Introduction
Cancer refers to a group of diseases resulting from
uncontrolled cellular growth. It is a major concern for public
healthcare, and data from GLOBOCAN revealed that ~14.1 million new
cancer cases and 8.2 million cancer-associated mortality occurred
worldwide in 2012 (1).
Na+/K+-ATPase (NKA), which
functions as a sodium-potassium pump, is a ubiquitous enzyme that
serves as an ion transporter and a signal transducer (2). This enzyme consists of one α and one β
subunit (2). The NKA pumps two
K+ into cells and three Na+ out of cells
using energy derived from ATP (2).
NKA serves a critical function in cellular growth, differentiation
and survival as well as cell migration and cell-cell interaction
(2). Since 1957, when Jens Christian
Skou discovered NKAs, increasing evidence suggests that NKAs not
only maintain cell membrane potential, but also serve an important
function in cancer (3–5). Alterations in NKA expression and
function have been documented in several types of cancer including
colorectal cancer and liver metastases (3). It has been reported that the
α1 and α3 NKA isoforms are overexpressed in
tumor cells and metastases, including hepatocellular carcinoma
(5).
Ouabain is a highly specific inhibitor of NKA and
has been used for the treatment of heart failure and atrial
fibrillation (6). There has been
renewed interest in the anticancer effect of ouabain as
epidemiological studies have revealed that administration of
ouabain in patients with cancer significantly improved survival
rates (7–12). A study by Xu et al (13) demonstrated that ouabain binds to the
NKA signalosome and activates multiple signaling pathways
associated with cell death and apoptosis. However, the molecular
mechanisms underlying the anticancer effect of ouabain remain
unclear. The results of the present study revealed that the
anticancer effect of ouabain is associated with inhibition of the
NKA α3 isoform rather than the α1
isoform.
Materials and methods
Cell culture
The human renal cancer cell line OS-RC-2 was
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). The human small cell lung cancer
cell line NCI-H446 was obtained from the Fujian Institute of
Hematology (Fuzhou, China). These cell lines were maintained at
37°C in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Equitech-Bio, Inc., Kerrville, TX, USA) and 1% penicillin G and
streptomycin (Invitrogen; Thermo Fisher Scientific, Inc.). Ouabain
was purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
All cells were maintained in 5% CO2 at 37°C.
MTT assay
Cells were seeded in 96-well plates (3,000
cells/well with 180 µl RPMI-1640) and treated with either DMSO or
ouabain (20, 40, 80, 160, 320 nM). Subsequently, cells were
incubated for the indicated period of time (24, 48 and 72 h), cell
viability was determined using the MTT assay kit (Roche Diagnostics
GmbH, Mannheim, Germany), according to the manufacturer's protocol.
The quantity of formazan was determined by recording changes in
absorbance at 490 nm. Each assay was performed in triplicate.
Comparisons were performed using one-way analysis of variance
(ANOVA).
Acridine orange/ethidium bromide
(AO/EB) staining
Cells were seeded in 6-well plates at a density of
1×105 cells per well. Cells were treated with ouabain
(0, 20, 40, 80 nM) and incubated in 5% CO2 at 37°C for
48 h and stained with the AO/EB dye solution containing 200 µg/ml
AO (Sigma-Aldrich; Merck KGaA) and 200 µg/ml EB (Sino-American
Biotechnology Co., Luoyang, China) at room temperature for 1 min.
Cells were then immediately observed using a fluorescence inverted
microscope (magnification, ×400; BX51-P; Olympus Corporation,
Tokyo, Japan) and 10 fields of views were assessed.
AnnexinV-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) flow cytometric analysis
Cells were seeded in 6-well plates at a density of
2×105 cells per well. 24 h later, cells were treated
with ouabain at 37°C for 48 h and then flow cytometric analysis was
performed to assess cellular apoptosis using the AnnexinV-FITC/PI
Apoptosis Detection kit (Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocol. Apoptotic
cells were analyzed using a flow cytometer and FlowJo software
(version 10; FlowJo LLC, Ashland, OR, USA).
Ca2+ and reactive oxygen
species (ROS) quantification
Cells were treated at 37°C with ouabain for 48 h and
then washed with PBS. The fluorescence probes Fura-3-acetoxymethyl
ester (AM) and dichloro-dihydro-fluorescein diacetate (DCFH-DA;
Beyotime Institute of Biotechnology) were used at concentrations of
10 and 2 µM, respectively. Cells were then incubated in RPMI-1640
medium containing the fluorescence probes in the dark for 20–40 min
at 37°C and washed for 30 min in serum-free RPMI-1640 medium.
Fluorescence images were captured using a confocal microscope
(magnification, ×400; C1SI; Nikon Corporation, Tokyo, Japan). The
excitation wavelength was 488 nm and the emission wavelength was
522–530 nm. The fluorescence intensity was assessed using Image-Pro
Plus software6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Isolation of apoptotic DNA
fragments
Cells treated at 37°C with different concentrations
(0, 10, 20, 40 nM) of ouabain for 48 h, cells were collected and
treated for 10 sec with lysis buffer (50 mM Tris-Cl, 150 mM NaCl,
1% nonylphenoxypolyethoxyl ethanol, 1% sodium deoxycholate and 1%
SDS) at room temperature (RT). Supernatant was collected by
centrifugation for 5 min at 14,000 × g, 1% SDS was added and
samples were then treated with ribonuclease A for 2 h at 56°C
followed by digestion with proteinase K for at least 2 h at 37°C.
