Introduction
Leukemia is a malignancy of the hematopoietic stem
cells that severely affects human health. Currently, the main
therapies available for the treatment of leukemia include
chemotherapy, bone marrow transplantation, allogeneic hematopoietic
stem cell transplantation, immunotherapy and targeted drug therapy
(1). Despite the advances made
through laboratory and clinical research, the prognosis for
patients with acute myeloid leukemia (AML) remains poor.
Furthermore, ~50% of patients with AML are not suitable for
induction chemotherapy to achieve complete remission, while a high
proportion of patients who do receive induction therapy may achieve
complete remission and subsequently relapse due to the presence of
chemo-refractory cells. Therefore, the development of novel
therapeutic strategies to treat patients with AML is urgently
required (1–3).
Tetrazine is a compound that consists of a
six-membered aromatic ring containing four nitrogen atoms, with the
molecular formula C2H2N4.
Tetrazine has three isomers: 1,2,4,5-tetrazine, 1,2,3,4-tetrazine,
and 1,2,3,5-tetrazine. Temozolomide, one of the imidazole
tetrazines belonging to the 1,2,3,5-tetrazine group, has been
approved by the Food and Drug Administration as a novel treatment
for malignant glioma, and works by inhibiting the growth and
proliferation of cancer cells (4).
Among the three isomers, 1,2,4,5-tetrazines are the
most stable compounds, and 1,2,4,5-tetrazine derivatives have
demonstrated potential therapeutic properties, such as anti-mite
(5), herbicidal (6), anti-malarial (7), antiviral (6), anti-inflammatory (6), antibacterial (8) and antitumor activities (6,9).
Furthermore, 1,2,4,5-tetramethyl-3,6-bis
(phenylethynyl)-1,2,4,5-tetrazine was one of the first
1,2,4,5-tetrazine derivatives to be identified as possessing
antitumor activity (6).
Thus, in order to identify other antitumor tetrazine
compounds, based on the novel structure of
3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine (China Invention Patent
no. ZL.98121915.2), dozens of different structural modifications of
tetrazine compounds have been synthesized by Professor W.X. Hu
(Pharmaceutical College of Zhejiang University of Technology,
Hangzhou, China). Among these tetrazine compounds, the derivative
N,N'-di-(m-methylphenyl)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide
(ZGDHu-1) was identified to exhibit the highest antitumor activity
(6,10–12). Its
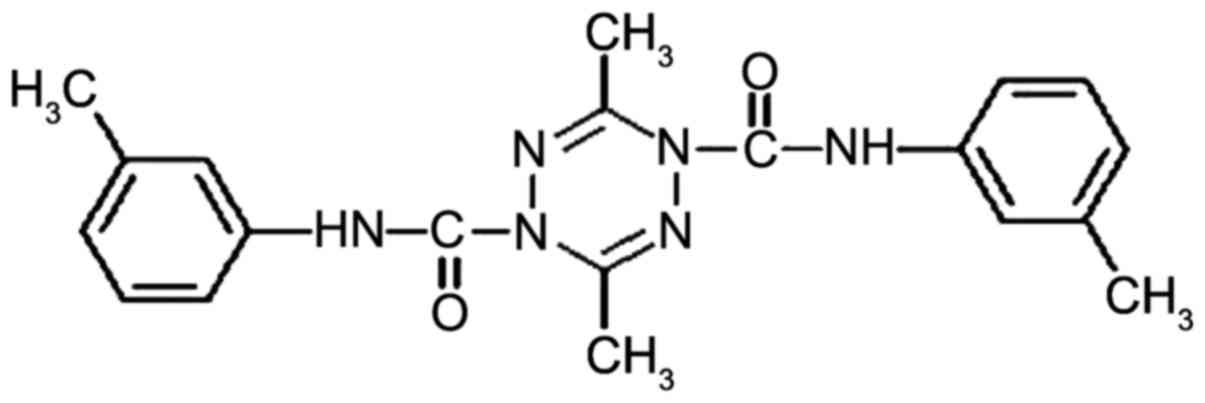
structure is shown in Fig. 1
(13). It was demonstrated that the
3,6-dimethyl group is essential for antitumor activity, and that
when the 3,6-dimethyl group was substituted for an ethyl or propyl
group, the antitumor activity decreased significantly (11,12). In
our previous studies, the antitumor activities of ZGDHu-1 were
evaluated in vitro in different types of tumor cells
(13–22), including monocyte leukemia, t(8;21)
AML, M3 leukemia, chronic lymphocytic leukemia (CLL), EBC-1 lung
carcinoma and PANC-1 pancreatic cancer, as well as a xenograft lung
cancer mouse model (Table I). Among
them, the antitumor activity of ZGDHu-1 was highest in Kasumi-1
cells, with a 50% inhibitory concentration (IC50) of 0.3
µM (22). Furthermore, it could
induce the apoptosis and differentiation of Kasumi-1 cells at
different concentrations (Fig. 2).
Notably, our unpublished data demonstrates that ZGDHu-1 exhibits
high antitumor activity with few adverse effects, and a 50% lethal
dose (LD50) of 5,000 mg/kg in mice.
