Introduction
According to the global cancer statistics in 2012,
hepatocellular carcinoma (HCC) is the sixth most common cancer
globally, and the survival outcomes are poor with 5-year overall
survival (OS) rates estimated at <12% (1,2). Surgery
or transplantation remain the mainstays of curative therapy for
early disease. Ablative strategies can also cure tumors. However,
relatively few patients are eligible for curative therapy due to
the late appearance of symptoms (3).
Medical strategies for treating HCC have advanced little during the
past 20 years. Traditional systemic chemotherapy represents a
limited treatment option associated with a small survival advantage
(4). Therefore, identifying novel
molecular biomarkers with the potential to evaluate tumor
recurrence and progression is crucial.
The doublecortin-like kinase 1 (DCLK1) gene, located
at human chromosome 13q13.3, encodes a member of the protein kinase
superfamily and the doublecortin family (5). The kinase encoded by this gene was first
described in the context of the nervous system, in which DCLK1
catalyzed the polymerization of tubulin into microtubules (6). Giannakis et al (7) were the first to demonstrate that DCLK1
regulated biological processes outside of the central nervous
system. This discovery revealed that DCLK1 was associated with
tumorigenesis and its progression. Immunohistochemical analysis
using a DCLK1 antibody revealed single cell staining in intestinal
crypt sections and gastric isthmus cells, which suggested that
DCLK1 represented a marker of adult gastric and small intestinal
stem cells (8). Nakanishi et
al (9) subsequently demonstrated
that DCLK1 marked cancer stem cells (CSCs) rather than normal stem
cells in the polyps of APC multiple intestinal neoplasia
(Min)/+ mice using lineage-tracing experiments. In addition,
DCLK1 was reported to be a putative CSC marker in pancreatic and
colon cancer via the same strategy (10,11).
CSCs were first identified in acute myeloid leukemia
(12) and subsequently revealed in
breast (13) and pancreatic (14) tumors, and in HCC (15). Accounting for 1–2% of total tumor
cells, CSCs exhibited similar characteristics to those of normal
stem cells, including self-renewal and unlimited proliferation and
differentiation (16), and
contributed to cancer progression, metastasis and therapeutic
resistance (17). CSCs may generate
more differentiated and rapidly proliferating cells, and thereby
form the majority of the tumor (18,19).
Despite the CSC marker hypothesis, multiple studies
demonstrated that DCLK1 negatively regulated tumor suppressor
microRNAs (miRNAs/miRs) associated with tumor initiation,
progression and metastasis (20–24).
Furthermore, previous studies have demonstrated DCLK1 expression in
multiple types of solid tumor, including colon, intestinal and
pancreatic cancer, and HCC (20,25–27). In
addition, it has been revealed that patients with a high (>4)
DCLK1 staining score are associated with increased cancer-specific
mortality rates compared with those with a low (0–4) DCLK1 staining
score in colorectal neoplasia (27).
To the best of our knowledge, no study has been performed to assess
the association between DCLK1 expression and survival outcome in
patients with HCC. Therefore, the present study evaluated DCLK1
expression in HCC using immunohistochemical analysis and assessed
its association with clinicopathological features and survival
outcome.
Materials and methods
Patients and tissue samples
A total of 96 HCC and 68 adjacent tissue samples
from patients with HCC who had not undergone chemotherapy, targeted
therapy, radiotherapy or immunotherapy were obtained from the
Department of Pathology of the Chinese People's Liberation Army
General Hospital (Beijing, China) between August 2011 and August
2012. Clinicopathological features of the patients are provided in
Table I. To analyze outcome data, the
date of surgery was defined as the beginning of disease-free
survival (DFS; time to disease progression) and overall survival
(OS; time to mortality). Follow-up ceased in November 2015 and the
median follow-up time was 30 months. The protocol of the present
study was approved by the Chinese Ministry of Health and the Ethics
Committee of the Chinese People's Liberation Army General Hospital
in accordance with the ethical principles of the Declaration of
Helsinki. Prior to the present study, all patients provided written
informed consent to participate.
 | Table I.Descriptive statistics for patients
with hepatocellular carcinoma. |
Table I.
Descriptive statistics for patients
with hepatocellular carcinoma.
| Characteristic | Value |
|---|
| Age, years |
51.7±9.5a |
| Tumor size, cm |
4.6±3.4a |
| Sex, n (%) |
|
|
Male | 78 (81) |
|
Female | 18 (19) |
| Grade, n (%) |
|
|
Well-differentiated | 38 (40) |
|
Moderately differentiated | 33 (34) |
| Poorly
differentiated | 25 (26) |
| Portal venous
metastasis, n (%) |
|
|
Negative | 54 (56) |
|
Positive | 42 (44) |
| Hepatic venous
metastasis, n (%) |
|
|
Negative | 72 (75) |
|
Positive | 24 (25) |
| Bile duct invasion,
n (%) |
|
|
Negative | 92 (96) |
|
Positive | 4 (4) |
| Intrahepatic
metastasis, n (%) |
|
|
Negative | 46 (48) |
|
Positive | 50 (52) |
| Cirrhosis, n
(%) |
|
| No | 43 (45) |
|
Yes | 53 (55) |
| Hepatitis B virus,
n (%) |
|
|
Negative | 85 (89) |
|
Positive | 11 (11) |
| Recurrence, n
(%) |
|
| No | 46 (48) |
|
Yes | 50 (52) |
| Mortality induced,
n (%) |
|
| No | 63 (66) |
|
Yes | 33 (34) |
| Tumor size, n
(%) |
|
| <5
cm | 75 (78) |
| >5
cm | 21 (22) |
| DCLK1 expression, n
(%) |
|
| No | 18 (19) |
|
Yes | 78 (81) |
Immunohistochemistry (IHC)
IHC analysis was conducted using Image-Pro Plus 6.0
offered by Media Cybernetics, Inc. (Rockville, MD, USA). DCLK1
expression in the 96 HCC and 68 adjacent tissue samples from
patients with HCC was assessed using IHC and the procedures were
the same (28). Subsequent to fixing
with 10% neutral formaldehyde at room temperature for 20 min (cat.
no. ZI-4002; OriGene Technologies, Inc., Rockville, MD, USA) and
paraffin embedding, the tissues were cut to prepare 4-µm sections
that were mounted on silane-coated glass slides. In the present
study, rabbit monoclonal anti-DCLK1 (dilution, 1:700; cat. no.,
ab109029; Abcam, Cambridge, MA, USA) was the primary antibody.