Subsequently, 0.5 volume 10 M ammonium acetate was added, the DNA
was precipitated with 2.5 volume ethanol, dissolved in gel loading
buffer (Sigma-Aldrich; Merck KGaA) and separated by electrophoresis
on 1% agarose gels.
Western blotting
Total protein was extracted from cells using radio
immunoprecipitation assay buffer (Beyotime Institute of
Biotechnology) supplemented with protease inhibitors (Roche
Diagnostics, Basel, Switzerland) at 4°C for 30 min, and western
blot analysis was performed as described previously (12). Primary antibodies against B-cell
lymphoma 2 (Bcl-2; cat. no. 12789-1-AP; 1:2,000; Protein Tech
Group, Inc., Chicago, IL, USA), Bcl-2-associated X protein (Bax;
cat. no. BM3964; 1:500; Boster Biological Technology, Pleasanton,
CA, USA) and β-actin (cat. no. 4967S; 1:2,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) were incubated with the
membranes overnight at 4°C. Following the primary incubation,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit IgG or anti-mouse IgG secondary antibodies
(Sigma-Aldrich; Merck KGaA).
Immunocytochemistry (ICC)
Cells were seeded on coverslips at a density of
1×105 cells. 24 h later cells were fixed with 0.4%
paraformaldehyde at room temperature for 20 min and endogenous
peroxidase activity was blocked with hydrogen peroxide for 30 min.
To prevent non-specific binding cells were blocked with fetal
bovine serum at room temperature for 30 min prior to primary
antibody (Na/K-ATPase α1 antibody; cat. no. sc-58629, 1:1,000;
Na/K-ATPase α3 antibody; cat. no. sc-71640; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) incubation overnight at 4°C
in a moist chamber. Cells were subsequently incubated with
secondary antibodies (horseradish peroxidase-conjugated goat
anti-mouse immunoglobulin G; cat. no, sc-2031; 1:2,000; Santa Cruz
Biotechnology, Inc.) for 30 min at 37°C and stained with
3,3′-diaminobenzidine for 5 min at RT. Cell nuclei were
counterstained with hematoxylin at room temperature for 3 min and
cells were finally dehydrated and mounted. Cells were visualized
using a fluorescence inverted microscope (magnification, ×400;
BX51-P; Olympus Corporation, Tokyo, Japan) and 10 fields of views
were assessed using Image-Pro Plus software 6.0 (Media Cybernetics,
Inc.).
Reverse transcription polymerase chain
reaction
Total RNA was extracted using TRIzol®
reagent (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. Total RNA was reverse transcribed into
cDNA using the Reverse Transcription System (Takara Bio, Inc.).
Subsequently, RT-qPCR was performed using miScript SYBR®
green PCR Kit (Qiagen GmbH, Hilden, Germany), according to the
manufacturer's protocol using specific primers for NKA isoform
α1, NKA α3 and β-actin. The PCR conditions
were as follows: 95°C for 30 sec and then 40 cycles of 95°C for 5
sec and 60°C for 34 sec. The expression levels of genes were
determined using the ΔΔCq method (14). The following primer pairs were used:
β-actin forward: 5′-AACACCCCAGCCATGTACG-3′ and reverse,
5′-ATGTCACGCACGATTTCCC-3′; NKA isoform α1 forward,
5′-TGTCCAGAATTGCAGGTCTTTG-3′ and reverse,
5′-TGCCCGCTTAAGAATAGGTAGGT-3′ and NKA isoform α3
forward, 5′-AAGGAGGTGGCTATGACAGAG-3′ and reverse,
5′-GTGAGTGCGTTAGGCCCAT-3′.
Small interfering (si)RNA
transfection
siRNA transfection was performed using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's guidelines. The control scramble siRNA (sc-37007),
Na+/K+-ATPase α1 siRNA (sc-36010) and
Na+/K+-ATPase α3 siRNA (sc-149790) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Statistical analysis
Data were analyzed using Prism 5.0 software
(Graphpad Software, Inc., La Jolla, CA, USA). Results are presented
as the mean ± standard deviation of three independent experiments.