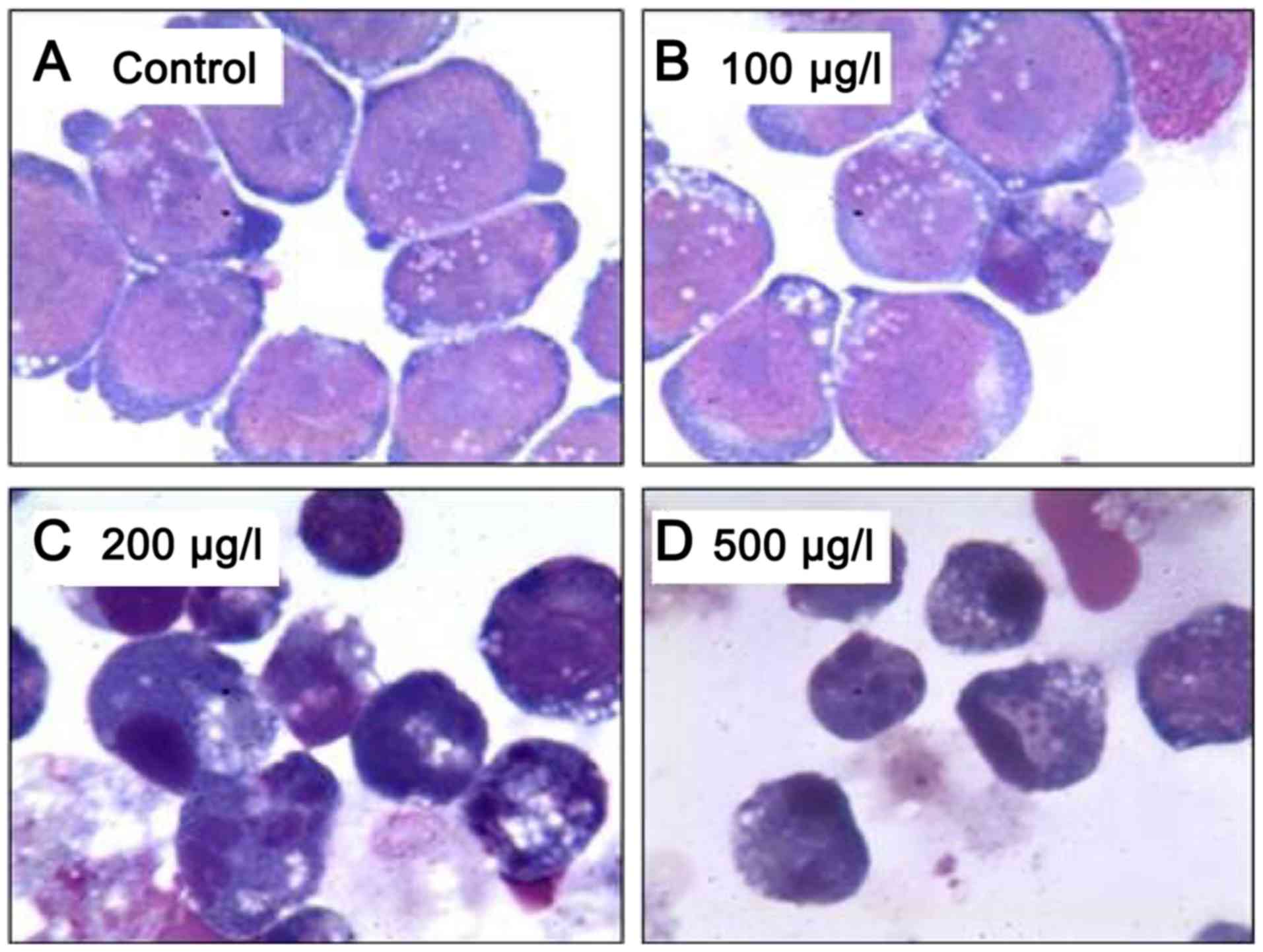 | Figure 2.Wright-Giemsa staining of Kasumi-1
cells following treatment with (A) dimethyl sulfoxide (control), or
ZGDHu-1 at concentrations of (B) 100, (C) 200 and (D) 500 µg/l for
48 h (magnification, ×1,000). The images illustrate that apoptotic
bodies among the Kasumi-1 cells were increased with higher
concentrations of ZGDHu-1, particularly at 200 and 500 µg/l. By
contrast, at 100 µg/l ZGDHu-1, no nucleolus, chromatin condensation
thickening, a small amount of azurophilic particles increased in
the cytoplasm, and a shift in the location of the nucleus to one
side with lobules were observed. ZGDHu-1,
N,N'-di-(m-methylphenyl)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide. |
 | Table I.Effects of ZGDHu-1 on different
cancer types. |
Table I.
Effects of ZGDHu-1 on different
cancer types.
| Cancer type | Type of study | Molecular
targets | Functions | (Refs.) |
|---|
| Monocyte
leukemia | In
vitro | Bax, p53, Fas,
Bcl-2, ΔΨm | Anti-proliferative
activity, induction of apoptosis, in vitro
differentiation | (13,16) |
| t(8;21) acute
myeloid leukemia | In
vitro | Apo 2.7, ΔΨm,
cyclin B1, cdc25c, CHK1, IκB, β5, β5i | Anti-proliferative
activity, induction of apoptosis, inactivated NF-κB, in
vitro differentiation, degradation of AML-ETO, proteasome
inhibition | (12,18) |
| Acute promyelocytic
leukemia | In
vitro | Bax, phospho-p38
MAPK | Anti-proliferative
activity, induction of apoptosis, in vitro
differentiation | (14) |
| Chronic lymphocytic
leukemia | In
vitro | ΔΨm, Bcl-2, ROS,
caspase-3 | Anti-proliferative
activity, induction of apoptosis, | (9,10) |
| Pancreatic
cancer | In
vitro | IκB, CHK1,
cyclinB1, cdc2 (CDK1), Bcl-2, caspase-3, PARP | Anti-proliferative
activity, induction of apoptosis | (11) |
| Lung cancer | In vitro/in
vivo | Bax, p53 and Fas,
caspase-3 | Anti-proliferative
activity, induction of apoptosis | (15,17) |
To date, the biological activity of ZGDHu-1 has only
been invesigated by our group during the past 10 years (13–22). One
important characteristic demonstrated by ZGDHu-1 is its ability to
arrest tumor cells at the G2/Mphase of the cell cycle
(Fig. 3) (16) and, in contrast to certain other
chemical compounds, ZGDHu-1 is able to induce apoptosis and inhibit
the proliferation of tumor cells at a high concentration, whereas
low concentrations induce leukemia cell differentiation (13–21).