Following deparaffinization in xylene solution and rehydration via
a reduced alcohol series (concentration 100, 95, 80 and 70%),
slides underwent epitope retrieval in 0.01 mol/l citrate buffer (pH
6.0) at 120°C for 4 min using a pressure cooker. Subsequently,
endogenous peroxidase activity was blocked using 3% hydrogen
peroxide at room temperature for 30 min. Goat serum (10%; BioSharp,
Hefei, China) was then used to block non-specific binding sites at
room temperature for 30 min. Sections were subsequently incubated
with the anti-DCLK1 antibody (1:700, diluted using PBS) overnight
at 4°C. Subsequent to washing with PBS and distilled water, the
sections were incubated with anti-rabbit secondary antibody
(dilution, 1:100; cat. no. PV-6001; OriGene Technologies, Inc.) at
37°C for 30 min. Sections were then treated with
3,3′-diaminobenzidine (dilution, 1:20; cat. no. ZLI-9017; OriGene
Technologies, Inc.) to visualize antibody reactions and
counterstained for ~4 min at room temperature with Mayer's
hematoxylin to develop cell nuclei. Subsequently, sections were
dehydrated in an ascending alcohol series (70, 80, 95 and 100%) and
mounted using neutral balsam (cat. no. GB590; Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). The negative
control experiment was performed according to the same IHC
procedure, except the anti-DCLK1 antibody (dilution, 1:500; cat.
no., EPR6085; Epitomics; Abcam), and the same IHC procedure was
performed on the positive control, which was human rectal
neuroendocrine tumor tissues from the Department of Pathology of
the Chinese People's Liberation Army General Hospital (Beijing,
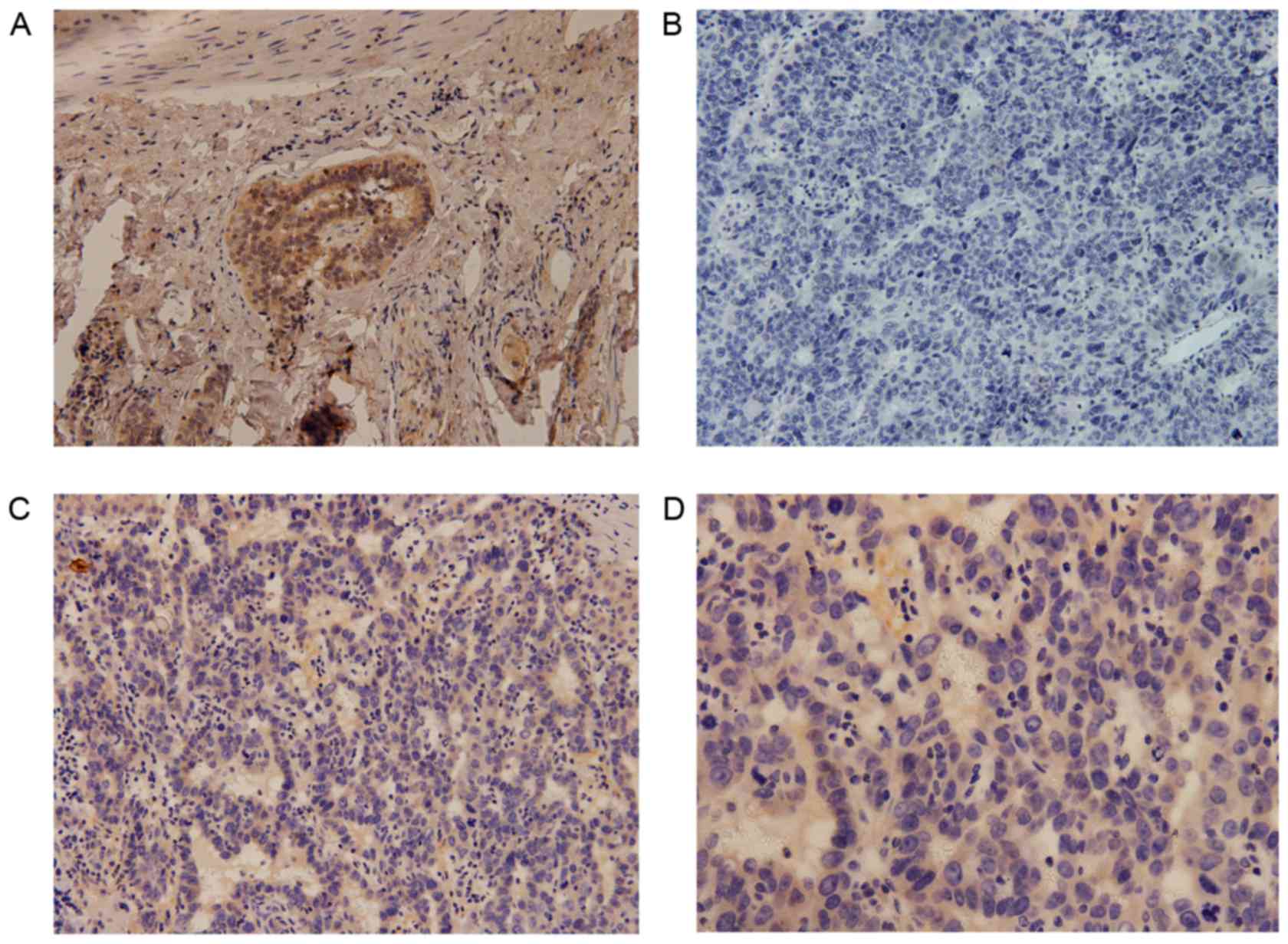
China; Fig. 1) (29).
IHC scoring
The results of DCLK1 IHC staining were analyzed by
two independent pathologists (the staff of the Chinese People's
Liberation Army General Hospital Pathology Department) blinded to
the other markers and the nature of the samples. In total, 5
microscopic fields were randomly selected for each slide. A
microscope (BX-53; Olympus Corporation; Japan; magnification, ×400)
was used to observe the staining of the target protein on the
tissues. The staining scoring was assessed for two parameters: i)
The percentage of stained cells and ii) staining intensity
(30). A score of 0, 1, 2 and 3 for
non-reactive, weak, moderate and strong, respectively, was used to
evaluate the staining intensity. Similarly, the percentage of
stained cells was scored as 0 (0%), 1 (<10%), 2 (10–40%), 3
(41–60%) and 4 (>60%). The DCLK1 staining score was the product
of the two scores. The expression of DCLK1 was defined as positive
when the composite score was >3 and as negative when the
composite score was 0–2 (31).
