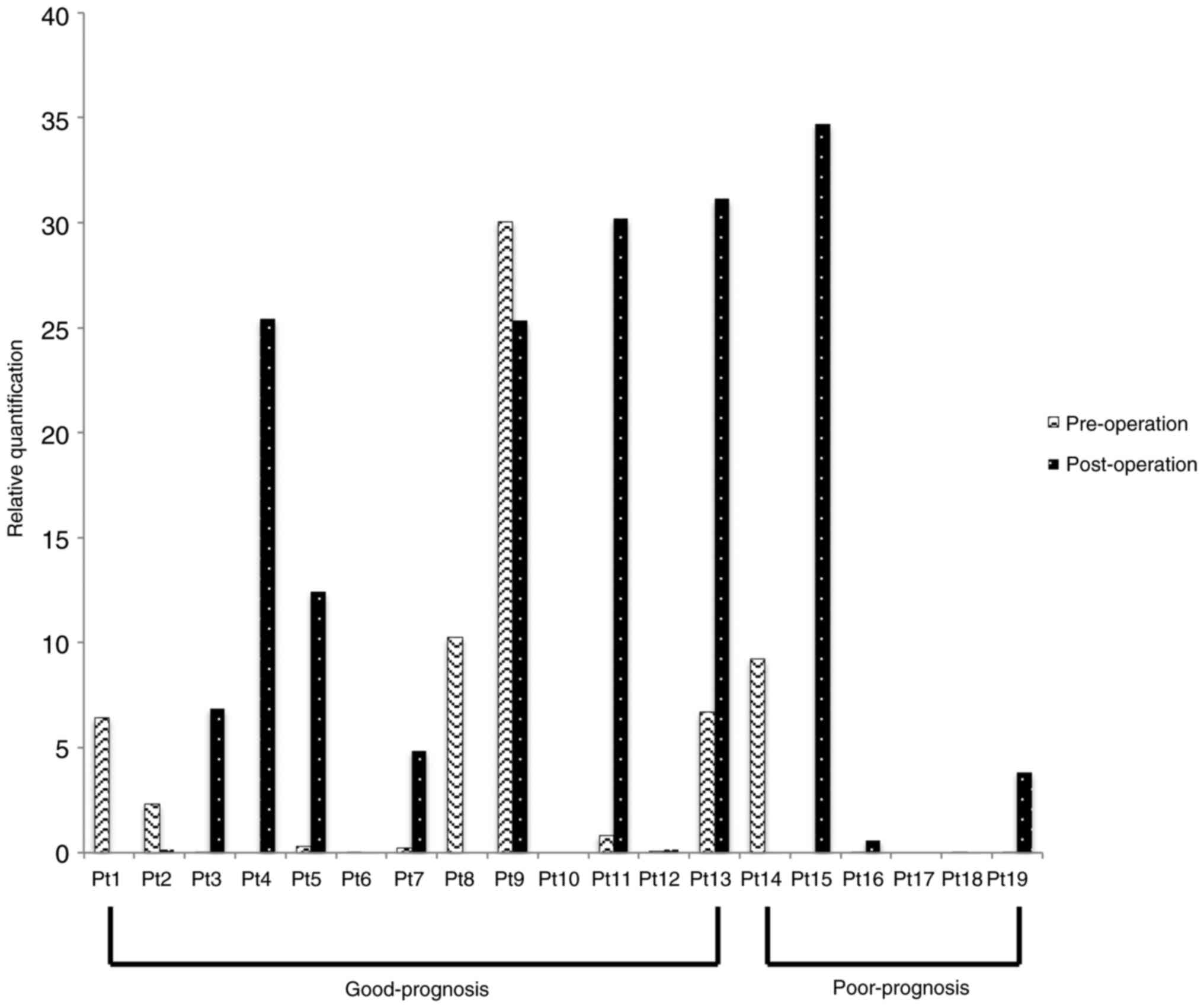
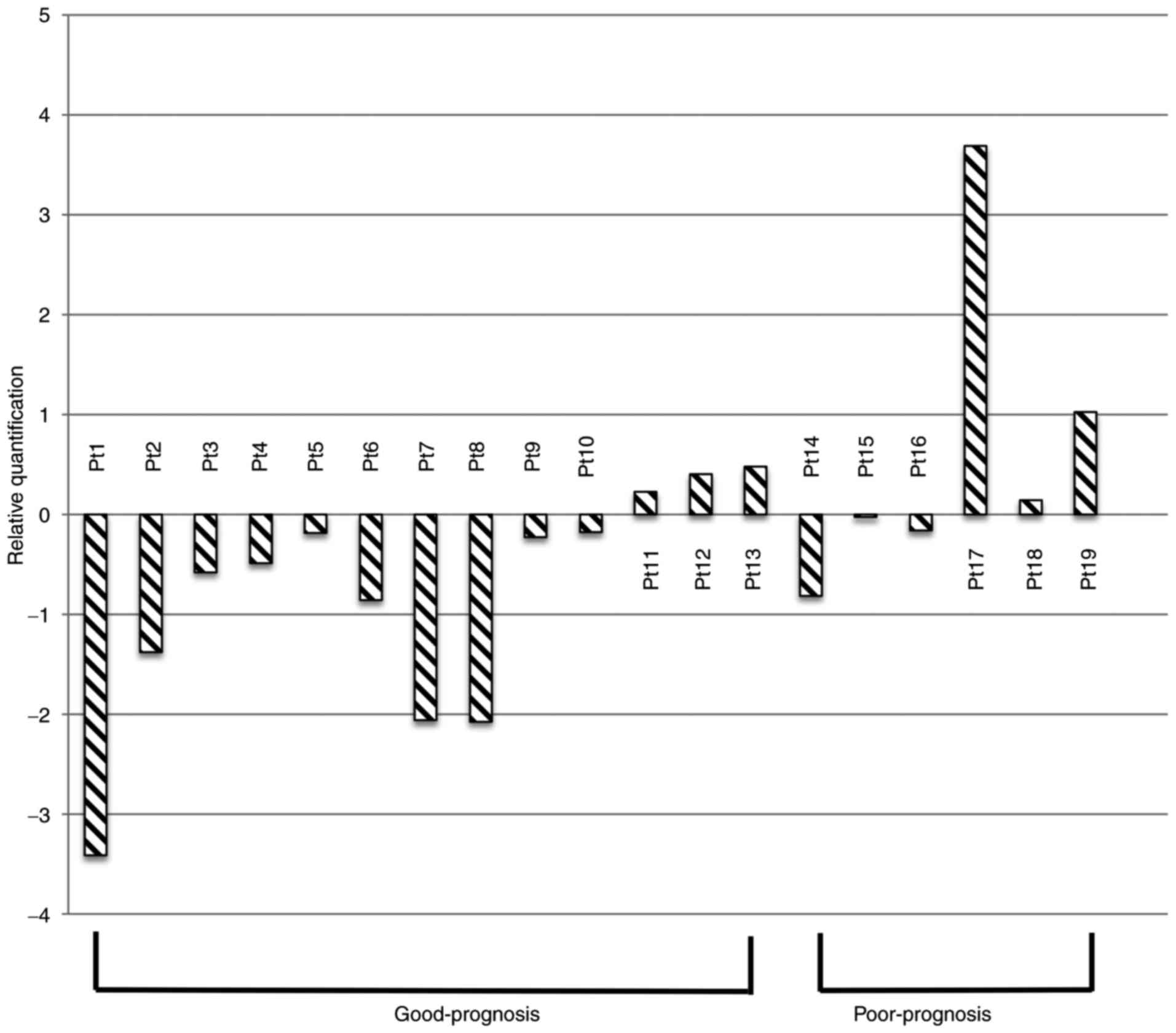
The real-time RT-PCR results for the different CK
mRNAs from the 19 patients are shown in Figs. 1–3. The
good-prognosis group consisted of patients completely cancer-free
after surgery, and the poor-prognosis group was composed of
patients with local recurrences or regional lymph node metastases.
Patients 1–13 and 14–19 comprised the good- and poor-prognosis
groups, respectively. CK17 mRNA was reduced in the post-operative
compared with the pre-operative samples for 10 of the 13 patients
in the good-prognosis group. Conversely, only three of the six
patients in the poor-prognosis group showed a post-operative
reduction in CK17 mRNA (Table II and
Fig. 4). When comparing pre- and
post-operative samples, there were no significant differences
between the good- and poor-prognosis groups in each CK19 mRNA or
CK20 mRNA levels.
 | Table II.Correlation between prognosis and
chronological change of CK17 mRNA in 19 patients. |
Table II.