Finally, it may be a proteasome inhibitor (22). Therefore, we recommend that other
research groups worldwide perform further studies investigate this
multi-target chemical compound to fully elucidate its antitumor
activity and its potential clinical utility.
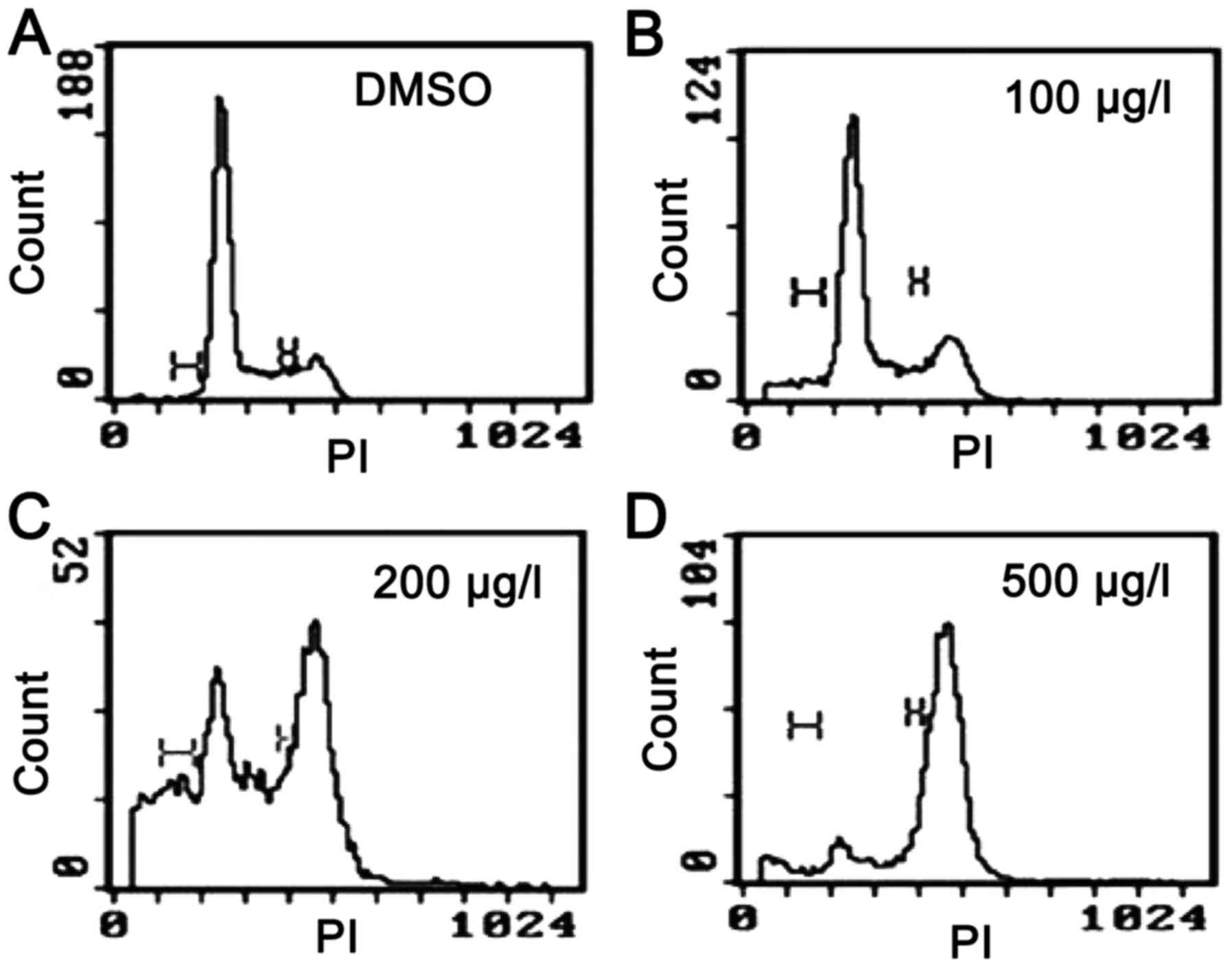 | Figure 3.Cell cycle effects on Kasumi-1 cells
following treatment with (A) DMSO (control), or ZGDHu-1 at (B) 100,
(C) 200 and (D) 500 µg/l for 48 h, as reported in Xia et al
(16). The cell cycle distribution
was analyzed using a fluorescence-activated cell sorting machine,
and the results demonstrated that cells were accumulated in the
G2/M phase following treatment with 200 and 500 µg/l
ZGDHu-1. DMSO, dimethyl sulfoxide; ZGDHu-1,
N,N'-di-(m-methylphenyl)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide;
PI, propidium iodide. |
Anti-leukemic activity of ZGDHu-1
Monocyte leukemia
Anti-proliferation activity
Over-proliferation is an essential process of cancer
cells, and uncontrolled cell division is considered a defining
characteristic (23). The cell cycle
is a complex process that ensures the controlled replication of
cells, and it can be divided into four stages: G1 phase
(first gap); S phase (DNA synthesis); G2 phase (second
gap); and M phase (mitosis). In our previous studies, SHI-1 cells
were selected as a suitable model for investigating monocyte
leukemia in vitro (24), and
the effect of ZGDHu-1 was evaluated in these cells using MTT
assays. Our results demonstrated that ZGDHu-1 could inhibit SHI-1
cell proliferation in a time- and dose-dependent manner; the
IC50 values at 48 and 72 h were 250 and 85 ng/ml,
respectively (17). Notably, the
majority of SHI-1 cells were arrested at the G2/M phase
(17).