Statistical analysis
Data are presented as the mean ± standard deviation,
or as frequency. IBM SPSS Statistics 22 (IBM Corp., Armonk, NY,
USA) was used for statistical analysis. The association between
different HCC pathologies and DCLK1 expression was evaluated using
the χ2 test or Fisher's exact test. DFS and OS curves
were estimated using the Kaplan-Meier method and the log-rank test.
Multivariate analysis was assessed using the Cox proportional
hazards model. P<0.05 was considered to indicate a statistically
significant difference.
Results
DCLK1 is expressed in HCC and adjacent
tissues
In the present study, the expression of DCLK1 was
analyzed using IHC performed on 96 resected HCC and 68 adjacent
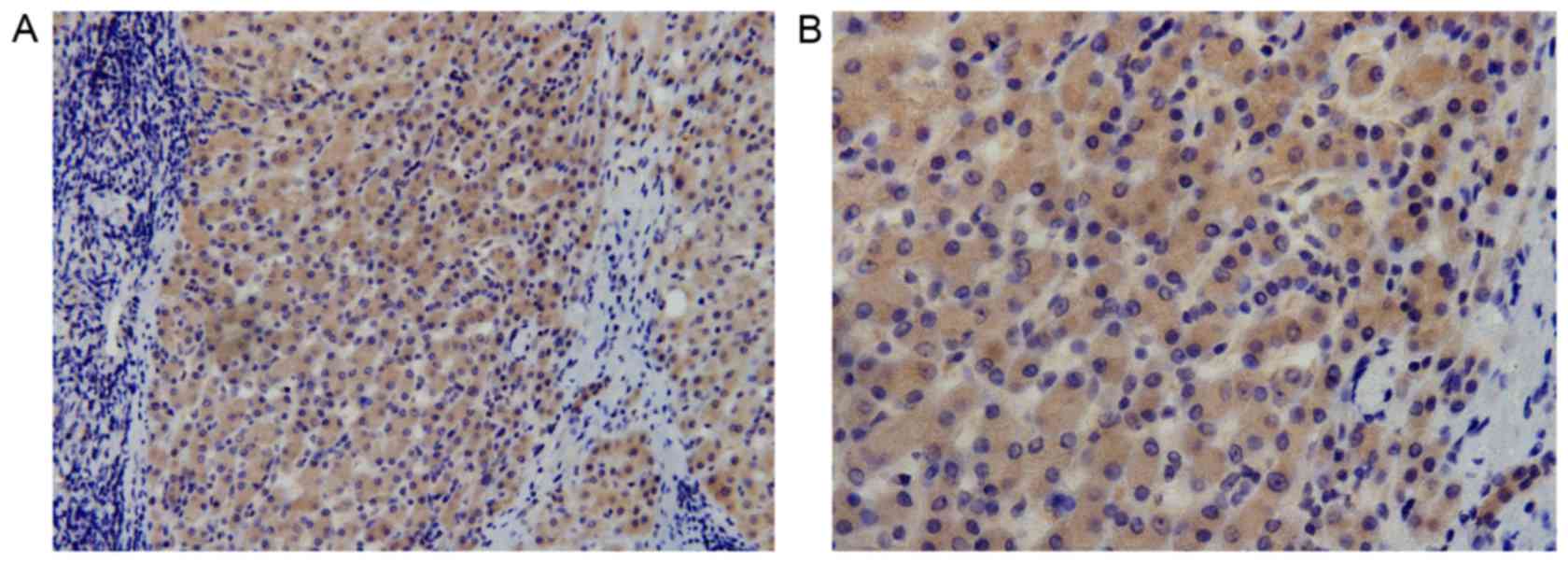
tissue specimens. Cytoplasmic expression of DCLK1 was observed in
HCC (Fig. 1) and adjacent tissue
specimens (Fig. 2); the positive
expression rate was 81% (78/96) and 74% (50/68), respectively,
while the median score was 4.6 and 3.9, respectively, with no
statistically significant difference observed between the HCC and
adjacent tissues (P=0.087).
Association between DCLK1 expression
and pathological parameters
The present study assessed the overall clinical
characteristics of 96 patients with HCC and their association with
the DCLK1 staining results. DCLK1 expression was positively
associated with intrahepatic metastasis (P=0.035), while no
association was identified with the other clinical predictor
variables, including sex, grade, hepatic venous metastasis, portal
venous metastasis, bile duct invasion and cirrhosis status
(P=0.739, P=0.189, P=0.763, P=0.605, P=0.571 and P=0.974,
respectively) (Table II).
 | Table II.Association between DCLK1 expression
and pathological parameters in patients with hepatocellular
carcinoma. |
Table II.
Association between DCLK1 expression
and pathological parameters in patients with hepatocellular
carcinoma.
|
|
| DCLK1
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total, n | Negative, n | Positive, n |
P-valuea |
|---|
| Tumor size, cm |
|
|
| 0.755 |
|
<5 | 75 | 15 | 60 |
|
|
>5 | 21 | 3 | 18 |
|
| Grade |
|
|
| 0.189 |
|
Well-differentiated | 38 | 10 | 28 |
|
|
Moderately differentiated | 33 | 6 | 27 |
|
| Poorly
differentiated | 25 | 2 | 23 |
|
| Portal venous
metastasis |
|
|
| 0.605 |
|
Negative | 54 | 9 | 45 |
|
|
Positive | 42 | 9 | 33 |
|
| Hepatic venous
metastasis |
|
|
| 0.763 |
|
Negative | 72 | 14 | 58 |
|
|
Positive | 24 | 4 | 20 |
|
| Bile duct
invasion |
|
|
| 0.571 |
|
Negative | 92 | 17 | 75 |
|
|
Positive | 4 | 1 | 3 |
|
| Intrahepatic
metastasis |
|
|
| 0.035 |
|
Negative | 46 | 14 | 38 |
|
|
Positive | 50 | 4 | 46 |
|
| Cirrhosis |
|
|
| 0.974 |
| No | 43 | 8 | 35 |
|
|
Yes | 53 | 10 | 43 |
|
| Hepatitis B
virus |
|
|
| 0.427 |
|
Negative | 85 | 15 | 70 |
|
|
Positive | 11 | 3 | 8 |
|
| Recurrence |
|
|
| 0.844 |
| No | 46 | 9 | 37 |
|
|
Yes | 50 | 9 | 41 |
|
| Sex |
|
|
| 0.739 |
|
Male | 78 | 14 | 64 |
|
|
Female | 18 | 4 | 14 |
|
Prognostic value of DCLK1
expression
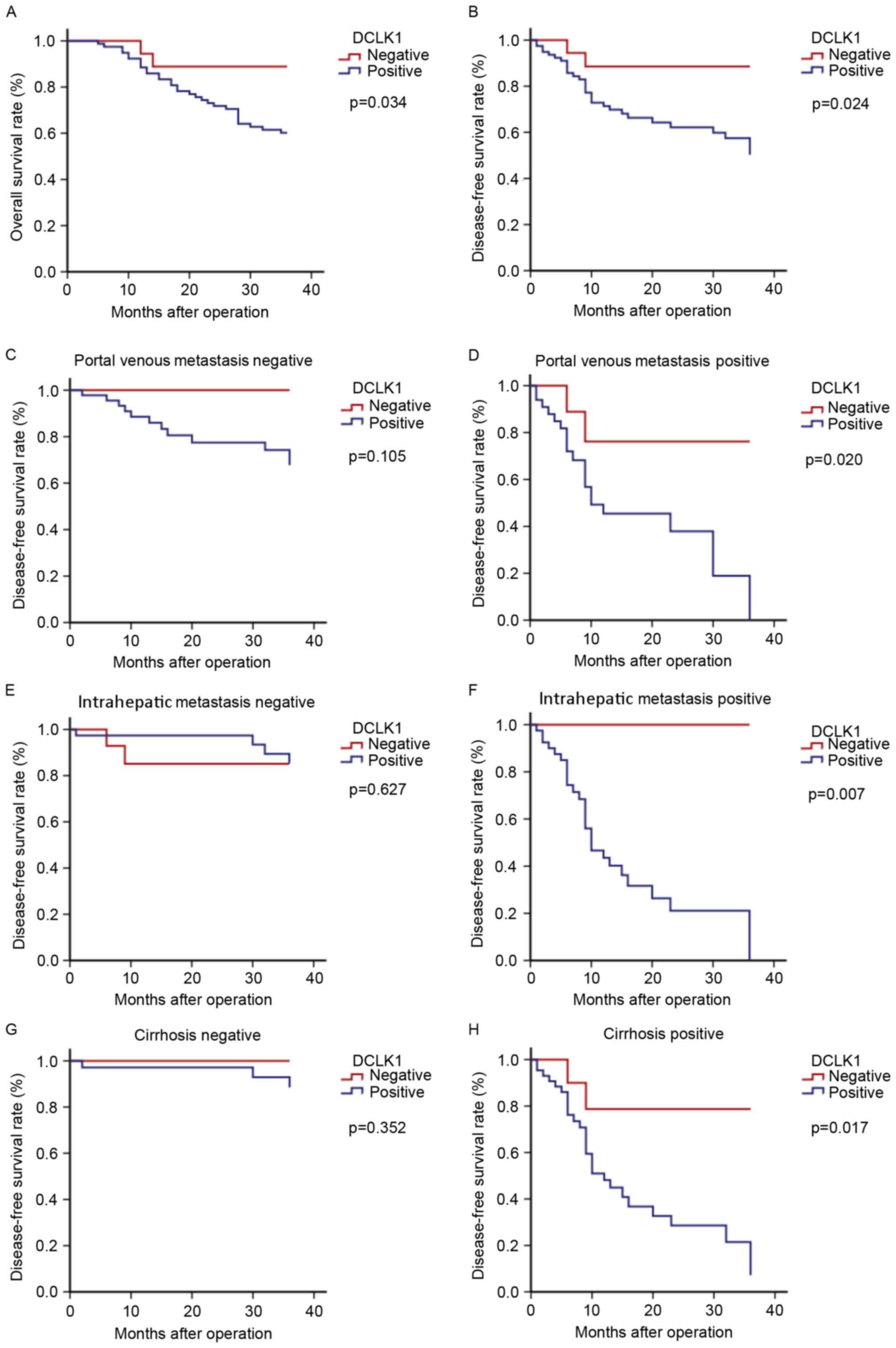
The median follow-up time was 30 months. Compared
with DCLK1 negative expression, the survival rate in the positive
expression were significantly reduced (both P<0.00; Fig. 3). Table
III provides the univariate analysis and the potential
prognostic factors. DCLK1 expression, tumor size (>5 cm), portal
venous metastasis, intrahepatic metastasis, hepatic venous
metastasis, grade and cirrhosis were associated with poor DFS
(P=0.024, P<0.001, P<0.001, P<0.001, P=0.004, P=0.008 and
P<0.001, respectively). No significant association was
identified between DFS and bile duct invasion, or hepatitis B virus
(Table III). Cox regression models
were then constructed, which contained the factors of DCLK1
expression, in order to realize the role of DCLK1 expression in
prognostic prediction. The results revealed that DCLK1 expression
was an independent prognostic parameter for the DFS of patients
with HCC (P=0.019; Table IV), with
an adjusted hazard ratio of 1.546 (95% confidence interval,
1.330–1.725). A significant association was also demonstrated
between DFS and portal venous metastasis, cirrhosis, hepatic venous
metastasis and tumor size (>5 cm; P=0.021, P=0.011, P=0027 and
P<0.001, respectively).
 | Table III.Univariate analysis. |
Table III.
Univariate analysis.
| Characteristic |
P-valuea |
|---|
| DFS |
|
| Tumor
size (<5 vs. >5 cm) | <0.001 |
| Grade
(1 vs. 2 vs. 3) | 0.008 |
| Portal
venous metastasis (negative vs. positive) | <0.001 |
| Hepatic
venous metastasis (negative vs. positive) | 0.004 |
| Bile
duct invasion (negative vs. positive) | 0.245 |
|
Intrahepatic metastasis
(negative vs. positive) | <0.001 |
|
Cirrhosis (no vs. yes) | <0.001 |
|
Hepatitis B virus (negative
vs. positive) | 0.176 |
| DCLK1
(negative vs. positive) | 0.024 |
| OS |
|
| Tumor
size (<5 vs. >5 cm) | <0.001 |
| Grade
(1 vs. 2 vs. 3) | 0.141 |
| Portal
venous metastasis (negative vs. positive) | 0.002 |
| Hepatic
venous metastasis (negative vs. positive) | 0.004 |
| Bile
duct invasion (negative vs. positive) | 0.270 |
|
Intrahepatic metastasis
(negative vs. positive) | <0.001 |
|
Cirrhosis (no vs. yes) | <0.001 |
|
Hepatitis B virus (negative
vs. positive) | 0.277 |
|
Recurrence (no vs. yes) | 0.013 |
| DCLK1
(negative vs. positive) | 0.034 |
 | Table IV.Cox regression analysis of factors
that could affect DFS and OS in patients with hepatocellular
carcinoma. |
Table IV.
Cox regression analysis of factors
that could affect DFS and OS in patients with hepatocellular
carcinoma.
| Factor | Hazard ratio | 95% CI |
P-valuea |
|---|
| DFS |
|
|
|
| Tumor
size, cm | 1.757 | 1.461–2.409 | <0.001 |
|
Grade |
|
|
|
|
Well-differentiated | 1.245 | 0.768–3.546 | 0.578 |
|
Moderately
differentiated | 1.831 | 0.598–5.602 | 0.298 |
|
Poorly
differentiated | 0.875 | 0.322–2.375 | 0.793 |
| Portal
venous metastasis | 1.380 | 1.168–1.857 | 0.021 |
| Hepatic
venous metastasis | 0.989 | 0.669–1.097 | 0.027 |
|
Intrahepatic metastasis | 0.540 | 0.492–0.739 | 0.080 |
|
Cirrhosis | 1.122 | 1.024–1.615 | 0.011 |
|
DCLK1 | 1.546 | 1.330–1.725 | 0.019 |
| OS |
|
|
|
| Tumor
size, cm | 1.401 | 1.188–1.856 | 0.018 |
| Portal
venous metastasis | 0.633 | 0.296–1.355 | 0.239 |
| Hepatic
venous metastasis | 2.300 | 2.139–2.651 | 0.002 |
|
Intrahepatic metastasis | 0.533 | 0.178–1.580 | 0.255 |
|
Cirrhosis | 0.607 | 0.346–0.731 | 0.040 |
|
DCLK1 | 0.278 | 0.062–1.215 | 0.089 |
|
Recurrence | 1.260 | 0.562–2.861 | 0.575 |
The present study also assessed the association
between DCLK1 expression and OS. Kaplan-Meier analysis demonstrated
that patients with DCLK1 expression were associated with decreased
OS compared with those without (P=0.034). The significant impact of
tumor size (>5 cm), portal venous metastasis, intrahepatic
metastasis, hepatic venous metastasis, recurrence and cirrhosis on
OS was also validated (P<0.001, P=0.002, P<0.001, P=0.004,
P=0.013 and P<0.001, respectively) (Table III). Cox regression analysis using
the aforementioned potential prognostic factors did not demonstrate
a significant association between DCLK1 expression and OS
(P=0.089), but showed the strong negative influence of tumor size
(>5 cm), hepatic venous metastasis and cirrhosis on OS (P=0.018,
P=0.002 and P=0.040, respectively).
The present study revealed that DCLK1 expression in
patients with HCC was an important independent prognostic factor of
DFS that was not associated with portal venous metastasis,
cirrhosis, hepatic venous metastasis or tumor size. However, DCLK1
expression was not demonstrated to be an independent predictor of
OS in patients with HCC.
Status of DCLK1 expression in portal
venous metastasis, intrahepatic metastasis and cirrhosis patient
subgroups
Previous studies have suggested that portal venous
metastasis, intrahepatic metastasis and cirrhosis may represent
critical predictors of disease recurrence, metastasis and poor
clinical outcome in patients with HCC (32–34).
Therefore, the present study conducted further subgroup survival
analysis stratified by portal venous metastasis, intrahepatic
metastasis and cirrhosis status. Kaplan-Meier survival curves
(Fig. 3) revealed that DCLK1
expression predicted poorer DFS in the patient subgroups positive
for portal venous metastasis, intrahepatic metastasis, and
cirrhosis (P=0.020, 0.007, and 0.017, respectively) compared with
their respective negative groups, which would provide evidence
supporting the use of early interventions with more aggressive
therapies following surgery in patients with HCC.
Discussion
In the present study, DCLK1 expression was analyzed
using IHC performed on 96 resected HCC and 68 adjacent tissue
specimens. DCLK1 expression was revealed in 81% (78/96) of the HCC
specimens, which was consistent with the 83% (19/23) result of a
previous study (31). Furthermore,
the clinical significance of DCLK1 expression and its association
with patient outcome were evaluated in 96 patients with HCC. The
present study identified a positive association between DCLK1
expression and tumor intrahepatic metastasis, while no association
was observed between DCLK1 expression and sex, grade, hepatic
venous metastasis, portal venous metastasis, bile duct invasion or
cirrhosis. Previous studies demonstrated that DCLK1 expression was
associated with T stage and lymphatic vessel involvement in
colorectal cancer (35). Although
DCLK1 expression has been studied in HCC, there is little data on
the expression and survival significance of DCLK1 in patients with
HCC. A previous study revealed that increased expression (staining
score >3) of DCLK1 was associated with a decreased 5-year OS
rate compared with decreased expression (staining score <3) in
patients with gastric cancer (36).
Consistent with this previous study, the present study demonstrated
that DCLK1 expression was an independent prognostic factor of DFS
in patients with HCC. To the best of our knowledge, the present
study revealed for the first time that DCLK1 expression predicts
poor DFS time in patients with HCC with portal venous metastasis,
intrahepatic metastasis or cirrhosis. Collectively, these results
suggested that DCLK1 expression may represent a novel potential
prognostic biomarker for patients with HCC.
Since these results suggested that DCLK1 could
represent a tumor promoter in HCC, an improved understanding of the
action of DCLK1 is required. DCLK1 possesses two N-termini that are
similar to doublecortin (DCX)-binding microtubules and regulate
neural progenitor cell migration (37). The C-terminal domain contains a
serine/threonine protein kinase. However, lacking definitive
evidence that DCLK1 resembles a cyclic adenosine monophosphate
(cAMP) dependent kinase, its structural homology to
Ca2+/calmodulin-dependent (CAM) kinase still warrants
consideration that this protein may show stimulation of its
activity by Ca-calmodulin (38,39). DCLK1
and DCX are members of the DCX family (37). DCX expressed in newly differentiated
neurons (40) has also been
implicated in regulating neuronal migration and axon growth
(41,42). A previous mouse study revealed that
DCLK1 and DCX exhibit a compensatory function in the formation of
axonal projections across the midline, and migration of cortical
neurons (39). Another study
suggested that phosphorylated DCX in vitro and in
vivo was associated with tumor invasion and progression
(43). However, the underlying
mechanism remains to be fully understood.
The present study suggested that DCLK1 expression
increased the aggressiveness of HCC, which requires further study.
One previous study revealed that DCLK1-expressing tumor cells with
stemness properties were associated with tumorigenesis and
metastasis, as regulated by specific miRNA pathways in HCC
(31). miRNA, a type of non-coding
RNA, functions primarily by binding to the 3′ untranslated region
of a target mRNA. miRNA serves important functions in numerous life
processes, including embryogenesis, stem cell differentiation,
tumorigenesis and tumor progression (44,45). In
HCC tumors, DCLK1-specific small interfering (si)RNA resulted in
tumor growth arrest, downregulation of DCLK1 and increased
expression of multiple tumor suppressor miRNAs, including miR-200,
miR-143/145 and miRNA let-7a (31).
The miR-200 family is a key regulator of the
epithelial-mesenchymal transition (EMT). EMT, the phenotypic
conversion of epithelial cells to mesenchymal cells (46), is a highly conserved process that is
essential in cancer initiation, invasion and metastasis (47). Increasing miR-200 expression resulted
in decreased expression of EMT-inducing transcription factors,
including snail family transcriptional repressor (SNAI)1, SNAI2,
zinc finger E-box binding homeobox (ZEB)1, ZEB2, twist family bHLH
transcription factor 1 and forkhead box C2, and increased
expression of EMT-inducing transcription factor epithelial cadherin
(20,31). These previous studies suggested that
DCLK1 serves a crucial function in promoting EMT and invasion by
regulating miR-200. miR-143/145 was revealed to possess tumor
suppressor properties, repressing the expression of POU class 5
homeobox 1, SRY-box 2 and Kruppel like factor 4, and thereby
repressing pluripotency, controlling differentiation and inhibiting
metastasis (48). The downregulation
of miRNA let-7a serves an important function in liver and
pancreatic tumor pathogenesis. MYC proto-oncogene (MYC), targeted
by miRNA let-7a, revealed decreased expression following knockdown
of DCLK1 in HCC cells (31).
Furthermore, a similar mechanism was detected in pancreatic and
colorectal cancer (20,23). The factors associated with EMT,
pluripotency and cancer stemness serve a multifaceted function in
tumorigenesis and metastasis in HCC, which are controlled by DCLK1
(31).
Histopathological assessment of tissues from chronic
liver diseases, in vitro experiments and murine models have
supported the existence of CSCs in HCC (15,49). A
previous study revealed that hepatitis C virus replication was
positively associated with DCLK1 expression (25). By contrast, siRNA knockdown of DCLK1
diminished hepatitis C virus replication and lowered the expression
of EMT-promoting factors (21,25,26).
Furthermore, DCLK1 served a crucial function in the development of
cirrhosis and HCC following sustained hepatitis C virus infection
(50). In FCA4 cell lines
(heterogeneous hepatoma cells with persistent replication of
hepatitis C virus RNAs), DCLK1 activated the inflammatory cascade,
as detected by S100 calcium binding protein A9 and nuclear factor
κB, and then promoted tumor proliferation, cell mortality, invasion
and EMT via MYC pathways (50).
Collectively, inflammation and neoplastic transformation are
regulated by a feed-forward-like loop of the DCLK1 signaling
pathway during chronic liver diseases (50). HCC associated with infection with HBV
has become one of the fastest-rising causes of cancer-associated
mortality in China (2,51). Given the importance of DCLK1
inflammatory and oncogenic functions in virus-induced chronic
diseases (25,31,50). the
present study assessed the association between DCLK1 expression and
hepatitis B virus and cirrhosis levels. The present study observed
that DCLK1 expression was a negative survival predictor in
cirrhosis subgroups. However, no association was observed between
DCLK1 expression and hepatitis B virus status, this may be
associated with the small number of specimens. Further study is
required to assess the association between DCLK1 expression and
hepatitis B virus in patients with HCC.
The present study revealed that DCLK1 expression was
an independent prognostic parameter for DFS, but not OS, in
patients with HCC. One of the main reasons for this was a lack of
long-term follow-up in the present study. At a median follow-up of
30 months, 50 patients developed recurrence, 30 of who succumbed.
The present study would have been improved with extended follow-up,
an increased number of samples and consecutive survival data.
Furthermore, in stratified survival analysis, the present study
observed that DCLK1 expression predicted early tumor recurrence and
poorer DFS rates with regard to portal venous metastasis,
intrahepatic metastasis, and cirrhosis patient subgroups, which was
consistent with the multi-functional role of DCLK1 in HCC. These
novel data revealed the potential of DCLK1-targeted therapy. DCLK1
may serve a function in multiple types of solid tumor. In a
previous study, siRNA-mediated blockade of DCLK1 resulted in
colorectal tumor xenograft growth arrest in nude mice and a
corresponding decrease in luciferase activity (23). Furthermore, another study demonstrated
that the ablation of DCLK1-expressing cells resulted in a decrease
in the number of intestinal polyps in APC (Min)/+ mice
(9). In addition, the stable
knockdown of DCLK1 resulted in the regression of liver metastasis
lesions in pancreatic cancer cells (52). Taken together, these data suggested a
function for DCLK1 in regulating tumor growth and indicate that
small molecular inhibitors of DCLK1 may prove useful as antitumor
drugs. Furthermore, DCLK1 expression may be used to predict early
tumor recurrence and poor clinical outcome across the three
subgroups of patients with HCC described in the present study.
However, the results of the present study require confirmation in
larger patient cohorts.
To conclude, the present study together with
previous study results have underscored the importance of DCLK1
expression in HCC. Progress in HCC treatments has stagnated over
recent decades, despite clinical trials of novel therapies.
Aggressive surgical therapies and early interventions following
surgery could be used to control local invasion and early
recurrence. The present study revealed that DCLK1 expression was
associated with a poorer prognostic outcome in patients with HCC,
and in the portal venous metastasis, intrahepatic metastasis and
cirrhosis patient subgroups. Therefore, DCLK1 may represent a
promising therapeutic target for HCC.
Acknowledgements
The authors would like to thank Dr Bing Li and Dr
Jing Yuan (Chinese People's Liberation Army General Hospital,
Pathology Department.) for their technical assistance. The present
study was supported by the Technology Nova Plan of Beijing City
(grant no. xx2013107).
Glossary
Abbreviations
Abbreviations:
|
DCLK1
|
doublecortin-like kinase 1
|
|
HCC
|
hepatocellular carcinoma
|
|
CSC
|
cancer stem cell
|
|
IHC
|
immunohistochemistry
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
EMT
|
epithelial-mesenchymal transition
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sarasin FP, Giostra E and Hadengue A:
Cost-effectiveness of screening for detection of small
hepatocellular carcinoma in western patients with Child-Pugh class
A cirrhosis. Am J Med. 101:422–434. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cervello M, McCubrey JA, Cusimano A,
Lampiasi N, Azzolina A and Montalto G: Targeted therapy for
hepatocellular carcinoma: Novel agents on the horizon. Oncotarget.
3:236–260. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin PT, Gleeson JG, Corbo JC, Flanagan L
and Walsh CA: DCAMKL1 encodes a protein kinase with homology to
doublecortin that regulates microtubule polymerization. J Neurosci.
20:9152–9161. 2000.PubMed/NCBI
|
|
6
|
Ohmae S, Takemoto-Kimura S, Okamura M,
Adachi-Morishima A, Nonaka M, Fuse T, Kida S, Tanji M, Furuyashiki
T, Arakawa Y, et al: Molecular identification and characterization
of a family of kinases with homology to Ca2+/calmodulin-dependent
protein kinases I/IV. J Biol Chem. 281:20427–20439. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giannakis M, Stappenbeck TS, Mills JC,
Leip DG, Lovett M, Clifton SW, Ippolito JE, Glasscock JI, Arumugam
M, Brent MR and Gordon JI: Molecular properties of adult mouse
gastric and intestinal epithelial progenitors in their niches. J
Biol Chem. 281:11292–11300. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
May R, Riehl TE, Hunt C, Sureban SM, Anant
S and Houchen CW: Identification of a novel putative
gastrointestinal stem cell and adenoma stem cell marker,
doublecortin and CaM kinase-like-1, following radiation injury and
in adenomatous polyposis coli/multiple intestinal neoplasia mice.
Stem Cells. 26:630–637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakanishi Y, Seno H, Fukuoka A, Ueo T,
Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et
al: Dclk1 distinguishes between tumor and normal stem cells in the
intestine. Nat Genet. 45:98–103. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Westphalen CB, Asfaha S, Hayakawa Y,
Takemoto Y, Lukin DJ, Nuber AH, Brandtner A, Setlik W, Remotti H,
Muley A, et al: Long-lived intestinal tuft cells serve as colon
cancer-initiating cells. J Clin Invest. 124:1283–1295. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Westphalen CB, Quante M, Worthley D,
Asfaha S, Remotti H, Olive KP and Wang TC: Dclk1 labels quiescent
pancreatic progenitor and cancer initiating cells. Cancer Res. 72
Suppl:Abstract 5220. 2012. View Article : Google Scholar
|
|
12
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 100:pp.
3983–3988. 2003, View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li C, Heidt DG, Dalerba P, Burant CF,
Zhang L, Adsy V, Wicha M, Clarke MF and Simeone DM: Identification
of pancreatic cancer stem cells. Cancer Res. 67:1030–1037. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mishra L, Banker T, Murray J, Byers S,
Thenappan A, He AR, Shetty K, Johnson L and Reddy EP: Liver stem
cells and hepatocellular carcinoma. Hepatology. 49:318–329. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lobo NA, Shimono Y, Qian D and Clarke MF:
The biology of cancer stem cells. Annu Rev Cell Dev Biol.
23:675–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumor initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Diehn M and Clarke MF: Cancer stem cells
and radiotherapy: New insights into tumor radioresistance. J Natl
Cancer Inst. 98:1755–1757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tang C, Ang BT and Pervaiz S: Cancer stem
cell: Target for anti-cancer therapy. FASEB J. 21:3777–3785. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sureban SM, May R, Lightfoot SA, Hoskins
AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao
CV, et al: DCAMKL-1 regulates epithelial-mesenchymal transition in
human pancreatic cells through a miR-200a-dependent mechanism.
Cancer Res. 71:2328–2338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sureban SM, May R, Mondalek FG, Qu D,
Ponnurangam S, Pantazis P, Anant S, Ramanujam RP and Houchen CW:
Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144
and inhibits colorectal cancer tumor growth via a Notch-1 dependent
mechanism. J Nanobiotechnology. 9:402011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sureban SM, May R, Qu D, Weygant N,
Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG
and Houchen CW: DCLK1 regulates pluripotency and angiogenic factors
via microRNA-dependent mechanisms in pancreatic cancer. PLoS One.
8:e739402013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sureban SM, May R, Ramalingam S,
Subramaniam D, Natarajan G, Anant S and Houchen CW: Selective
blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a
MicroRNA-dependent mechanism. Gastroenterology. 137(649–659): e1–2.
2009.
|
|
24
|
Sureban SM, May R, Weygant N, Qu D,
Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen
CB, Wang TC and Houchen CW: XMD8-92 inhibits pancreatic tumor
xenograft growth via a DCLK1-dependent mechanism. Cancer Lett.
351:151–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ali N, Allam H, May R, Sureban SM, Bronze
MS, Bader T, Umar S, Anant S and Houchen CW: Hepatitis C
virus-induced cancer stem cell-like signatures in cell culture and
murine tumor xenografts. J Virol. 85:12292–12303. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
May R, Sureban SM, Hoang N, Riehl TE,
Lightfoot SA, Ramanujam R, Wyche JH, Anant S and Houchen CW:
Doublecortin and CaM kinase-like-1 and
leucine-rich-repeat-containing G-protein-coupled receptor mark
quiescent and cycling intestinal stem cells, respectively. Stem
Cells. 27:2571–2579. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gagliardi G, Goswami M, Passera R and
Bellows CF: DCLK1 immunoreactivity in colorectal neoplasia. Clin
Exp Gastroenterol. 5:35–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saqui-Salces M, Keeley TM, Grosse AS, Qiao
XT, EL-Zaatari M, Gumucio DL, Samuelson LC and Merchant JL: Gastric
tuft cells express DCLK1 and are expanded in hyperplasia. Histochem
Cell Biol. 136:191–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ikezono Y, Koga H, Abe M, Akiba J,
Kawahara A, Yoshida T, Nakamura T, Iwamoto H, Yano H, Kage M, et
al: High expression of the putative cancer stem cell marker, DCLK1,
in rectal neuroendocrine tumors. Oncol Lett. 10:2015–2020.
2015.PubMed/NCBI
|
|
30
|
Hao XP, Willis JE, Pretlow TG, Rao JS,
MacLennan GT, Talbot IC and Pretlow TP: Loss of fragile histidine
triad expression in colorectal carcinomas and premalignant lesions.
Cancer Res. 60:18–21. 2000.PubMed/NCBI
|
|
31
|
Sureban SM, Madhoun MF, May R, Qu D, Ali
N, Fazili J, Weygant N, Chandrakesan P, Ding K, Lightfoot SA and
Houchen CW: Plasma DCLK1 is a marker of hepatocellular carcinoma
(HCC): Targeting DCLK1 prevents HCC tumor xenograft growth via a
microRNA-dependent mechanism. Oncotarget. 6:37200–37215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fattovivh G, Stroffoloni T, Zagni I and
Donato F: Hepatocellular carcinoma in cirrhosis: Incidence and risk
factors. Gastroenterology. 127 5 Suppl 1:S35–S50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Torzilli G, Belghiti J, Kokudo N, Takayama
T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E,
Donadon M, et al: A snapshot of the effective indications and
results of surgery for hepatocellular carcinoma in tertiary
referral centers: Is it adherent to the EASL/AASLD recommendations?
An observational study of the HCC East-West study group. Ann Sugr.
257:929–937. 2013. View Article : Google Scholar
|
|
34
|
Fan ST, Poon RT, Yeung C, Lam CM, Lo CM,
Yuen WK, Ng KK, Liu CL and Chan SC: Outcome after partial
hepatectomy for hepatocellular cancer within the Milan criteria. Br
J Surg. 98:1292–1300. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
O'Connell MR, Sarkar S, Luthra GK, Okugawa
Y, Toiyama Y, Gajjar AH, Qiu S, Goel A and Singh P: Epigenetic
changes and alternate promoter usage by human colon cancers for
expressing DCLK1-isoforms: Clinical Implications. Sci Rep.
5:149832015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng QB, Yu JC, Kang WM, Ma ZQ, Zhou WX,
Li J, Zhou L, Cao ZJ and Tian SB: Expression of doublecortin-like
kinase 1 in human gastric cancer and its correlation with
prognosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 35:639–644.
2013.(In Chinese). PubMed/NCBI
|
|
37
|
Reiner O, Coquelle FM, Peter B, Levy T,
Kaplan A, Sapir T, Orr I, Barkai N, Eichele G and Bergmann S: The
evolving doublecortin (DCX) superfamily. BMC Genomics. 7:1882006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lin PT, Gleeson JG, Corbo JC, Flanagan L
and Walsh CA: DCAMKL1 encodes a protein kinase with homology to
doublecortin that regulates microtubule polymerization. J Neurosci.
20:9152–9161. 2000.PubMed/NCBI
|
|
39
|
Koizumi H, Tanaka T and Gleeson JG:
Doublecortin-like kinase functions with doublecortin to mediate
fiber tract decussation and neuronal migration. Neuron. 49:55–66.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gleeson JG, Minnerath SR, Fox JW, Allen
KM, Luo RF, Hong SE, Berg MJ, Kuzniecky R, Reitnauer PJ, Borgatti
R, et al: Characterization of mutations in the gene doublecortin in
patients with double cortex syndrome. Ann Neurol. 45:146–153. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gleeson JG, Lin PT, Flanagan LA and Walsh
CA: Doublecortin is a microtubule-associated protein and is
expressed widely by migrating neurons. Neuron. 23:257–271. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schaar BT, Kinoshita K and McConnell SK:
Doublecortin microtubule affinity is regulated by a balance of
kinase and phosphatase activity at the leading edge of migrating
neurons. Neuron. 41:203–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Clark EA, Golub TR, Lander ES and Hynes
RO: Genomic analysis of metastasis reveals an essential role for
RhoC. Nature. 406:532–535. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McManus MT: MicroRNAs and cancer. Semin
Cancer Biol. 13:253–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microRNAs in human lung
cancers in association with shortened postoperative survival.
Cancer Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Turley EA, Veiseh M, Radisky DC and
Bissell MJ: Mechanisms of disease: Epithelial-mesenchymal
transition-does cellular plasticity fuel neoplastic progression?
Nat Clin Pract Oncol. 5:280–290. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Diehn M and Clarke MF: Cancer stem cells
and radiotherapy: New insights into tumor radioresistance. J Natl
Cancer Inst. 98:1755–1757. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu N, Papagiannakopoulos T, Pan G, Thomson
JA and Kosik KS: MicroRNA-145 regulates OCT4, SOX2, and KLF4 and
represses pluripotency in human embryonic stem cells. Cell.
137:647–658. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamashita T and Wang XW: Cancer stem cells
in the development of liver cancer. J Clin Invest. 123:1911–1918.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ali N, Chandrakesan P, Nguyen CB, Husain
S, Gillaspy AF, Huycke M, Berry WL, May R, Qu D, Weygant N, et al:
Inflammatory and oncogenic roles of a tumor stem cell marker
doublecortin-like kinase (DCLK1) in virus-induced chronic liver
diseases. Oncotaarget. 6:20327–20344. 2015. View Article : Google Scholar
|
|
51
|
Lata J: Chronic liver diseases as liver
tumor precursors. Dig Dis. 28:596–599. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ito H, Tanaka S, Akiyama Y, Shimada S,
Adikrisna R, Matsumura S, Aihara A, Mitsunori Y, Ban D, Ochiai T,
et al: Dominant expression of DCLK1 in human pancreatic cancer stem
cells accelerates tumor invasion and metastasis. PLoS One.
11:e01465642016. View Article : Google Scholar : PubMed/NCBI
|