Comparisons were performed by one-way analysis of variance followed
by Dunnett's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
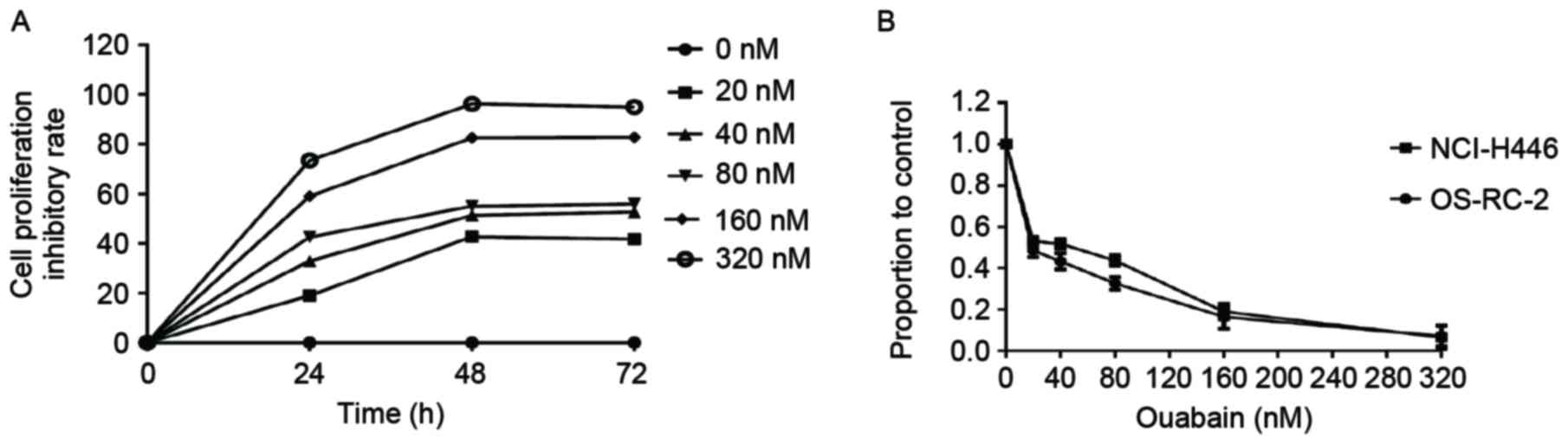
Ouabain inhibits proliferation of
OS-RC-2 and NCI-H446 cells
To examine the effect of ouabain on cellular
proliferation, OS-RC-2 cells were treated with different
concentrations of ouabain (0, 20, 40, 80, 160, 320 nM) for 24, 48
and 72 h (Fig. 1A). Ouabain inhibited
cancer cell proliferation in a time-dependent manner. The
proportion of viable cells following ouabain treatment were
measured using MTT assay. As the effect on cell proliferation was
greater at 48 h in OS-RC-2 cells, this time point was selected for
the experiments of this study, unless otherwise stated. The
half-maximal inhibitory concentration n(IC50) value of
ouabainin OS-RC-2 cells, determined using the MTT assay, was ~39 nM
(Fig. 1B). These results indicated
that ouabain inhibited proliferation of OS-RC-2 cells in a dose-
and time-dependent manner. Similar experiments were performed in
NCI-H446 cells generating similar results (Fig. 1B). This suggests that the
anti-proliferative effect of ouabain may apply to other cancer cell
lines.
Ouabain induces cell death in OS-RC-2
and NCI-H446 cells
To investigate the underlying molecular mechanism of
cell death induced by ouabain, treated cells were observed under an
inverted and a fluorescent microscope. Morphological changes were
induced inOS-RC-2 (Fig. 2A) and
NCI-H446 (Fig. 2B) cells treated with
a range of ouabain concentrations for 48 h. Cells treated with
different concentrations of ouabain presented typical cell death
features including membrane blebbing, cell shrinkage, detachment,
nuclear condensation and fragmentation. AO/EB staining was then
performed to confirm cell death. Red-orange fluorescence was
enhanced in OS-RC-2 (Fig. 2C) and
NCI-H446 (Fig. 2D) cells treated with
increasing concentrations of ouabain; indicating that ouabain
induces cell death.
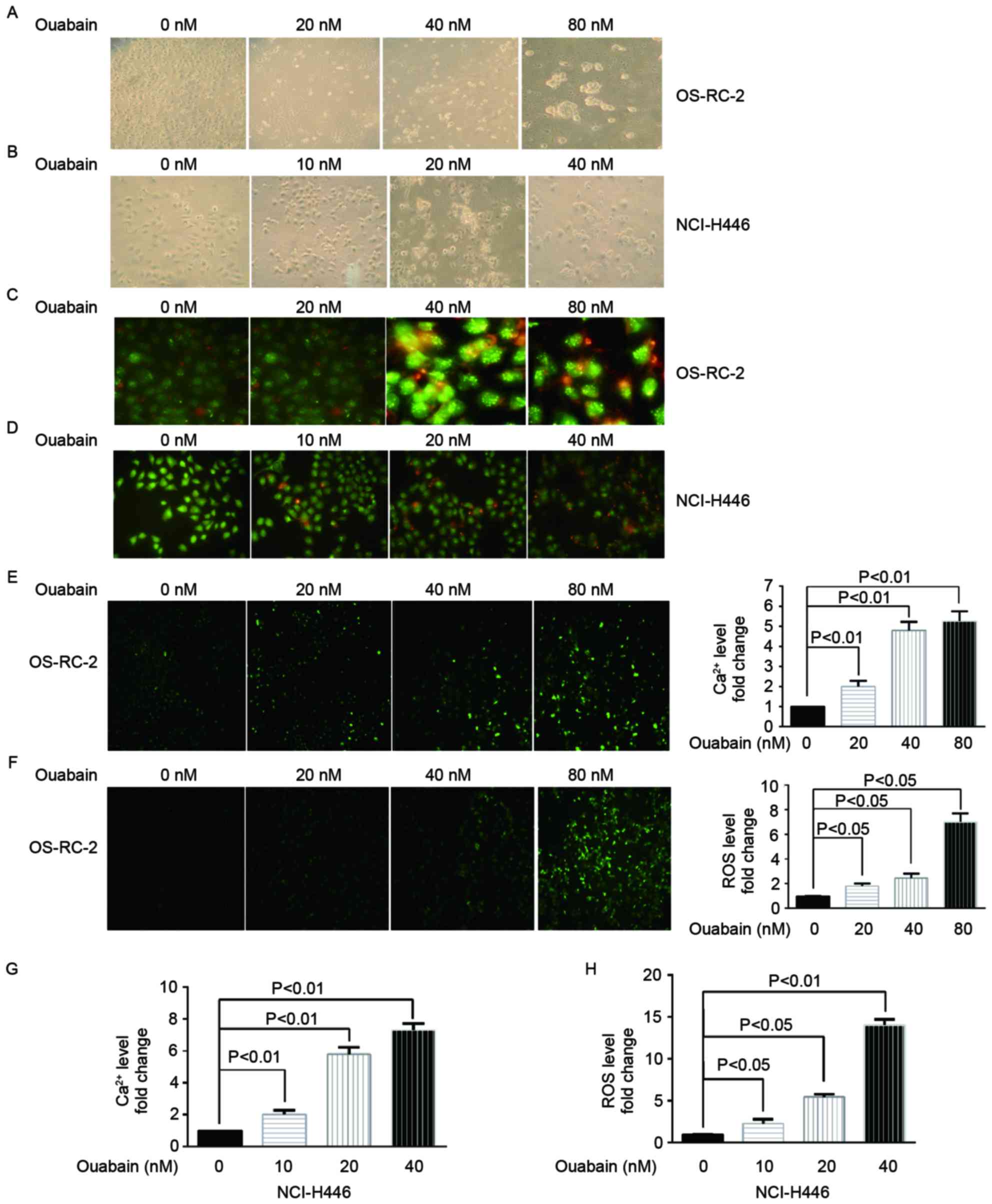 | Figure 2.Ouabain induces cell death. (A)
OS-RC-2 cells treated with a range of ouabain concentrations (0,
20, 40 and 80 nM) for 48 h were observed under an inverted
microscope (magnification, ×400). (B) NCI-H446 cells treated with a
range of ouabain concentrations (0, 10, 20 and 40 nM) for 48 h were
observed under an inverted microscope (magnification, ×400). (C)
OS-RC-2 cells treated with a range of ouabain concentrations (0,
20, 40 and 80 nM) for 48 h were stained with AO/EB dye solution and
observed under a confocal microscope (magnification, ×400). (D)
NCI-H446 cells treated with a range of oubain concentrations (0,
10, 20 and 40 nM) for 48 h were stained with AO/EB dye solution and
observed under a confocal microscope (magnification, ×400). (E)
Intracellular Ca2+ levels determined using a Fura-3/AM
probe in OS-RC-2 cells treated with a range of ouabain
concentrations (0, 20, 40 and 80 nM) for 48 h, using a confocal
microscope (magnification, ×400). (F) Intracellular ROS levels
determined using a DCFH-DA probe in OS-RC-2 cells treated with a
range of ouabain concentrations (0, 20, 40 and 80 nM) for 48 h,
using a confocal microscope (magnification, ×400). (G)
Intracellular Ca2+ levels determined using a Fura-3/AM
probe in NCI-H446 cells treated with a range of ouabain
concentrations (0, 10, 20 and 40 nM) for 48 h, using a confocal
microscope. (H) Intracellular ROS levels determined using a DCFH-DA
probe in NCI-H446 cells treated with a range of ouabain
concentrations (0, 10, 20 and 40 nM) for 48 h, using a confocal
microscope. AO/EB, acridine orange/ethidium bromide; DCFH-DA,
dichloro-dihydro-fluorescein diacetate; AM, acetoxymethylester;
ROS, reactive oxygen species. |
Ouabain increases the intracellular
Ca2+ and ROS levels (15)
Majno and Joris (16)
reported that an increasing concentrations of intracellular
Ca2+ and ROS serves a key function in cell death. Thus,
to investigate whether ouabain caused changes in Ca2+
and ROS levels, OS-RC-2 and NCI-H446 cells were treated with a
range of ouabain concentrations and the Ca2+ and ROS
levels were examined using Fura-3-AM and DCFH-DA probes,
respectively. OS-RC-2 (Fig. 2E and F)
and NCI-H446 (Fig. 2G and H) cells
treated with ouabain presented significantly higher Ca2+
and ROS fluorescence intensity compared with the untreated control
group (P<0.05), suggesting that ouabain induces cell death.
Ouabain induces apoptosis
To investigate whether ouabain induces apoptosis,
flow cytometric analysis with annexin V staining was performed. As
presented in Fig. 3A and B,
increasing concentration of ouabain significantly induced apoptosis
(P<0.01). In addition, a key feature of apoptosis is DNA
fragmentation, which it is possible to visualize as DNA laddering
following separation by gel electrophoresis (17). OS-RC-2 and NCI-H446 cells were treated
with a range of ouabain concentrations and DNA laddering was
visualized following separation by gel electrophoresis (Fig. 3C and D). Apoptosis regulator Bax is a
pro-apoptotic member of the Bcl-2 family, while other members
including Bcl-2 inhibit apoptosis (17). As presented in Fig. 3E, OS-RC-2 cells treated with a range
of ouabain concentrations for 48 h demonstrated a dose-dependent
increase in Bax protein levels and a decrease in Bcl-2 protein
levels. These results suggested that ouabain induces apoptosis in
cancer cells.
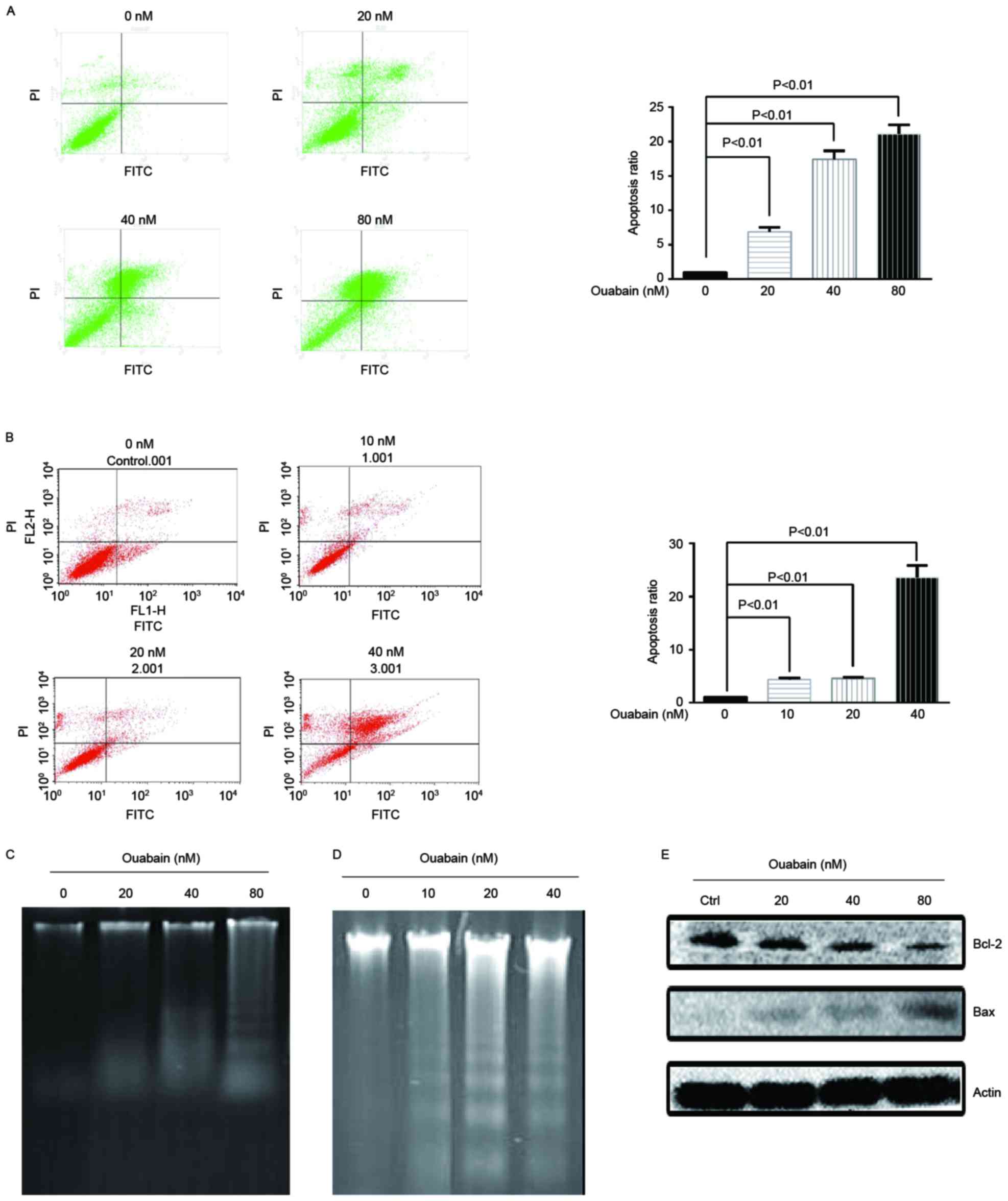 | Figure 3.Ouabain induces apoptosis. (A) OS-RC-2
cells were treated with a range of ouabain concentrations (0, 20,
40 and 80 nM) for 48 h and flow cytometric analysis was performed
to assess apoptosis using an AnnexinV-FITC/PI Apoptosis Detection
kit. (B) NCI-H446 cells were treated with a range of ouabain
concentrations (0, 10, 20 and 40 nM) for 48 h and flow cytometric
analysis was performed to assess apoptosis using an
AnnexinV-FITC/PI Apoptosis Detection kit. (C) OS-RC-2 cells were
treated with a range of ouabain concentrations (0, 20, 40 and 80
nM) for 48 h and then DNA laddering was visualized following
separation by gel electrophoresis. (D) NCI-H446 cells were treated
with a range of ouabain concentrations (0, 10, 20 and 40 nM) for 48
h and then DNA laddering was visualized following separation by gel
electrophoresis. (E) Bax and Bcl-2 protein expression was
determined in OS-RC-2 cells treated with a range of ouabain
concentrations (0, 20, 40 and 80 nM) for 48 h, using western
blotting. FITC, fluorescein isothiocyanate; PI, propidium iodide;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein. |
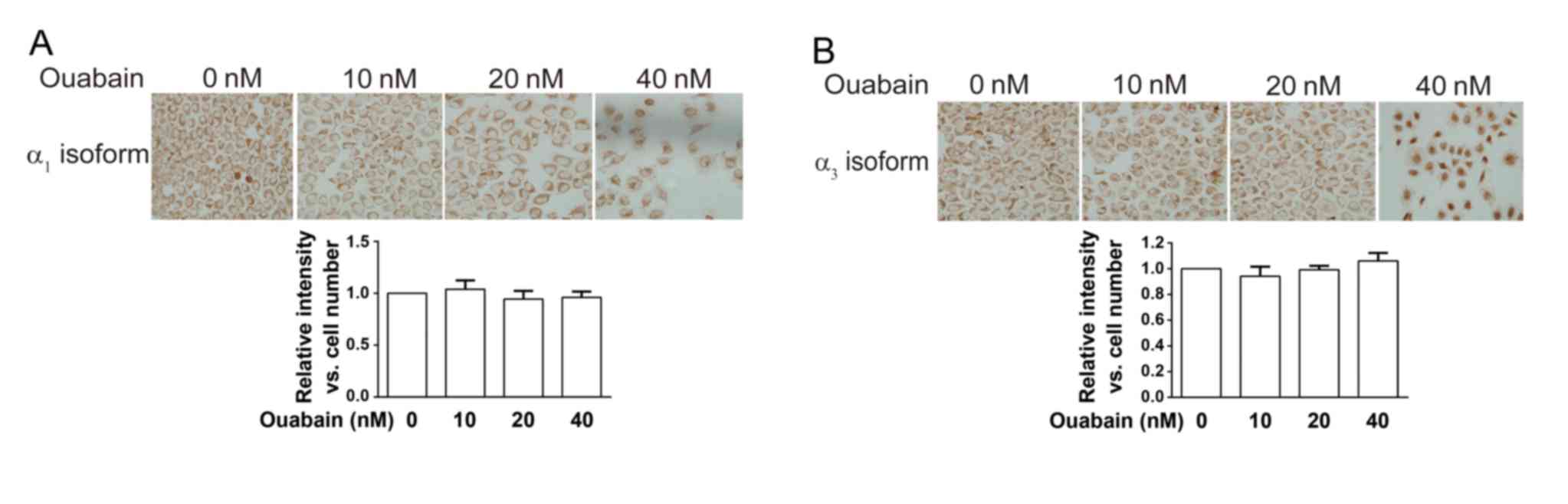
The anticancer effect of ouabain was
associated with think α3 isoform rather than the α1 isoform
To investigate the involvement of NKA in the
anticancer effect of ouabain, the expression of the NKA
α1 and α3 isoforms was determined using ICC
staining in NCI-H446 cells treated with a range of ouabain
concentrations (Fig. 4A and B). The
expression levels of the NKA α1 and α3
isoformswere determined using Image-Pro Plus software 6.0 and no
significant difference was observed between treated and untreated
cells. These results indicated that ouabain had no effect on the
expression of NKA α1 and α3 isoforms.
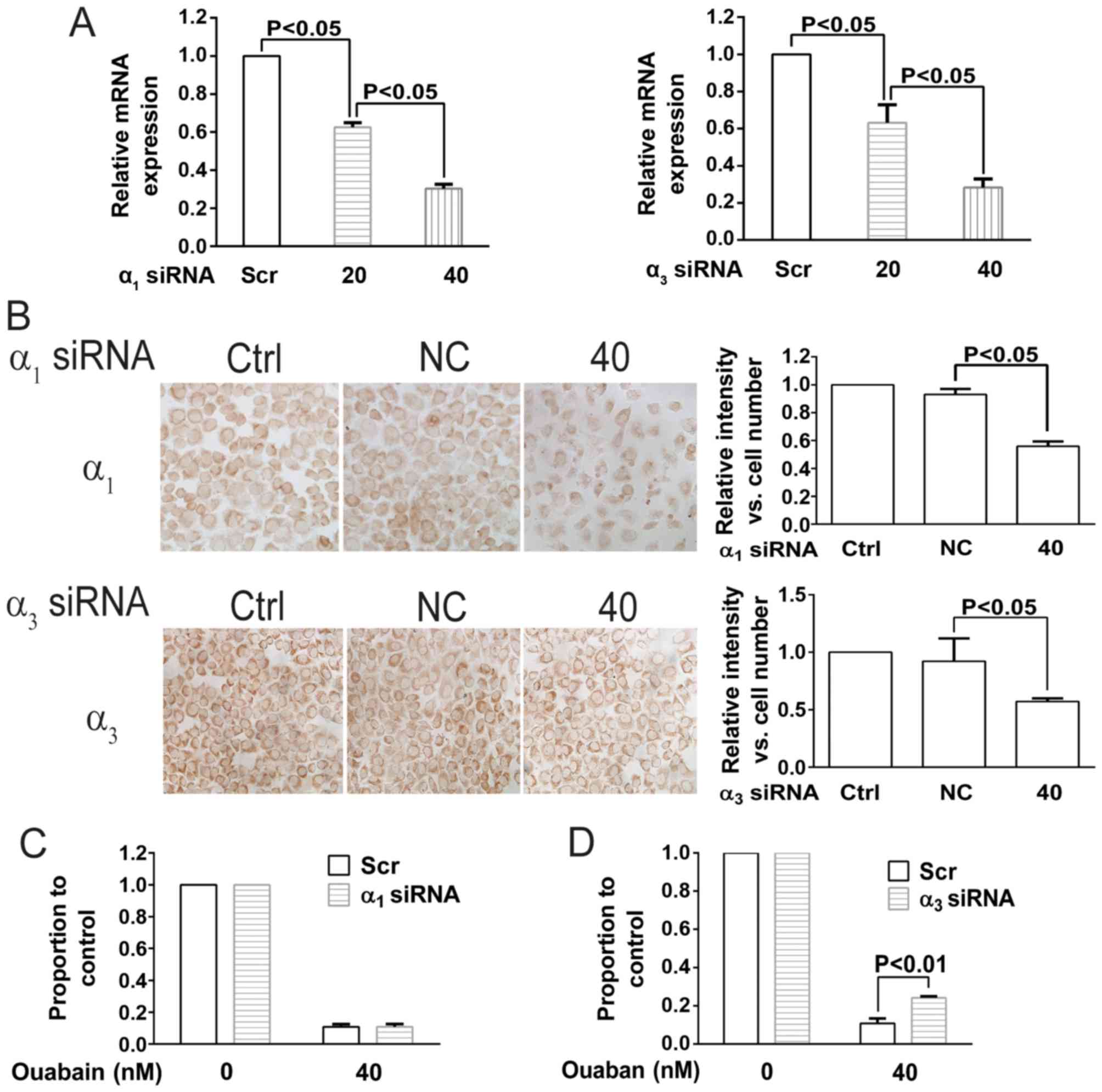
To further investigate the underlying molecular
mechanism, transfection with siRNAs targeting the NKA α1
and α3 isoforms was performed in NCI-H446 cells. As
presented in Fig. 5A and B, mRNA and
protein expression of the NKA α1 and α3
isoforms was significantly decreased following siRNA transfection.
NCI-H446 siRNA transfected cells were then treated with ouabain. As
presented in Fig. 5C and D, only the
siRNA targeting the NKA α3 isoform antagonized the
effect of ouabain; indicating that ouabain sensitivity is
associated with the NKA α3 isoform rather than
α1 isoform.
Discussion
Targeted therapy is expected to be more effective
than conventional treatments and less toxic to normal cells.
Several studies have demonstrated that NKA expression is associated
with cancer mortality rates (3,5,18). Therefore, NKA has attracted a lot of
interest as an anticancer target. The clinical use of ouabain for
the treatment of heart failure and atrial fibrillation is well
established. Additionally, a number of studies have demonstrated
that ouabain possesses antitumor activity (11,18–20).
However, several concerns, including high cytotoxicity (20), remain to be addressed and little is
known about the anticancer mechanism of ouabain.
The results of the present study demonstrated that
NKA inhibition by ouabain inhibits cell proliferation and induces
apoptosis, indicating that NKA serves a critical function in cell
growth and survival. To examine the associations of NKA isoforms
with ouabain sensitivity, siRNA-mediated knockdown of NKA
α1 and α3 isoforms was performed. siRNAs
targeting the NKA α1 and α3 isoforms
downregulated the mRNA and protein expression of each isoform,
respectively. However, only the NKA α3 isoform siRNA
partially rescued the cells from ouabain-induced growth inhibition,
suggesting that the anticancer effect of ouabain may be associated
with the NKA α3 isoform. NKA α3
isoform-knockdown did not fully reverse the growth inhibition, even
though the effect was statistically significant; suggesting that
other factors may be involved in the anticancer effect of ouabain.
Further research is required to elucidate the underlying molecular
mechanisms. The results of the present study demonstrated that NKA
inhibition attenuates cellular proliferation and induces apoptosis,
mediated by increased Ca2+ and ROS intracellular levels.
NKA α3 isoform siRNA knockdown impaired the
antiproliferative effect of ouabain, suggesting that ouabain
preferentially binds to the NKA α3 isoform. These
results indicated that the NKA α3 isoform may be the
anticancer molecular target of ouabain. Future research, should
concentrate on further investigating the anticancer mechanism of
ouabain and reducing its cardiotoxicity.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (grant no. 31640053), the
Natural Science Foundation of Fujian Province (grant nos.
2016Y0029, 2016J01149 and 2016J01146) and the open Scientific
Foundation of Fujian Key Laboratory (grant no. 2014ZDSY2002).
Glossary
Abbreviations
Abbreviations:
|
NKA
|
Na+/K+-ATPase
|
|
ICC
|
immunocytochemistry
|
|
ROS
|
reactive oxygen species
|
|
IC50
|
half-maximal inhibitory
concentration
|
|
AO/EB
|
acridine orange/ethidium bromide
|
|
DCFH-DA
|
dichloro-dihydro-fluorescein
diacetate
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
Bax
|
Bcl-2-associated X protein
|
|
RT
|
room temperature
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Jemal A, Bray F, Ferlay J, Ward E and
Forman D: Global cancer statistics. CA Cancer J Clin. 61:69–90.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jorgensen PL, Hakansson KO and Karlish SJ:
Structure and Mechanism of Na, K-ATPase: Functional Sites and Their
Interactions. Ann Rev Physiol. 65:817–849. 2003. View Article : Google Scholar
|
|
3
|
Bechmann M Baker, Rotoli D, Morales M,
Mdel C Maeso, García Mdel P, Ávila J, Mobasheri A and
Martín-Vasallo P: Na, K-ATPase isozymes in colorectal cancer and
liver metastases. Front Physiol. 7:92016.PubMed/NCBI
|
|
4
|
Skou JC: The influence of some cations on
an adenosine triphosphatase from peripheral nerves. Biochim Biophys
Acta. 23:394–401. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhuang L, Xu L, Wang P, Jiang Y, Yong P,
Zhang C, Zhang H, Meng Z and Yang P: Na+/K+
-ATPase α1 subunit, a novel therapeutic target for hepatocellular
carcinoma. Oncotarget. 6:28183–28193. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prassas I and Diamandis EP: Novel
therapeutic applications of cardiac glycosides. Nat Rev Drug
Discov. 7:926–935. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kong D, Li J, Zhao B, Xia B, Zhang L, He
Y, Wang X, Gao L, Wang Y, Jin X and Lou G: The effect of SCF and
ouabain on small intestinal motility dysfunction induced by gastric
cancer peritoneal metastasis. Clin Exp Metastasis. 32:267–277.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin HK, Ryu BJ, Choi SW, Kim SH and Lee
K: Inactivation of Src-to-ezrin pathway: A possible mechanism in
the ouabain-mediated inhibition of A549 cell migration. Biomed Res
Int. 2015:5371362015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan X, Liang F, Li D and Zheng J: Ouabain
elicits human glioblastoma cells apoptosis by generating reactive
oxygen species in ERK-p66SHC-dependent pathway. Mol Cell Biochem.
398:95–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ninsontia C and Chanvorachote P: Ouabain
mediates integrin switch in human lung cancer cells. Anticancer
Res. 34:5495–5502. 2014.PubMed/NCBI
|
|
11
|
Mijatovic T, Van Quaquebeke E, Delest B,
Debeir O, Darro F and Kiss R: Cardiotonic steroids on the road to
anti-cancer therapy. Biochim Biophys Acta. 1776:32–57.
2007.PubMed/NCBI
|
|
12
|
Newman RA, Yang P, Pawlus AD and Block KI:
Cardiac glycosides as novel cancer therapeutic agents. Mol Interv.
8:36–49. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu ZW, Wang FM, Gao MJ, Chen XY, Shan NN,
Cheng SX, Mai X, Zala GH, Hu WL and Xu RC: Cardiotonic steroids
attenuate ERK phosphorylation and generate cell cycle arrest to
block human hepatoma cell growth. J Steroid Biochem Mol Biol.
125:181–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Tian J, Haas M, Shapiro JI, Askari
A and Xie Z: Ouabain interaction with cardiac
Na+/K+-ATPase initiates signal cascades
independent of changes in intracellular Na+ and
Ca2+ concentrations. J Biol Chem. 275:27838–27844.
2000.PubMed/NCBI
|
|
16
|
Majno G and Joris I: Apoptosis, oncosis,
and necrosis: An overview of cell death. Am J Pathol. 146:3–15.
1995.PubMed/NCBI
|
|
17
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stenkvist B: Is digitalis a therapy for
breast carcinoma? Oncol Rep. 6:493–499. 1999.PubMed/NCBI
|
|
19
|
Haux J: Digitoxin is a potential
anticancer agent for several types of cancer. Med Hypotheses.
53:543–548. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Winnicka K, Bielawski K and Bielawska A:
Cardiac glycosides in cancer research and cancer therapy. Acta Pol
Pharm. 63:109–115. 2006.PubMed/NCBI
|