Correlation between prognosis and
chronological change of CK17 mRNA in 19 patients.
|
| No. of cases |
|
|
|---|
|
|
|
|
|
|---|
| Prognosis | CK17↓b | CK17↑c | Total |
Significanced |
|---|
| Good
prognosisa | 10 | 3 | 13 |
|
| Poor prognosis | 3 | 3 | 6 | P<0.01 |
Uni and multiivariate analysis of
prognostic factors
Patient follow-up was for a maximum of 50 months
(range, 14–50 months; median, 38.6 months), and survival analysis
showed that gender, age, tumor size, clinical stage and
differentiation grade were not associated with survival rate. The
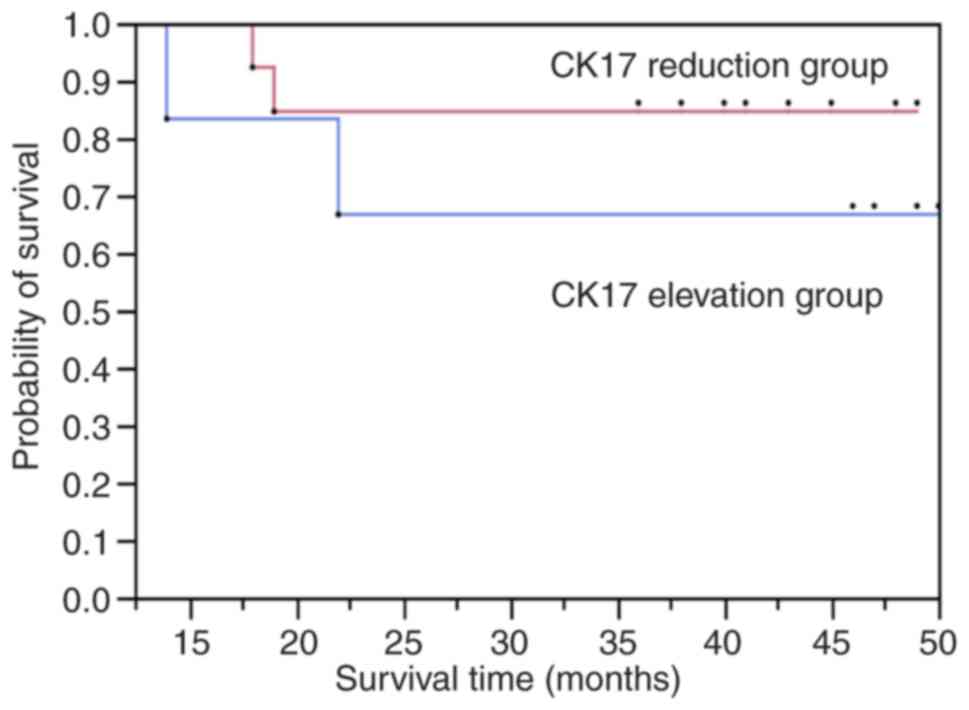
patients were then divided into the CK17-reduced and CK17-elevated
groups. The CK17-reduced group comprised patients whose CK17 mRNA
level decreased postoperatively and the CK17-elevated group
comprised patients whose preoperative CK17 mRNA level was lower
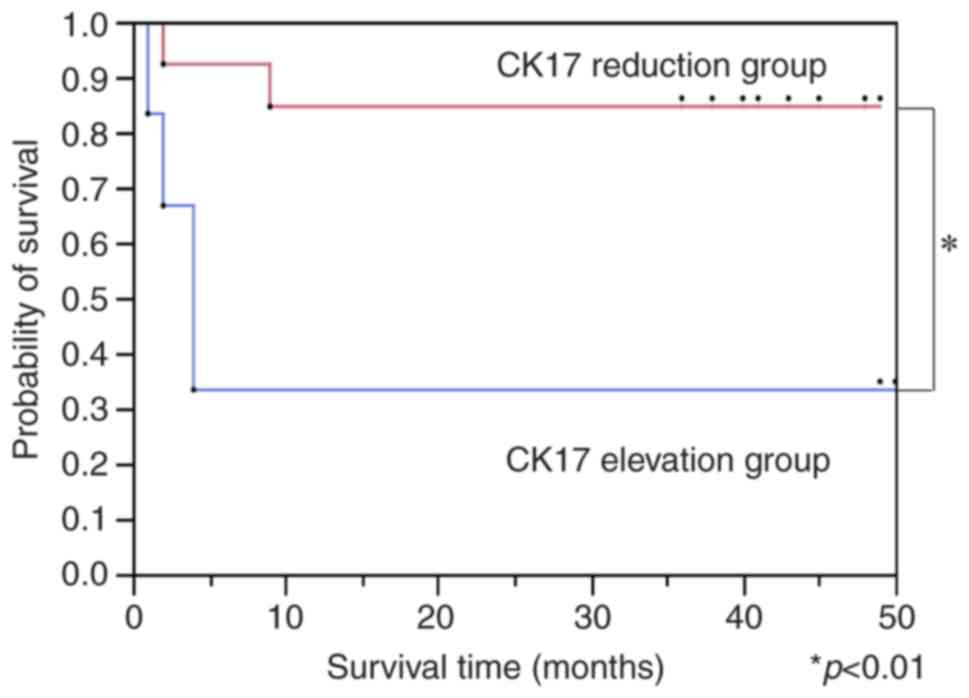
than in postoperative samples. The disease-free survival rate of
the CK17-reduced group was significantly higher than the
CK17-elevated group (Fig. 5).