Induction of apoptosis
Apoptosis refers to programmed cell death, which
serves an essential role in the regulation of cell growth, and is
regarded as a natural barrier to cancer cell progression (25). Resistance to apoptosis may decrease
the sensitivity of cancer cells to diverse chemotherapy agents and
induce multidrug resistance. Thus, apoptosis induction is
considered to be an essential strategy for the elimination of
cancer cells as part of cancer treatment (26). Apoptosis is classified into two major
signaling pathways, termed the extracellular (extrinsic inducers)
and intracellular (intrinsic inducers) signaling pathways (26).
In SHI-1 cells, ZGDHu-1 was observed to
significantly induce the apoptosis in a time- and dose-dependent
manner through increasing Bcl-2-associated X, apoptosis regulator
(Bax), tumor protein p53 (p53) and Fas cell surface death receptor
(Fas) gene expression, and decreasing B-cell lymphoma-2 (Bcl-2)
expression (20). Additionally,
ZGDHu-1 was demonstrated to increase the expression of
mitochondrial membrane protein apo2.7 in a dose-dependent manner,
whilemitochondrial membrane potential (ΔΨm) was significantly
reduced (20).
Induction of differentiation
Following treatment with a low concentration of
ZGDHu-1, SHI-1 cells were observed to be reduced in size but with
an enlarged cytoplasm, resulting in a decrease in the
nucleus/cytoplasm ratio. Additionally, the cells possessed no
nucleolus, and exhibited thickened condensed chromatin, a small
amount of azurophilic particles increased in the cytoplasm, and a
shift in the location of the nucleus to one side with lobules
following treatment with ZGDHu-1. Furthermore, following culturing
with 2–100 ng/ml ZGDHu-1 for 3 days, the morphology of SHI-1 cells
matured, as indicated by a higher rate of staining with nitro blue
tetrazolium chloride (NBT), a marker for functionally
differentiated myeloid cells (17).
In addition, CD11b, CD14 and CD64 were upregulated, and the effect
on CD14 and CD64 expression levels was dose-dependent (17). Overall, these results indicate that a
low concentration ZGDHu-1 can induce SHI-1 cells to differentiate
into mature monocytes.
t(8;21) acute myeloid leukemia
Anti-proliferative activity
Presently, aggressive cytosine arabinoside-based
chemotherapy is the standard treatment for t(8;21) AML. However,
clinical observations have demonstrated that the 5-year survival
rate of patients with AML is <40% (27). Thus, the development of novel
therapies is required for the treatment of patients with t(8;21)
AML (27). To date, investigations of
the effects of ZGDHu-1 on t(8;21) AML cells have comprised the most
extensive study of ZGDHu-1 by our group (Figs. 2 and 3).
Our previous study demonstrated that ZGDHu-1 could inhibit the
proliferation of Kasumi-1 cells in a time- and dose-dependent
manner, and the IC50values at 48 and 72 h were 450 and
300 ng/ml, respectively (22).
Arrest of the cell cycle at the G2/M
phase
The cell cycle is strictly regulated by cyclins,
cyclin-dependent kinases (CDKs) and cyclin-dependent kinase
inhibitors (CKIs) (28). Furthermore,
the uncontrolled proliferation that is characteristic of human
cancer cells has been associated with the dysregulation of
CDKs/cyclins (28). Hyperactivation
of CDK activity confers a cell growth advantage, while inactivation
of tumor suppressor genes, checkpoint regulators or CKIs results in
dysregulation of the cell cycle (29). Among these regulators, the CDK1
(cdc2)/cyclin B complex is a major determinant of early M phase
progression, and the combination of cyclin B1 and CDK1 is essential
for eukaryotic cells entering mitosis (30). Additionally, cell division cycle (cdc)
25c is a protein phosphatase that is responsible for activating and
dephosphorylating CDK1 (30). At the
end of this signaling cascade, CDK1/cyclin B activity is
attenuated, thereby blocking entry into mitosis. Thus, inhibition
of CDK1/cyclin B is achieved by the downregulation of cdc25
phosphatase and upregulation of WEE1 G2 checkpoint kinase, which
together results in the accumulation of inhibitory phosphate groups
on tyrosine 15 of CDK1. This checkpoint activation results in cell
cycle arrest at the G2/M phase (29).
In our study, it was revealed that treatment of
Kasumi-1 cells with ZGDHu-1 results in significant dysregulation of
G2/M regulatory molecules, including the downregulation
of cyclin B1, CDK1 and cdc25c, and the upregulation of checkpoint
kinase 1 (CHK1), phospho-CHK1, phospho-cdc25c, phospho-p53, p27 and
p53 (16). Additionally, pretreatment
with a selective CHK1 inhibitor, CHIR-124, significantly abrogated
G2/M arrest via ZGDHu-1. Overall, this study indicates
that ZGDHu-1 could arrest t(8;21) AML cells at the G2
phase through G2/M checkpoint-associated CDKs and
CKIs.
Induction of apoptosis
The mitochondrial signaling pathway serves an
essential role in intracellular apoptosis. In ZGDHu-1-treated SHI-1
cells (20), changes in Apo 2.7 and
ΔΨm indicated that the integrity of the mitochondrial membrane was
destroyed. Additionally, dysregulation of Bcl-2-associated agonist
of cell death, Bax and Bcl-2 expression further supports the notion
that ZGDHu-1 induces apoptosis through the mitochondrial signaling
pathway (16). ZGDHu-1 was identified
to be able to decrease caspase-3 expression and markedly increase
cleaved caspase-3 expression in a dose-dependent manner, and
cleaved fragments of poly ADP-ribose polymerase (PARP) were
observed, which indicates that caspase-3 is activated by ZGDHu-1
treatment (16).
Inactivation of nuclear factor κB (NF-κB)
over-activity
Over the last decade, studies have reported that
constitutive activation of NF-κB may beobserved in AML. NF-κB
serves an important role inapoptosis and survival of leukemia
cells, which is also dependent on Bcl-2 and Bcl-extra large
(31,32). In our preliminary study, the results
suggested that the expression pattern of NF-κB was significantly
dysregulated by ZGDHu-1 treatment (16).
Induction of differentiation and the AML1-ETO
(A/E) fusion gene target
ZGDHu-1-treated Kasumi-1 cells demonstrated reduced
cell size and an increased amount of cytoplasm, resulting in the
decrease in the nucleus/cytoplasm ratio. Additionally, the cells
exhibited no nucleolus, chromatin condensation thickening, a small
amount of azurophilic particles increased in the cytoplasm, and a
shift in the location of the nucleus to one side with lobules
following ZGDHu-1 treatment. Furthermore, ZGDHu-1-treated Kasumi-1
cells exhibited a significant reduction in NBT staining. Similarly
to all-transretinoicacid (ATRA) treatment, ZGDHu-1 increased the
percentage of CD11b+ and CD13+ cells,
partially supporting the notion that ZGDHu-1 induces the
differentiation of Kasumi-1 cells (22).
The A/E oncoprotein can inhibit the differentiation
and enhance the self-renewal of hematopoietic stem cells, which are
essential features of Kasumi-1 cells and serve a major role in the
progression of AML disease (33). In
our previous study, A/E oncoprotein expression was significantly
dysregulated when treated with different concentrations of ZGDHu-1
(16). The results demonstrated that
A/E oncoproteins were not alteredat the mRNA level, but were
degraded at the protein level (16),
indicating that ZGDHu-1 exerts its effect on the A/E fusion protein
through a post-translational signaling pathway or other unknown
mechanism.
Inhibition of proteasome activity
The ubiquitin-proteasome signaling pathway is
responsible for the degradation of mutated and misfolded proteins,
which involves two essential steps: The attachment of multiple
ubiquitin molecules to a protein substrate, and the degradation of
the tagged substrate by the 26S proteasome (34). The 26S proteasome contains a 20S
catalytic core proteasome and two 19S regulatory subunits, which
serve as recognition sites for proteolysis (35). The 20S core is composed of a total of
28 subunits, comprising 14 α and 14 β subunits (33).
Currently, bortezomib is the only proteasomal
inhibitor for the treatment of patients with multiple myeloma or
mantle cell lymphoma (36), wherein
it acts as a reversible inhibitor of the 26S proteasome (37). Bortezomib can bind to the active site
of the β5-subunit proteasome and inhibit the chymotrypsin-like
activity, which is essential for cell death-inducing capability
(38). Numerous important proteasome
target proteins have been demonstrated to be affected, including
cyclins (39,40), p53 (41), the retinoblastoma (Rb) family
(42), pro-apoptotic Bax (43), CKIp27 (44), and NF-κB inhibitorα (45). In our preliminary study in Kasumi-1
cells, it was revealed that ZGDHu-1 could significantly decrease
the protein expression of the β5 and β5 isubunits of the 20S
proteasome, and partially decrease the expression of the β1 and β1
isubunits, but not the β2 and β2 isubunits (22). However, further studies are warranted
in order to elucidate the association between the apoptotic effects
of ZGDHu-1 as a proteasome inhibitor and the underlying signaling
pathway involved.
Acute promyelocytic (M3) leukemia
(APL)
Anti-proliferative activity and induction of
apoptosis
AML is a heterogeneous and aggressive disease, which
is characterized by the rapid growth of abnormal white blood cells
in the bone marrow. Inhibition of cellular differentiation at
specific stages during their development is the most prominent
characteristic of AML (46). Since
the success of ATRA in the treatment of APL, differentiation
therapy has been regarded as a promising method for AML treatment
(47), and the investigations of APL
has benefited from ATRA-maturation sensitive and resistant cell
lines (NB4 and UF-1) derived from leukemia cells from a patient
with APL (48).
The results of our previous study revealed that
ZGDHu-1 could inhibit NB4 cell proliferation; the IC50
values were 450 ng/ml (48 h) and 200 ng/ml (72 h) (18). Notably, the majority of NB4 cells were
also arrested at G2/M phase, and phospho-p38 and Bax
expression were increased while Bcl-2 and phospho-STAT3 were
unchanged when treated with ZGDHu-1 (18). Furthermore, the effect of the ZGDHu-1
on the apoptosis of NB4 cells was time- and dose-dependent, and the
apoptotic effect of 100 ng/ml ZGDHu-1 on NB4cells was similar to
the effect of 10 µg/ml ATRA, implying that ZGDHu-1 exerts a
stronger apoptotic effect compared with ATRA (18). Additionally, another APL cell line,
HL-60, was also investigated. The IC50 values at 48 and
72 h were both 180 ng/ml, and the majority of HL-60 cells were also
arrested at G2/M phase (18).
Induction of differentiation
At a low concentration (2–100 ng/ml) of ZGDHu-1, NB4
cells exhibit more mature features after 3 days, with higher NBT
positivity and CD11b and CD13 expression compared with the
untreated control groups (18). In
HL-60 cells, the expression levels of CD11b, CD13, CD14 and CD64
were demonstrated to be significantly upregulated following ZGDHu-1
treatment, which suggests that ZGDHu-1 induces the differentiation
of Kasumi-1 cells into mature granulocytes or monocytes.
CLL
CLL is a type of cancer in which the bone marrow
produces an excessive amount of lymphocytes. Combined regimens,
such as fludarabine, cyclophosphamide and rituximab, have become
the standard treatment for patients with CLL. However, the majority
of patients with CLL are elderly and not all patients are eligible
for aggressive chemoimmunotherapy (49). CLL cells are arrested at
G0/G1and cannot overcome the differentiation
hurdle (50). Notably, impaired cell
death, rather than excessive proliferation, is regarded as
essential for the accumulation of CLL cells and their resistance to
chemotherapy (50).
Additionally, abnormal apoptosis was demonstrated to
be associated with the clinical progression of patients with CLL
(51). The activated survival
signaling pathways, such as the phosphoinositide 3-kinase/Akt or
NF-κB pathways in CLL cells, upregulate important anti-apoptotic
Bcl-2 family members, leading to chemotherapy resistance and
disease progression (52,53). Furthermore, higher Bcl-2/Bax or
Mcl-1/Bax ratios indicate a resistance to fludarabine and
significantly shorter survival time in patients with CLL (50,54–57).
Recently, ABT-199 has demonstrated the most promising clinical
results of all the putative agents targeting Bcl-2 (58).
In our previous study, ZGDHu-1 dose-dependently
inhibited the viability of primary CLL cells. However, this effect
was not identified in the peripheral B cells of healthy people at
the same concentrations, indicating that the cytotoxic effects of
ZGDHu-1 are specific to CLL cells (13).
Induction of apoptosis
The results of a previous study suggest that ZGDHu-1
induces the apoptosis of malignant B lymphocytes of patients with
CLL, but cannot induce the apoptosis of healthy B lymphocytes
(13). This indicates that the
pro-apoptotic activity of ZGDHu-1 is specifically against CLL
cells, and the apoptotic effect was partially dependent on a loss
of ΔΨm, phosphatidylserine (PS) translocation across the plasma
membrane, and reactive oxygen species (ROS) accumulation. Lastly,
ZGDHu-1 has been demonstrated to significantly induce caspase-3
cleavage and decrease anti-apoptotic Bcl-2 expression, without
affecting Bax expression (13).
ZGDHu-1 and fludarabine exert a synergistic
effect on the apoptosis of CLL cells
Currently, fludarabine is widely used for the
treatment of patients with CLL (59,60). It
has been demonstrated to serve multiple functions, such as
interference with DNA synthesis and repair, apoptosis induction and
cell cycle regulation in leukemia cells (60). However, toxic effects of fludarabine,
such as severe opportunistic infections, myelosuppression and
gastrointestinal toxicities (including vomiting, nausea and hepatic
lesions) have been reported (60).
Thus, reducing the toxicity of fludarabine by reducing its dose and
the identification of novel candidate drugs are warranted. In our
study, it was revealed that ZGDHu-1 and fludarabine had a
synergistic effect on the cytotoxicity and apoptosis of CLL cells,
and this effect was also dependent on the loss of ΔΨm, PS
translocation and ROS accumulation (14). The combination also significantly
induced the cleavage of caspase-3 and significantly decreased
anti-apoptotic Bcl-2 expression in CLL cells (14).
Therefore, the combination of ZGDHu-1 and
fludarabine may be useful for the maintenance therapy of patients
with CLL, as it can sensitize CLL cells to low doses of fludarabine
without increasing the risk of long-term side effects on the immune
system or other opportunistic infections (14). However, numerous important questions
remain unresolved regarding what type of biomarkers that
distinguish lymphoid malignancies will respond to ZGDHu-1 as a
monotherapy or combination with other chemotherapy drugs, and
whether relapsed or refractory patients with CLL are sensitive to
the ZGDHu-1. In combination settings, the highest priority question
must be to determine whether ZGDHu-1 can synergize with cytotoxic
agents to overcome chemoresistance.
Anti-solid tumor activity of ZGDHu-1
Pancreatic cancer
Pancreatic cancer, which is characterized by early
metastasis, late diagnosis, high mortality rate and <5% 5-year
survival rate, is regarded as ‘the king of cancer’ (61). Previously, using the MTT method, it
was identified that ZGDHu-1 suppressed the proliferation of PANC-1
cells in a time- and dose-dependent manner; the
IC50values at 48 and 72 h were 295 and 150 ng/ml,
respectively (15). Furthermore,
ZGDHu-1 could arrest cells at the G2/M phase and induce
the apoptosis of PANC-1 cells in a dose-dependent manner.
Additionally, ZGDHu-1 was demonstrated to upregulate Bax expression
and downregulate Bcl-2 expression, also activating pro-caspase-3
and PARP. Cyclin B1 and CDK1 expression levels were identified to
be decreased whereasCHK1 expression was increased, all of which are
G2/M regulatory molecules (15). Overall, our research revealed that
ZGDHu-1 could effectively suppress cell proliferation and induce
the apoptosis of PANC-1 cells. However, further in vivo
models are required to validate the efficacious antitumor
activities of ZGDHu-1 described.
Lung cancer
Anti-proliferative activity and induction of
apoptosis
ZGDHu-1 was demonstrated to inhibit cell
proliferation and induce apoptosis in human lung carcinoma EBC-1
cells (21). ZGDHu-1 inhibited EBC-1
cell proliferation with anIC50 at 24 h of 295 ng/ml, at
48 h of 112 ng/ml and at 72 h of 23 ng/ml. Bax, p53 and Fas
expression were significantly increased, whereas Bcl-2 expression
was unaltered andcaspase-3 expression was significantly decreased
following treatment with ZGDHu-1 (21).
Induction of apoptosis of A549 cells in vitro and
antitumor activity in vivo
In a previous study, ZGDHu-1 was demonstrated to
inhibit A549 cell proliferation, and the majority of A549 cells
were arrested at the G2/M phase. Bax, Bax/Bcl-2 and p53
expression levels were increased significantly, with a marked
decrease in Bcl-2 expression (19).
Additionally, ZGDHu-1 was revealed to induce tumor cell apoptosis
in vitro and significantly suppress the growth of a A549
xenograft tumors in vivo (19). When the xenograft tumor mouse models
were treated with 10, 20 and 40 mg/kg ZGDHu-1 for 14 days, the
tumor growth inhibition rates were 43.7, 56.9 and 60.0%,
respectively (19). However, the
efficacy of ZGDHu-1 has only been investigated in the A549 lung
cancer xenograft mouse model, and further studies are warranted to
elucidate the direct and cellular targets of the ZGDHu-1 in
vivo. Similar to temozolomide, one of the imidazole tetrazines,
which is now an orally administered alkylating agent widely used as
a novel treatment for malignant glioma, ZGDHu-1 has demonstrated
promising anticancer results.
Therapeutic perspectives and
conclusions
The biological activity of ZGDHu-1 has onlybeen
invesigated by our group for the past 10 years (1–14). So far,
the most notable characteristic of ZGDHu-1 is its ability to arrest
all tumor cells at the G2/M phase; however,
caspase-dependent and proteasome inhibitory activity has alsobeen
reported. Until now, the ZGDHu-1 appears to be an efficacious drug
on tumor cell lines in vitro; however, its antitumor effect
in vivo has only been investigated in the lung cancer animal
model. The effect of ZGDHu-1 in other solid tumor cells and
leukemia animal models are still under investigation as ZGDHu-1 is
difficult to synthesize. Notably, the IC50 of ZGDHu-1 in
Kasumi-1 leukemia cells was 0.3 µM, which was the lowest among all
other investigated tumor cells, and the drug had few adverse
effects, with an LD50 of 5,000 mg/kg in the mouse model.
However, after 10 years of research, the exact target of ZGDHu-1 in
the tumor cells remains unclear. The arrest of all tumor cells at
the G2/M phaseand induction of leukemia cell
differention at low concentrations are just some of the effects of
ZGDHu-1. Further investigations into the direct or cellular targets
of ZGDHu-1 are warranted. Ultimately, novel targets will be
identified and may hold great promise for clinical cancer
therapy.
Acknowledgements
The present study was supported by the Zhejiang
Province Health Bureau (grant no. 2013ZDA005). In addition, the
study was supported by the National Natural Science Foundation of
China (grant no. 81502472), Zhejiang Province Health Bureau (grant
no. 2014KYA015) and Outstanding Youth Foundation of Zhejiang
Provincial People's Hospital (grant no. 2015 A level).
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
ZGDHu-1
|
N,N'-di-(m-methylphenyl)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide
|
|
ΔΨm
|
mitochondrial membrane potential
|
|
PS
|
phosphatidylserine
|
|
ROS
|
reactive oxygen species
|
|
CLL
|
chronic lymphocytic leukemia
|
|
CDK
|
cyclin-dependent kinase
|
|
CKI
|
cyclin-dependent kinase inhibitor
|
|
A/E
|
AML1-ETO
|
|
ATRA
|
all-transretinoic acid
|
|
APL
|
acute promyelocytic leukemia
|
References
|
1
|
Estey E: Why is progress in acute myeloid
leukemia so slow? Semin Hematol. 52:243–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lichtenegger FS, Krupka C, Köhnke T and
Subklewe M: Immunotherapy for acute myeloid leukemia. Semin
Hematol. 52:207–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coombs CC, Tallman MS and Levine RL:
Molecular therapy for acute myeloid leukaemia. Nat Rev Clin Oncol.
13:305–318. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marucci G: Treatment of pituitary
neoplasms with temozolomide: A review. Cancer. 117:4101–4102. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Falfushynska HI, Gnatyshyna LL and Stoliar
OB: Population-related molecular responses on the effect of
pesticides in Carassius auratus gibelio. Comp Biochem Physiol C
Toxicol Pharmacol. 155:396–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rao GW, Wang C, Wang J, Zhao ZG and Hu WX:
Synthesis, structure analysis, antitumor evaluation and 3D-QSAR
studies of 3,6-disubstituted-dihydro-1,2,4,5-tetrazine derivatives.
Bioorg Med Chem Lett. 23:6474–6480. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nhu D, Duffy S, Avery VM, Hughes A and
Baell JB: Antimalarial
3-arylamino-6-benzylamino-1,2,4,5-tetrazines. Bioorg Med Chem Lett.
20:4496–4498. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tabassum S, Parveen M, Ali A, Alama M,
Ahmad Anis, UKhan A and Khana RA: Synthesis of
Aryl-1,2,4,5-tetrazinane-3-thiones, in vitro DNA binding studies,
nuclease activity and its antimicrobial activity. J Mol Structure.
1020:33–40. 2012. View Article : Google Scholar
|
|
9
|
Stanovnik B, Grošelj U and Svete J:
9.12–1,2,4,5-TetrazinesComprehensive Heterocyclic Chemistry III.
Katritzky AR, Ramsden CA, Scriven EFV and Taylor RJK: Elsevier;
Oxford: pp. 641–714. 2008
|
|
10
|
Rao GW and Hu WX: Synthesis, X-ray
crystallographic analysis and antitumor activity of
1-acyl-3,6-disubstituted phenyl-1,4-dihydro-1,2,4,5-tetrazines.
Bioorg Med Chem Lett. 15:3174–3176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rao GW and Hu WX: Synthesis, structure
analysis and antitumor activity of
3,6-disubstituted-1,4-dihydro-1,2,4,5-tetrazine derivatives. Bioorg
Med Chem Lett. 16:3702–3705. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu WX, Rao GW and Sun YQ: Synthesis and
antitumor activity of s-tetrazine derivatives. Bioorg Med Chem
Lett. 14:1177–1181. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu LN, Zhou YL, Wang ZN, Huang Q and Hu
WX: ZGDHu-1 promotes apoptosis of chronic lymphocytic leukemia
cells. Int J Oncol. 41:533–540. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu L, Liu J, Wang Z, Hu W, Huang Q and
Zhou Y: ZGDHu-1 and fludarabine have a synergistic effect on
apoptosis of chronic lymphocytic leukemia cells. Oncol Rep.
34:1239–1248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SF, Xia J, Lv YP, Liu JL, Li WX, Yu
XP, Hu WX and Zhou YL:
N,N'-di-(m-methylphenyi)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide
(ZGDHu-1) suppresses the proliferation of PANC-1 pancreatic cancer
cells via apoptosis and G2/M cell cycle arrest. Oncol Rep.
33:1915–1921. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xia J, Chen SF, Lv YP, Lu LN, Hu WX and
Zhou YL: ZGDHu-1 induces G2/M phase arrest and apoptosis
in Kasumi-1 cells. Mol Med Rep. 11:3398–3404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou YL, Lu YP, Hu WX, Qiu LN, Wang WS and
Liu JD: Effects of N, N-di-(m-methylphenyl)-3,6-dimethyl-1,
4-dihydro-1,2,4,5-tetrazine-1,4-dicarboxamide (ZGDhu-1) on SHI-1
leukemia cells in vitro. Zhonghua Xue Ye Xue Za Zhi. 27:361–365.
2006.(In Chinese). PubMed/NCBI
|
|
18
|
Zhou YL, Lü YP, Hu WX, Qiu LN, Wang WS, Wu
JG and Liu JD: Effects of N, N'-Di-(m-methylphenyi)-3,6-dimethyl-1,
4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamide on proliferation,
apoptosis and differentiation of NB4 leukemia cells in vitro.
Zhongguo Shi Yan Xue Ye Xue Za Zhi. 14:880–886. 2006.(In Chinese).
PubMed/NCBI
|
|
19
|
Zhou YL, Hu WX, Lü YP, Qiu LN, Wang WS,
Yang ZY, Liu JD and Rao GW: Effect of ZGDHu-1 on proliferation and
apoptosis of A549 cells in vitro and antitumor activity in vivo.
Yao Xue Xue Bao. 42:26–34. 2007.(In Chinese). PubMed/NCBI
|
|
20
|
Zhou YL, Lü YP, Hu WX, Qiu LN, Wang WS,
Liu JD and Wu JG: ZGDHu-1-inducing apoptosis of SHI-1 leukemia
cells and its molecular mechanism. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 15:483–489. 2007.(In Chinese). PubMed/NCBI
|
|
21
|
Zhou YL, Xu WL, Wang ZN, Lü YP and Hu WX:
Apoptosis of human lung carcinoma cell line EBC-1 induced by
N,N'-di-(m-methylphenyl)-3,6-dimethyl-1,4-dihydro-1,2,4,5-tetrazine-1,4-dicarboamid
e and its molecular mechanism. Zhonghua Zhong Liu Za Zhi.
32:886–891. 2010.(In Chinese). PubMed/NCBI
|
|
22
|
Zhou YL, Chen LC and Lü YP: Inhibition
effects of ZGDHu-1 on proteasome in Kasumi-1 cells. Zhonghua Xue Ye
Xue Za Zhi. 33:61–63. 2012.(In Chinese). PubMed/NCBI
|
|
23
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Xue Y, Zhang X, