Introduction
Pancreatic neuroendocrine tumors (pNETs) are tumors
arising from the endocrine cells of the pancreas; pNETs comprise
<3% of novel pancreatic neoplasms (1). Its incidence has increased in recent
years since the introduction of novel diagnostic procedures
(2). pNETs can be generally divided
into functional and nonfunctional tumors. Nonfunctional pNETs
constitute ~85% of all pNETs and are more aggressive compared with
the functional pNETs (1,3). Although they are generally viewed as
indolent tumors, pNETs are highly heterogenous neoplasms and
certain subgroups may demonstrate aggressive characteristics
(4,5).
Currently, therapeutic methods for pNETs are diverse, including
surgical resection and non-surgical interventions (targeted
therapies, chemotherapy, somatostatin analogues, peptide receptor
radionuclide therapy and liver-directed therapies) (6–8). Close
observation may be required to small pNETs (9,10).
Therefore, biomarkers that reflect the aggressive features of pNETs
are urgently required in order to aid therapeutic decisions and
follow-up observations (11).
Carbohydrate antigen 19-9 (CA19-9) is a
tumor-associated carbohydrate biomarker that was derived from a
human colorectal cancer cell line targeted by the monoclonal
antibody 1116-NS-19-9 (12). It has
been widely used in the management of gastrointestinal
malignancies, particularly for pancreatic cancer (11,13). In a
pool data analysis of CA19-9 for the diagnosis of pancreatic
cancer, the median sensitivity was 79% (70–90%) and the median
specificity was 82% (68–91%) (14).
It is a sialylated Lewis blood group antigen and its secretion is
influenced by Lewis antigen phenotypes (13). The elevation of CA19-9 expression
levels has also been observed in other conditions, including
biliary obstruction and inflammation, digestive tract inflammation
and other gastrointestinal malignancies, which limits its clinical
application in pancreatic cancer (13,14).
CA19-9 is not generally considered to be a
diagnostic or prognostic biomarker in pNETs as the majority of
pNETs present with normal range of CA19-9 (15,16).
Conversely, in view of its abnormal increased expression level in
common pancreatic cancer; CA19-9 has been used as a diagnostic
marker to differentiate pancreatic cancer from pNETs (15,16).
However, few previous studies have demonstrated that CA19-9 may be
used as a prognostic biomarker in neuroendocrine tumors (17,18). For
example, Elisei et al (17)
reported a case of multiple endocrine neoplasia type 2B with
significant elevation of CA19-9 expression levels. The patient
experienced rapid disease progression and survived for only a short
period, indicating that CA19-9 may be a biomarker of aggressiveness
(17). A further study conducted by
Elisei et al (18) revealed
that 16/100 advanced structural recurrent/persistent medullary
thyroid cancer tissues exhibited high expression levels of CA19-9,
and the CA19-9 positive group demonstrated a higher mortality rate
compared with the normal CA19-9 expression group.
The aim of current study was to evaluate the role of
serum CA19-9 expression levels as a prognostic factor in a
relatively large cohort of patients with pNETs at various clinical
stages. Potential factors associated with abnormally increased
expression levels of CA19-9 in pNETs were also investigated.
Materials and methods
Study design and treatment
Patients (156 cases) were retrospectively retrieved
from a single institution (Shanghai Cancer Center, Fudan
University, Shanghai, China) between June 2006 and February 2015.
The male-female ratio was ~1.1:1, with a mean age of 53 (range,
15–77). Specimens were collected prior to initiating major
treatment by the Tissue Bank, Shanghai Cancer Center (Shanghai,
China). The enrollment criteria included subjects who had baseline
CA19-9 information. In addition, patients were included if they had
complete demographics information. Patients were excluded if the
diagnosis of pNET was not pathologically confirmed. All the cases
were staged according to the modified European Neuroendocrine Tumor
Society Tumor-Node-Metastasis (TNM) staging system (19). Tumors were classified as G1, G2 or G3
according to the 2010 World Health Organization classification
(based on the mitotic index and the Ki-67 index) (20). All clinical and pathological data were
collected from patient medical records obtained from the Shanghai
Cancer Center, Fudan University. Incidental pNETs were detected
during health check-ups or evaluations for unassociated symptoms
(15). Functional pNETs were also
included in incidental pNETs in the present study. The primary end
point was set as overall survival. Follow-up information was
updated in December 2016, with a median follow-up time of 32
months. Survival time was determined from the date of final
diagnosis to the date of the last follow-up or mortality. The
present study was approved by the Ethical Committee of Shanghai
Cancer Center, Fudan University. Informed written consent was
obtained from all patients prior to enrollment in the current
study.
CA19-9 evaluation
Serum CA19-9 expression levels were examined within
2 weeks prior to major treatment initiation using an
electrochemiluminescence immunoassay on the Roche Cobas e601 (Roche
MODU D+P model, D2400-P800) immunoassay analyzer (Roche Diagnostics
GmbH, Mannheim, Germany) according to the manufacturer's protocol.
The recommended upper limit for the serum CA19-9 expression level
was <37 U/ml. CA19-9 has been reported to be affected by biliary
obstruction, inflammation and other conditions (14); thus, all patients with serum bilirubin
>2 mg/ml, biliary obstruction and inflammation, digestive tract
inflammation or other gastrointestinal malignancies at the time of
CA19-9 evaluation were excluded (165 cases were included at the
start and 156 cases were included subsequent to the
exclusions).
Statistical analysis
The receiver operating characteristic (ROC) curve
and area under the ROC curve were used to select the optimal
cut-off values for baseline CA19-9 expression levels. Time-to-event
variables were determined using the Kaplan-Meier method. Arms
stratified by potential prognostic factors (age, gender, size,
location, TNM stage, CA19-9 levels, grade and symptom) were
analyzed by the log-rank tests. The Cox's proportional hazard ratio
(HR) with a 95% confidence interval (CI) was used to estimate the
difference between the stratified arms using the Stata®
version 12.0 statistical software package (StataCorp LP, College
Station, TX, USA). Categorical data were analyzed using Pearson's
χ2 test or Fisher's exact test as appropriate. A
two-sided P<0.05 was considered to indicate a statistically
significant difference.
Results
Data and survival analysis of
patients
A total of 156 patients with pathologically
confirmed pNETs were included in the final analysis (Table I). The male-female ratio was ~1.1:1,
with 55.8% of patients being >50-years old (range, 15–77). A
total of 65 (41.7%) patients had tumors located at the head of the
pancreas and 53 (34.0%) patients had tumors <3 cm in diameter.
In the current series, 65.4% of patients had stage I or II tumors.
A majority (73.1%) of patients had G1 or G2 diseases, and 15 (9.6%)
patients had functional diseases. For all the patients, ~59.0% of
the patients had pNETs with symptoms and the other 41% were
asymptomatic.
 | Table I.Demographics and clinical
characteristics. |
Table I.
Demographics and clinical
characteristics.
| Demographic/clinical
characteristics | Patients, n (%) |
|---|
| Age |
|
| ≤50
years | 69 (44.2) |
| >50
years | 87 (55.8) |
| Gender |
|
| Male | 73 (46.8) |
|
Female | 83 (53.2) |
| Location |
|
| Head | 65 (41.7) |
|
Other | 91 (58.3) |
| Size |
|
| ≤3
cm | 53 (34.0) |
| >3
cm | 103 (66.0) |
| TNM stage |
|
| I,
II | 102 (65.4) |
| III,
IV | 54 (34.6) |
| Grade |
|
| G1,
G2 | 114 (73.1) |
| G3 | 16 (10.3) |
|
Unknown | 26 (16.7) |
| Functional |
|
|
Positive | 15 (9.6) |
|
Negative | 141 (90.4) |
| Symptomatic |
|
|
Positive | 92 (59.0) |
|
Negative | 64 (41.0) |
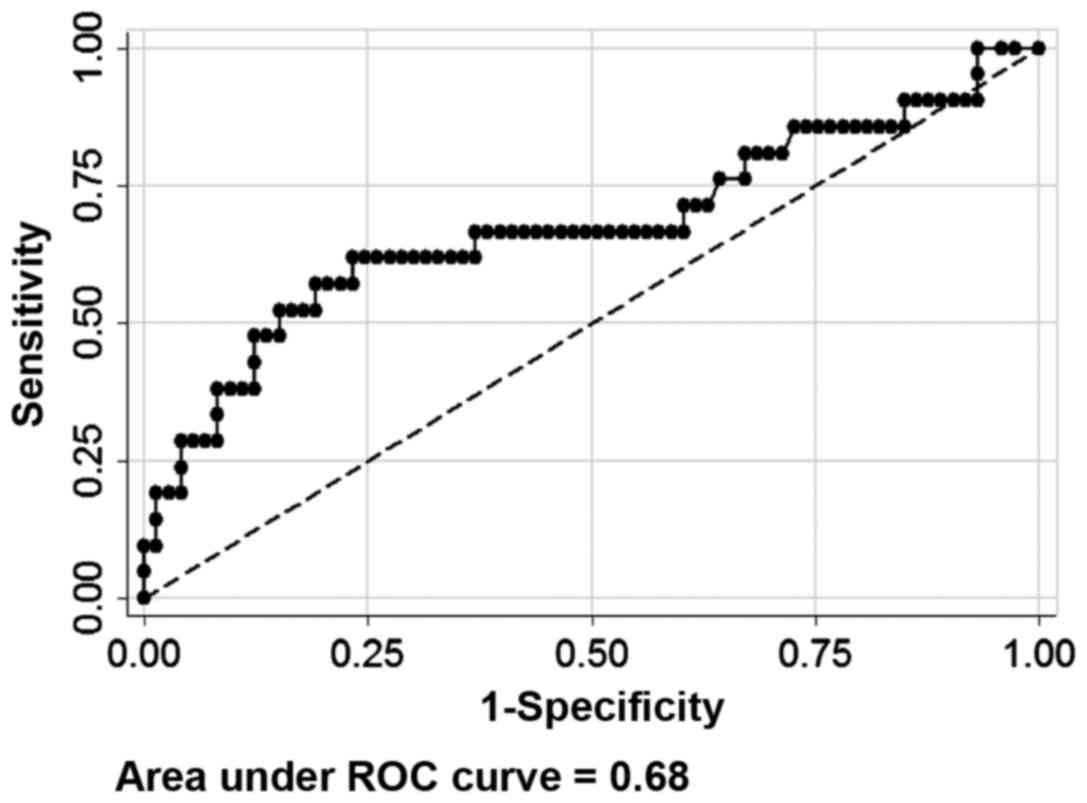
The selected cut-off value for CA19-9 as a
prognostic predictor of pNETs was 16 U/ml by the ROC curve (area
under ROC curve, 0.68; sensitivity 61.9%; specificity 76.7%;
Fig. 1), with 32.7% of cases having
CA19-9 expression levels higher than the cut-off value. A total of
8 (5.1%) cases had a CA19-9 expression level >100 U/ml and 22
(14.1%) cases were >37 U/ml. Univariate analysis was performed
in order to evaluate factors associated with overall survival using
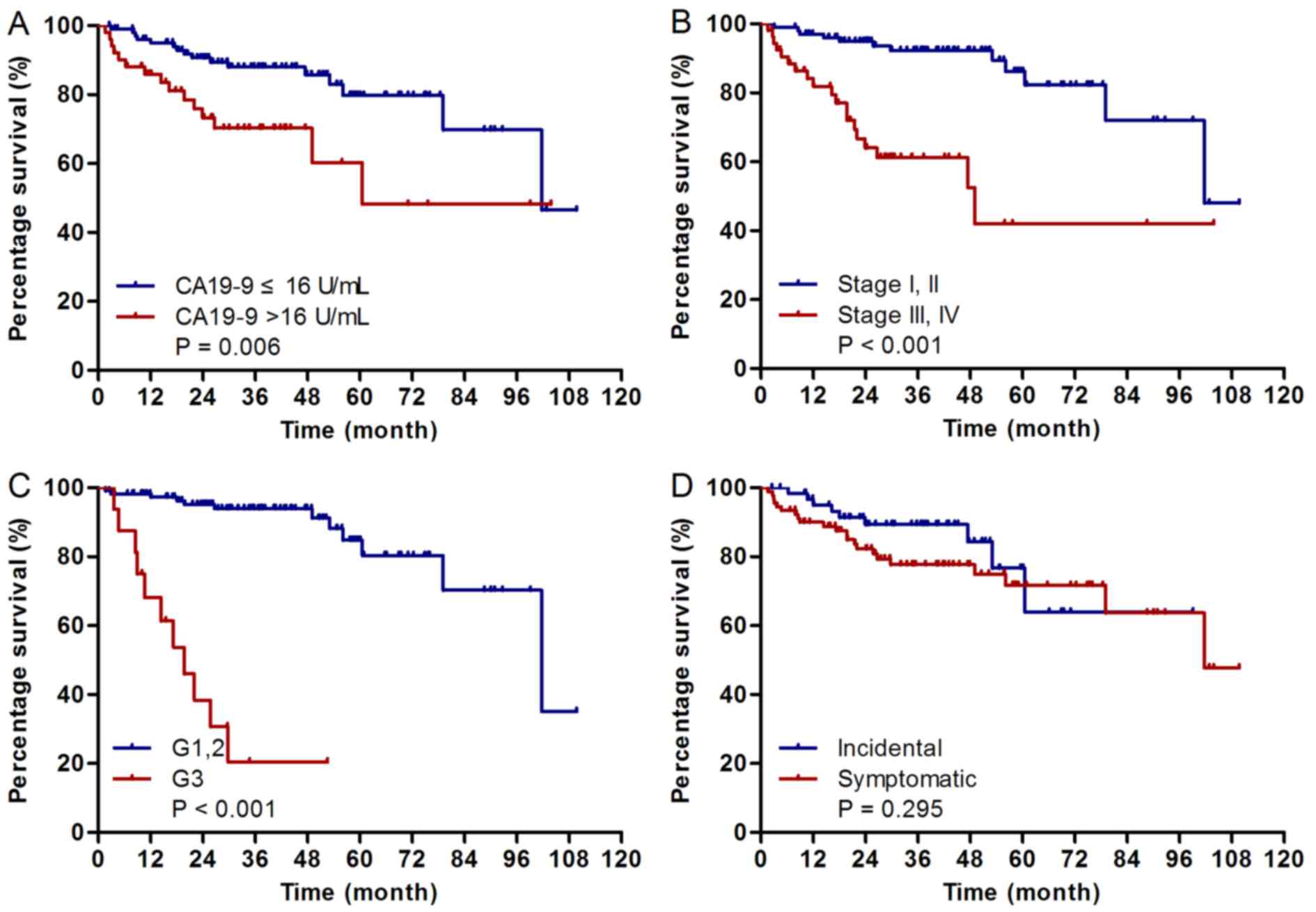
the Cox's proportional hazards model. The results demonstrated that
TNM stage III or IV (HR=5.08; P<0.001), CA19-9 >16 U/ml
(HR=2.60; P=0.006) and G3 diseases (HR=13.43; P<0.001) are
adverse prognostic factors for patients' overall survival, whereas
age, gender, tumor size and tumor location were not significantly
associated with overall survival (Table
II; Fig. 2). In multivariate
analysis, TNM stage III or IV (HR=2.88; P=0.018) and G3 diseases
(HR=8.79; P<0.001) were determined to be adverse prognostic
factors for patients' overall survival (Table II).
 | Table II.Univariate and multivariate analysis
for overall survival of all patients using the Cox proportional
hazards model. |
Table II.
Univariate and multivariate analysis
for overall survival of all patients using the Cox proportional
hazards model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
|
≤50 | 1 | / | / |
|
|
|
|
>50 | 0.82 | 0.40–1.67 | 0.581 |
|
|
|
| Gender |
|
|
|
|
|
|
|
Male | 1 | / | / |
|
|
|
|
Female | 0.52 | 0.25–1.08 | 0.075 |
|
|
|
| Size (cm) |
|
|
|
|
|
|
| ≤3 | 1 | / | / |
|
|
|
|
>3 | 1.88 | 0. 81–4.38 | 0.135 |
|
|
|
| Location |
|
|
|
|
|
|
|
Head | 1 | / | / |
|
|
|
|
Other | 1.16 | 0.56–2.41 | 0.682 |
|
|
|
| TNM stage |
|
|
|
|
|
|
| I,
II | 1 | / | / | 1 | / | / |
| III,
IV | 5.08 | 2.42–10.67 | <0.001 | 2.88 | 1.20–6.93 | 0.018a |
| CA19-9 (U/ml) |
|
|
|
|
|
|
|
|
≤16 | 1 | / | / | 1 | / | / |
|
>16 | 2.60 | 1.28–5.28 | 0.006 | 1.52 | 0.70–3.29 | 0.286 |
| Grade |
|
|
|
|
|
|
| G1,
G2 | 1 | / | / | 1 | / | / |
| G3 | 13.43 | 5.65–31.93 | <0.001 | 8.79 | 3.47–22.28 | <0.001a |
|
Unknown | 3.10 | 1.25–7.69 | 0.015 | 1.77 | 0.65–4.80 | 0.262 |
| Incidental |
|
|
|
|
|
|
|
Negative | 1 | / | / |
|
|
|
|
Positive | 0.67 | 0.30–1.45 | 0.295 |
|
|
|
Parameters associated with baseline
NLR levels
A χ2 test was performed in order to
compare clinicopathological characteristics between the CA19-9 ≤16
U/ml group and the CA19-9 >16 U/ml group (Table III). The CA19-9 >16 U/ml group
had a significantly higher proportion of patients with TNM stage
III/IV (P=0.001), but not age (P=0.379), gender (P=0.465), location
(P=0.931), nerve invasion (P=0.429), functional (P=0.085) and
symptomatic status (P=0.310). Although not statistically
significant, the CA19-9 >16 U/ml group also demonstrated a trend
to have a greater proportion of G3 tumors (P=0.075), positive lymph
status (P=0.057), tumor size (P=0.119) and vessel invasion
(P=0.093).
 | Table III.Serum CA19-9 expression levels,
patient demographics and clinical characteristics. |
Table III.
Serum CA19-9 expression levels,
patient demographics and clinical characteristics.
| Demographic or
clinical characteristic | CA19-9 ≤16
U/ml | CA19-9 >16
U/ml | P-value |
|---|
| Age |
|
| 0.379 |
| ≤50
years | 49 | 20 |
|
| >50
years | 56 | 31 |
|
| Gender |
|
| 0.465 |
|
Male | 47 | 26 |
|
|
Female | 58 | 25 |
|
| Location |
|
| 0.931 |
|
Head | 44 | 21 |
|
|
Others | 61 | 30 |
|
| Size |
|
| 0.119 |
| ≤3
cm | 40 | 13 |
|
| >3
cm | 65 | 38 |
|
| TNM stage |
|
| 0.001a |
| I,
II | 78 | 24 |
|
| III,
IV | 27 | 27 |
|
| Grade |
|
| 0.075 |
| G1,
G2 | 82 | 32 |
|
| G3 | 8 | 8 |
|
| Lymph
statusb |
|
| 0.057 |
|
Positive | 12 | 10 |
|
|
Negative | 45 | 14 |
|
| Vessel
invasionb |
|
| 0.093 |
|
Positive | 10 | 8 |
|
|
Negative | 41 | 15 |
|
| Nerve
invasionb |
|
| 0.429 |
|
Positive | 7 | 5 |
|
|
Negative | 42 | 18 |
|
| Functional |
|
| 0.085 |
|
Positive | 13 | 2 |
|
|
Negative | 92 | 49 |
|
| Symptomatic |
|
| 0.310 |
|
Positive | 59 | 33 |
|
|
Negative | 46 | 18 |
|
Discussion
The present study determined the cut-off value for
CA19-9 as a prognostic predictor of pNETs to be 16 U/ml, by ROC
curve. Univariate analysis demonstrated that CA19-9 >16 U/ml was
an adverse prognostic factor for patients' overall survival. It was
also revealed that the CA19-9 >16 U/ml group had a statistically
higher proportion of TNM stage III/IV, as compared with the CA19-9
≤16 U/ml group. These findings indicate that CA19-9 may be a
prognostic biomarker of pNETs, which may be able to reflect the
aggressiveness and severity of the disease.
Chromogranin A (CgA) is currently the most widely
used and most characterized biomarker of pNETs, and is detected in
the circulation (16,21–23). In
contrast to CgA, which is known to be elevated in well- and
moderately-differentiated NETs (23),
the present study demonstrated that increased CA19-9 expression
levels indicated s poor prognosis, G3 disease, advanced stage and
aggressive features. Therefore, for cases with abnormal elevation
of the CA19-9 expression level, active treatment, including
surgical resection and adjuvant treatments, and close follow-up
must be strongly recommended and observation alone should not be
used. In addition, CA19-9 may supplement CgA as a prognostic
biomarker for pNETs. Furthermore, CgA should be combined with
CA19-9 to reflect the tumor volume and severity of pNETs.
Compared with symptomatic non-functional pNETs,
incidental pNETs are more frequently <2 cm in diameter, stage
T1, node negative, grade I and associated with improved prognosis
(15). Cheema et al (15) evaluated 143 patients with stage I–III
pNETs, and the 5-year progression-free survival rate of
incidentally diagnosed tumors was significantly higher compared
with symptomatic tumors (86.0 vs. 59.0%; P=0.007). The present
study revealed that 41.0% of pNETs were incidental pNETs, and these
demonstrated no significantly improved survival compared with
symptomatic tumors (HR=0.67; P=0.295), contrary to the results of a
previous study (15).
In the present study cohort, only 8 (5.1%) cases had
a CA19-9 expression level >100 U/ml and 22 (14.1%) cases were
>37 U/ml, which is a lower frequency compared with that in
pancreatic cancer (14). However, the
use of CA19-9 as a differentiating diagnostic marker for pancreatic
cancer must be utilized with caution, as >14% of pNETs were
found to have aberrant CA19-9 secretion (>37 U/ml). The
molecular mechanisms underlying the abnormal elevation of CA19-9
expression levels in pNETs are largely undefined. Previous studies
have demonstrated that CA19-9 abnormal secretion may be explained
by tumor hypoxia and glycosylation (24,25). The
observation that CA19-9 was correlated with TNM stage and vessel
invasion in pNETs in the present study also indicates that tumor
hypoxia and glycosylation may be potential associated mechanisms.
Further studies are required in order to confirm this
hypothesis.
In the present study, pNETs with a CA19-9 >16
U/ml demonstrated a trend towards having a higher proportion of G3
tumors, as compared with pNETs with a CA19-9 ≤16 U/ml (P=0.075). G3
pNETs are well known for their aggressiveness and poor response to
major treatment strategies, including surgery (26). Considering the differing management
strategy between G1, G2 and G3 pNETs in clinical practice, the
aberrant elevation of CA19-9 may serve as an indication of G3 pNETs
for clinicians (26). However,
further studies with larger sample cohort are required.
The novelty of the present study is in that, to the
best of our knowledge, it is the first to reveal that CA19-9 is a
prognostic biomarker of pNETs, which may be able to reflect poor
prognosis, advanced stage and aggressive characteristics. This
result is in contrast to the results of a previous study that
indicated that CA19-9 has limited value in the management of pNETs
(27). In addition, CA19-9 may
supplement CgA as a biomarker to guide the management of pNETs.
However, as the present study was retrospective, the results must
be interpreted with caution. Further prospective studies with
larges sample sizes are urgently required in order to confirm these
findings.
Acknowledgements
The present study was supported by the National
Science Foundation for Distinguished Young Scholars of China (grant
no. 81625016), the National Natural Science Foundation of China
(grant nos. 81372649, 81172276, 81370065 and 81372653), the
Shanghai Municipal Commission of Health and Family Planning
Scientific Research (grant no. 20144Y0170) and the Basic Research
Projects of the Science and Technology Commission of Shanghai
Municipality (grant no. 15JC1401200).
References
|
1
|
Halfdanarson TR, Rabe KG, Rubin J and
Petersen GM: Pancreatic neuroendocrine tumors (PNETs): Incidence,
prognosis and recent trend toward improved survival. Ann Oncol.
19:1727–1733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luo G, Liu Z, Guo M, Jin K, Xiao Z, Liu L,
Xu J, Zhang B, Liu C, Huang D, et al: (18)F-FDG PET/CT can be used
to detect non-functioning pancreatic neuroendocrine tumors. Int J
Oncol. 45:1531–1536. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bilimoria KY, Tomlinson JS, Merkow RP,
Stewart AK, Ko CY, Talamonti MS and Bentrem DJ: Clinicopathologic
features and treatment trends of pancreatic neuroendocrine tumors:
Analysis of 9,821 patients. J Gastrointest Surg. 11:1460–1469.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du S, Wang Z, Sang X, Lu X, Zheng Y, Xu H,
Xu Y, Chi T, Zhao H, Wang W, et al: Surgical resection improves the
outcome of the patients with neuroendocrine tumor liver metastases:
Large data from Asia. Medicine (Baltimore). 9:e3882015. View Article : Google Scholar
|
|
5
|
Frilling A, Akerström G, Falconi M, Pavel
M, Ramos J, Kidd M and Modlin IM: Neuroendocrine tumor disease: An
evolving landscape. Endocr Relat Cancer. 19:R163–R185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Falconi M, Zerbi A, Crippa S, Balzano G,
Boninsegna L, Capitanio V, Bassi C, Di Carlo V and Pederzoli P:
Parenchyma-preserving resections for small nonfunctioning
pancreatic endocrine tumors. Ann Surg Oncol. 17:1621–1627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valle JW, Eatock M, Clueit B, Gabriel Z,
Ferdinand R and Mitchell S: A systematic review of non-surgical
treatments for pancreatic neuroendocrine tumours. Cancer Treat Rev.
40:376–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang M, Zeng L, Zhang Y, Su AP, Yue PJ and
Tian BL: Surgical treatment and clinical outcome of nonfunctional
pancreatic neuroendocrine tumors: A 14-year experience from one
single center. Medicine (Baltimore). 93:e942014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuo EJ and Salem RR: Population-level
analysis of pancreatic neuroendocrine tumors 2 cm or less in size.
Ann Surg Oncol. 20:2815–2821. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee LC, Grant CS, Salomao DR, Fletcher JG,
Takahashi N, Fidler JL, Levy MJ and Huebner M: Small,
nonfunctioning, asymptomatic pancreatic neuroendocrine tumors
(PNETs): Role for nonoperative management. Surgery. 152:965–974.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu B, Tang LH, Liu Z, Mei M, Yu R, Dhall
D, Qiao XW, Zhang TP, Zhao YP, Liu TH, et al: α-Internexin: A novel
biomarker for pancreatic neuroendocrine tumor aggressiveness. J
Clin Endocrinol Metab. 99:E786–E795. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koprowski H, Steplewski Z, Mitchell K,
Herlyn M, Herlyn D and Fuhrer P: Colorectal carcinoma antigens
detected by hybridoma antibodies. Somatic Cell Genet. 5:957–971.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo G, Xiao Z, Long J, Liu Z, Liu L, Liu
C, Xu J, Ni Q and Yu X: CA125 is superior to CA19-9 in predicting
the resectability of pancreatic cancer. J Gastrointest Surg.
17:2092–2098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goonetilleke KS and Siriwardena AK:
Systematic review of carbohydrate antigen (CA 19-9) as a
biochemical marker in the diagnosis of pancreatic cancer. Eur J
Surg Oncol. 33:266–270. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheema A, Weber J and Strosberg JR:
Incidental detection of pancreatic neuroendocrine tumors: An
analysis of incidence and outcomes. Ann Surg Oncol. 19:2932–2936.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hijioka M, Ito T, Igarashi H, Fujimori N,
Lee L, Nakamura T, Jensen RT and Takayanagi R: Serum chromogranin A
is a useful marker for Japanese patients with pancreatic
neuroendocrine tumors. Cancer Sci. 105:1464–1471. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elisei R, Lorusso L, Romei C, Bottici V,
Mazzeo S, Giani C, Fiore E, Torregrossa L, Insilla AC, Basolo F, et
al: Medullary thyroid cancer secreting carbohydrate antigen 19-9
(Ca 19-9): A fatal case report. J Clin Endocrinol Metab.
98:3550–3554. 2012. View Article : Google Scholar
|
|
18
|
Elisei R, Lorusso L, Piaggi P, Torregrossa
L, Pellegrini G, Molinaro E, Agate L, Bottici V, Pani F, Insilla A
Cacciato, et al: Elevated serum levels of carbohydrate antigen 19.9
(Ca 19.9) is a prognostic factor of death in patients with advanced
medullary thyroid cancer. Eur J Endocrinol. 173:297–304. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo G, Javed A, Strosberg JR, Jin K, Zhang
Y, Liu C, Xu J, Soares K, Weiss MJ, Zheng L, et al: Modified
staging classification for pancreatic neuroendocrine tumors on the
basis of the American Joint Committee on Cancer and European
Neuroendocrine Tumor Society Systems. J Clin Oncol. 35:274–280.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rindi G, Arnold R, Bosman FT, Capella C,
Klimstra D and Klöppel G: Nomenclature and classification of
neuroendocrine neoplasms of the digestive systemBosman TF, Carneiro
F, Hruban RH and Theise ND: WHO classification of tumours of the
digestive system. Lyon, France: International Agency for Research
on Cancer (IARC); 2010
|
|
21
|
Campana D, Nori F, Piscitelli L,
Morselli-Labate AM, Pezzilli R, Corinaldesi R and Tomassetti P:
Chromogranin A: Is it a useful marker of neuroendocrine tumors? J
Clin Oncol. 25:1967–1973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zatelli MC, Torta M, Leon A, Ambrosio MR,
Gion M, Tomassetti P, De Braud F, Fave G Delle, Dogliotti L and
Uberti EC degli; Italian CromaNet Working Group, : Chromogranin A
as a marker of neuroendocrine neoplasia: An Italian Multicenter
Study. Endocr Relat Cancer. 14:473–482. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Modlin IM, Gustafsson BI, Moss SF, Pavel
M, Tsolakis AV and Kidd M: Chromogranin A-biological function and
clinical utility in neuro endocrine tumor disease. Ann Surg Oncol.
17:2427–2443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kannagi R: Carbohydrate antigen sialyl
Lewis a-its pathophysiological significance and induction mechanism
in cancer progression. Chang Gung Med J. 30:189–209.
2007.PubMed/NCBI
|
|
25
|
Kannagi R, Sakuma K, Miyazaki K, Lim KT,
Yusa A, Yin J and Izawa M: Altered expression of glycan genes in
cancers induced by epigenetic silencing and tumor hypoxia: Clues in
the ongoing search for new tumor markers. Cancer Sci. 101:586–593.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin K, Xu J, Chen J, Chen M, Chen R, Chen
Y, Chen Z, Cheng B, Chi Y, Feng ST, et al: Surgical management for
non-functional pancreatic neuroendocrine neoplasms with synchronous
liver metastasis: A consensus from the Chinese Study Group for
Neuroendocrine Tumors (CSNET). Int J Oncol. 49:1991–2000.
2016.PubMed/NCBI
|
|
27
|
Li J, Luo G, Fu D, Jin C, Hao S, Yang F,
Wang X, Yao L and Ni Q: Preoperative diagnosis of nonfunctioning
pancreatic neuroendocrine tumors. Med Oncol. 28:1027–1031. 2011.
View Article : Google Scholar : PubMed/NCBI
|