However, the overall survival rates the two groups were not
significantly different (Fig. 6).
Hence, a post-operative elevation of CK17 was associated with poor
prognosis. As a result of multivariate analysis, it was found that
the elevation and reduction of CK17 mRNA affected disease-free
survival, but other confounding factors did not affect disease-free
survival. As for the overall survival rate, it was found that all
factors had no effect (data not shown).
Discussion
Our results showed that the CK17 mRNA level in the
PBMC from OSCC patients has the potential to be a prognostic
marker. CK17 mRNA expression was post-operatively increased in some
OSCC patients whose morbidity was poor. Therefore, patients with
elevated CK17 mRNA should receive more aggressive post-operative
treatments. Although CK19 mRNA and CK20 mRNA have been reported to
be potential prognostic markers for some head and neck carcinomas
(27,34), they were not significantly
overexpressed in the PBMC and were not prognostic markers for OSCC.
The time-dependent change in average CK17 mRNA and CK19 mRNA
expressions were reduced in the good-prognosis but increased in the
poor-prognosis group. The Kaplan-Meier curve for disease-free
survival showed a significant improvement for the patients with
reduced post-operative CK17 mRNA expression; there was no
significant difference based on CK19 mRNA expression. These changes
in OSCC outcomes suggest that this new clinical parameter may be
associated with diagnosis, prognosis and lymphoid node metastasis.
DTC is confirmed in the blood is T2 or more (27) and the population is small. This time
we reported it as a preliminary report. No other confounding
factors showed significant expression against prognosis.
The disease-free survival rate of the CK17-reduced
group was significantly higher than the CK17-elevated group;
however, CK17 mRNA was detected in every patient and healthy
volunteer, so we cannot consider CK17 mRNA a diagnostic marker. We
can, however, compare the CK17 mRNA levels in pre- and
post-operative samples to forecast prognosis. Even CK17 mRNA was
detected in healthy condition, a significant difference was found
between good-prognosis and poor-prognosis relative chronosical time
dependent manner. The overall survival rate in the CK17-reduced
group was not significantly higher than in the CK17-elevated group.
This may suggest that recurrences and metastases are often
successfully treated post-operatively. Recurrences are more likely
to be related to CK mRNA expression in the PBMC than lymph node
metastases because, in general, OSCC lymph node metastases do not
involve DTCs, but lymph node metastases are associated with DTCs in
breast carcinoma (35). Additionally,
CK17 mRNA in PBMC may be relevant to cervical lymph node metastases
in OSCC. Previously, CK17 mRNA has not been studied as a diagnostic
or prognostic marker; our data suggest that detecting CK17 mRNA
expression may warrant initiating post-operative treatment. Many of
the past reports were retrospective studies and many dates are
concluded false negatives (13).
However, this study is a prospective study so it is difficult to
consider our dates to false negatives only in the observation
period of this research. Therefore, we will continue to observe
whether recurrence or metastasis occurs in both of good- and
poor-prognosis patients.
In previous studies, CK19 mRNA has been shown to be
a diagnostic marker for OSCC, which is in agreement with this
study. Wang et al reported that circulation tumor cells were
detected in PBMC of breast cancer patients by immunohistochemistry
using CK19 (36). However, CK17 mRNA
has been more readily detected in OSCC patients than CK19 mRNA and
is considered a more useful diagnostic marker. CK19 mRNA is a
marker for non-small cell carcinoma and squamous cell carcinoma of
the lung, and uterine cervical carcinoma (37,38).
Contrary to previous reports, we detected CK19 mRNA in OSCC
patients, regardless of clinicopathological parameters. We conclude
that CK19 mRNA does not link to the clinical progression or
differentiation of OSCC. In a previous study, CK20 mRNA
overexpression in primary OSCC tissues was significantly associated
with the occurrence of metastases to cervical lymph nodes (27). However, in this study, CK20 mRNA
expression was only detected in 13 of 19 patients, and was not
significantly associated with clinicopathological parameters.
Therefore, we suggest that CK20 mRNA is not an efficient diagnostic
or prognostic marker for OSCC. A limitation exists in this study,
because there were no examples that CK17 mRNA in PBMC matched CK17
mRNA of tumor tissue in the past. We could not examine the CK17
status in PBMC matched tumor tissues. Toyoshima et al
conducted a retrospective study and suggested a prognostic factor
for CK20 mRNA (27), but with regard
to CK17 mRNA, there was no significant expression in the
clinicopathological parameters of primary tumor. In this study,
CK17 mRNA is in agreement with this result, but unlike in previous
studies (18), novel points are that
this time we have made a prospective study based on the results so
far and that it is suggested that CK17 mRNA has possibly a
prognostic factor.
We defined blood sampling one month after surgery in
that surgical invasion calmed down and can be confirmed before
recurrence or metastasis occurred. After that, because of
recurrence and metastasis, postoperative treatment was underway and
conditions were not aligned. It is ideal to take multiple times,
but in order to grasp recurrence and metastasis early, it is very
important to draw blood one month after surgery. As in this study,
recurrence and metastasis often occur within 10 months, and it is
important to examine at an early stage postoperatively. If there
are residual cancer cells, CK17 mRNA in PBMCs is expected to
increase, even if there is no recurrence or metastasis, over time.
Certainly OSCC has complicated onset and it seems difficult to
discuss everything with our results. It is too simplistic to pay
attention only to CK17 mRNA, but it was also found that the
expression of CK17 mRNA was also significantly related to prognosis
in multivariate analysis. For those with little change even with
good prognosis this time, follow-up observation is necessary with
particular attention from now.
Toyoshima et al reported that detection of
CK20 mRNA in PBMC by real-time RT-PCR was related lymph node
metastasis (27). In addition, Wang
et al reported that circulating tumor cells were detected
CK19 in PBMC of breast cancer patients by immunohistochemistry.
DTCs are not derived from PBMC, but they showed DTCs in PBMC
isolated from the blood by immunohistochemisty (36). Therefore, it is possible to detect CKs
mRNA without separating DTC from PMBC. However, it is generally
recognized that DTCs occur at low frequencies and cannot easily be
detected in peripheral blood circulation, even when advanced
metastatic disease is present in patients (39–41).
Therefore, it is necessary to conduct a high-sensitivity
examination. Real-time RT-PCR is a sensitive and specific method to
analyze gene expression patterns in tissues and body fluids. CKs
are constitutively expressed in epithelial cells and highly
overexpressed in tumors. Therefore, it should be possible to detect
epithelial tissue-derived DTCs based on PBMC CKs mRNA levels. In
this study, a sensitive real-time RT-PCR was performed, offering
the possibility to detect 1 to 10 tumor cells within 1 million
normal mononuclear cells (42). As
healthy volunteers also produce tumor cells slightly, they may be
positive by real-time RT-PCR. According to Frank Macfarlane Burnet,
3,000 cancer cells are made a day on healthy people due to gene
transcription mistake etc (43). Each
CK mRNA RQ was compared with those of healthy volunteers to
determine the specific level of overexpression in the PBMCs of OSCC
patients. Rinsing the catheter and removing the first 5 ml of blood
aspirate avoided false-positive results due to contaminating PBMCs
samples with dermal cells from the needle. Thus, the occurrence of
normal epithelial cells in peripheral circulation was ruled out. CK
mRNAs are not detected in PBMC because there are no epithelial
cell, so the detection of CK mRNA in isolated PBMCs indicated the
occurrence of tumor cell disseminated into the peripheral
circulation from the primary site with high probability.
While this study did not directly detect DTCs, our
findings could be suggested possibility indirect evaluation methods
for DTCs. The multiplexed positive detection of CK17 transcripts in
the PBMC could define a possible risk for the presence of systemic
disease, which might warrant the use of adjuvant chemotherapy in
OSCC patients. Hematological examination is a brief test and might
also be a useful method for monitoring OSCC patients for disease
relapse and progression after surgery and chemotherapy. This method
should be tested and validated for predicting disease-free and
overall survival in OSCC patients in a future study with a greater
patient cohort.
Acknowledgements
The present study was supported by a Grant-in-Aid
for Scientific Research from the Japanese Ministry of Education,
Culture, Science, Sports and Technology of Japan (no. 25861955,
15K20539, 16K20584).
Glossary
Abbreviations
Abbreviations:
|
OSCC
|
oral squamous cell carcinoma
|
|
CKs
|
cytokeratins
|
|
PBMC
|
peripheral blood mononuclear cells
|
|
DTCs
|
disseminated tumor cells
|
References
|
1
|
Gorsky M, Epstein JB, Oakley C, Le ND, Hay
J and Stevenson-Moore P: Carcinoma of the tongue: A case series
analysis of clinical presentation, risk factors, staging, and
outcome. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
98:546–552. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Willén R, Nathanson A, Moberger G and
Anneroth G: Squamous cell carcinoma of the gingiva. Histological
classification and grading of malignancy. Acta Otolaryngol.
79:146–154. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasaki M, Aoki T, Karakida K, Otsuru M,
Takahashi M, Akamatsu T, Sakamoto H and Ota Y: Postoperative
follow-up strategy in patients with oral squamous cell carcinoma. J
Oral Maxillofac Surg. 69:e105–e111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pantel K and Brakenhoff RH: Dissecting the
metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burchill SA, Bradbury MF, Pittman K,
Southgate J, Smith B and Selby P: Detection of epithelial cancer
cells in peripheral blood by reverse transcriptase-polymerase chain
reaction. Br J Cancer. 71:278–281. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noguchi T, Shibata T, Fumoto S, Sato T,
Uchida Y, Daa T, Yokoyama S, Gabbert HE, Mueller W and Takeno S:
Detection of disseminated cancer cells in rib marrow of patients
with esophageal cancer. Oncol Rep. 10:623–627. 2003.PubMed/NCBI
|
|
7
|
Davelaar EM, van de Lande J, von
Mensdorff-Pouilly S, Blankenstein MA, Verheijen RH and Kenemans P:
A combination of serum tumor markers identifies high-risk patients
with early-stage squamous cervical cancer. Tumour Biol. 29:9–17.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Benoy IH, Elst H, Philips M, Wuyts H, Van
Dam P, Scharpé S, Van Marck E, Vermeulen PB and Dirix LY: Real-time
RT-PCR detection of disseminated tumour cells in bone marrow has
superior prognostic significance in comparison with circulating
tumour cells in patients with breast cancer. Br J Cancer.
94:672–680. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Soeth E, Grigoleit U, Moellmann B, Röder
C, Schniewind B, Kremer B, Kalthoff H and Vogel I: Detection of
tumor cell dissemination in pancreatic ductal carcinoma patients by
CK 20 RT-PCR indicates poor survival. J Cancer Res Clin Oncol.
131:669–676. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Partridge M, Brakenhoff R, Phillips E, Ali
K, Francis R, Hooper R, Lavery K, Brown A and Langdon J: Detection
of rare disseminated tumor cells identifies head and neck cancer
patients at risk of treatment failure. Clin Cancer Res.
9:5287–5294. 2003.PubMed/NCBI
|
|
11
|
Gath HJ, Heissler E, Hell B, Bier J,
Riethmüller G and Pantel K: Immunocytologic detection of isolated
tumor cells in bone marrow of patients with squamous cell
carcinomas of thehead and neck region. Int J Oral Maxillofac Surg.
24:351–355. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iinuma H, Okinaga K, Egami H, Mimori K,
Hayashi N, Nishida K, Adachi M, Mori M and Sasako M: Usefulness and
clinical significance of quantitative real-time RT-PCR to detect
isolated tumor cells in the peripheral blood and tumor drainage
blood of patients with colorectal cancer. Int J Oncol. 28:297–306.
2006.PubMed/NCBI
|
|
13
|
Gradilone A, Gazzaniga P, Silvestri I,
Gandini O, Trasatti L, Lauro S, Frati L and Aglianò A: Detection of
CK19, CK20 and EGFR mRNAs in peripheral blood of carcinoma
patients: Correlation with clinical stage of disease. Oncol Rep.
10:217–222. 2003.PubMed/NCBI
|
|
14
|
Lin JC, Chen KY, Wang WY, Jan JS and Wei
YH: PCR detection of circulating tumor cells in nasopharyngeal
carcinoma patients with distant metastasis: Effect of enzyme and
sampling. Head Neck. 24:591–596. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaubal S, Wollenberg B, Kastenbauer E and
Zeidler R: Ep-CAM-a marker for the detection of disseminated tumor
cells in patients suffering from SCCHN. Anticancer Res.
19:2237–2242. 1999.PubMed/NCBI
|
|
16
|
Brakenhoff RH, Stroomer JG, ten Brink C,
de Bree R, Weima SM, Snow GB and van Dongen GA: Sensitive detection
of squamous cells in bone marrow and blood of head and neck cancer
patients by E48 reverse transcriptase-polymerase chain reaction.
Clin Cancer Res. 5:725–732. 1999.PubMed/NCBI
|
|
17
|
Xu XC, Lee JS, Lippman SM, Ro JY, Hong WK
and Lotan R: Increased expression of cytokeratins CK8 and CK19 is
associated with head and neck carcinogenesis. Cancer Epidemiol
Biomarkers Prev. 4:871–876. 1995.PubMed/NCBI
|
|
18
|
Kitamura R, Toyoshima T, Tanaka H, Kawano
S, Kiyosue T, Matsubara R, Goto Y, Hirano M, Oobu K and Nakamura S:
Association of cytokeratin 17 expression with differentiation in
oral squamous cell carcinoma. J Cancer Res Clin Oncol.
138:1299–1310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Toyoshima T, Vairaktaris E, Nkenke E,
Schlegel KA, Neukam FW and Ries J: Cytokeratin 17 mRNA expression
has potential for diagnostic marker of oral squamous cell
carcinoma. J Cancer Res Clin Oncol. 134:515–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cohen-Kerem R, Madah W, Sabo E, Rahat MA,
Greenberg E and Elmalah I: Cytokeratin-17 as a potential marker for
squamous cell carcinoma of the larynx. Ann Otol Rhinol Laryngol.
113:821–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van de Rijn M, Perou CM, Tibshirani R,
Haas P, Kallioniemi O, Kononen J, Torhorst J, Sauter G, Zuber M,
Köchli OR, et al: Expression of cytokeratins 17 and 5 identifies a
group of breast carcinomas with poor clinical outcome. Am J Pathol.
161:1991–1996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ikeda K, Tate G, Suzuki T and Mitsuya T:
Coordinate expression of cytokeratin 8 and cytokeratin 17
immunohistochemical staining in cervical intraepithelial neoplasia
and cervical squamous cell carcinoma: An immunohistochemical
analysis and review of the literature. Gynecol Oncol. 108:598–602.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong LP, Chen WT, Zhang CP and Zhang ZY:
Increased CK19 expression correlated with pathologic
differentiation grade and prognosis in oral squamous cell carcinoma
patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
104:377–384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Katsumata K, Sumi T, Mori Y, Hisada M,
Tsuchida A and Aoki T: Detection and evaluation of epithelial cells
in the blood of colon cancer patients using RT-PCR. Int J Clin
Oncol. 11:385–389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chausovsky G, Luchansky M, Figer A,
Shapira J, Gottfried M, Novis B, Bogelman G, Zemer R, Zimlichman S
and Klein A: Expression of cytokeratin 20 in the blood of patients
with disseminated carcinoma of the pancreas, colon, stomach, and
lung. Cancer. 86:2398–2405. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kawamata H, Uchida D, Nakashiro K, Hino S,
Omotehara F, Yoshida H and Sato M: Haematogenous cytokeratin 20
mRNA as a predictive marker for recurrence in oral cancer patients.
Br J Cancer. 80:448–452. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Toyoshima T, Vairaktaris E, Nkenke E,
Schlegel KA, Neukam FW and Ries J: Hematogenous cytokeratin 20 mRNA
detection has prognostic impact in oral squamous cell carcinoma:
Preliminary results. Anticancer Res. 29:291–297. 2009.PubMed/NCBI
|
|
28
|
Imai Y, Yamagishi H, Fukuda K, Okamura T,
Ono Y, Ban S, Inoue T and Ueda Y: Expression of cytokeratin 20
indicates invasive histological phenotype in poorly differentiated
colorectal adenocarcinoma. Anticancer Res. 34:159–167.
2014.PubMed/NCBI
|
|
29
|
Wahi PN, Cohen B, Luthra UK and Torloni H:
Histological considerationHistological Typing of Oral and
Oropharyngeal Tumors. World Health Organization; Geneva: pp. 15–19.
1977
|
|
30
|
Gale N, Pilch BZ, Sindramsky D, et al:
Epithelium precursor lesionsWorld Health Organization
Classification of Tumors. Pathology and Genetics of Head and Neck
Tumors. Barnes L, Eveson J, Reichart P and Sidransky D: IARC Press;
Lyon: pp. 177–e179. 2005
|
|
31
|
Sobin LH, Sobin LH Witte and Wittekind CH:
TNM Classification of Malignant Tumors. Wiley-Liss, Inc.; New York,
NY: 2002
|
|
32
|
Yamamoto E, Miyakawa A and Kohama G: Mode
of invasion and lymph node metastasis in squamous cell carcinoma of
the oral cavity. Head Neck Surg. 6:938–947. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nagler RM, Barak M, Peled M, Ben-Aryeh H,
Filatov M and Laufer D: Early diagnosis and treatment monitoring
roles of tumor markers Cyfra 21-1 and TPS in oral squamous cell
carcinoma. Cancer. 85:1018–1025. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hartkopf AD, Taran FA, Wallwiener M, Hahn
M, Becker S, Solomayer EF, Brucker SY, Fehm TN and Wallwiener D:
Prognostic relevance of disseminated tumour cells from the bone
marrow of early stage breast cancer patients-results from a large
single-centre analysis. Eur J Cancer. 50:2550–2559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang F, Li YC, Liu LP, Zhang HM and Tong
S: Circulating tumor cells and tumor stem cells detection in the
peripheral blood mononuclear cells of breast cancer. J Clin Lab
Anal. 30:616–622. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hamakawa H, Bao Y, Takarada M, Fukuzumi M
and Tanioka H: Cytokeratin expression in squamous cell carcinoma of
the lung and oral cavity: An immunohistochemical study with
possible clinical relevance. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 85:438–443. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Callet N, Cohen-Solal Le Nir CC, Berthelot
E and Pichon MF: Cancer of the uterine cervix: Sensitivity and
specificity of serum Cyfra 21.1 determinations. Eur J Gynaecol
Oncol. 19:50–56. 1998.PubMed/NCBI
|
|
39
|
Smith B, Selby P, Southgate J, Pittman K,
Bradley C and Blair GE: Detection of melanoma cells in peripheral
blood by means of reverse transcriptase and polymerase chain
reaction. Lancet. 338:1227–1229. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gläser R, Rass K, Seiter S, Hauschild A,
Christophers E and Tilgen W: Detection of circulating melanoma
cells by specific amplification of tyrosinase complementary DNA is
not a reliable tumor marker in melanoma patients: A clinical
two-center study. J Clin Oncol. 15:2818–2825. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jung R, Krüger W, Hosch S, Holweg M,
Kröger N, Gutensohn K, Wagener C, Neumaier M and Zander AR:
Specificity of reverse transcriptase polymerase chain reaction
assays designed for the detection of circulating cancer cells is
influenced by cytokines in vivo and in vitro. Br J Cancer.
78:1194–1198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yeh KH, Chen YC, Yeh SH, Chen CP, Lin JT
and Cheng AL: Detection of circulating cancer cells by nested
reverse transcription-polymerase chain reaction of cytokeratin-19
(K19)-possible clinical significance in advanced gastric cancer.
Anticancer Res. 18:1283–1286. 1998.PubMed/NCBI
|
|
43
|
Corthay A: Does the immune system
naturally protect against cancer? Front Immunol. 5:1972014.
View Article : Google Scholar : PubMed/NCBI
|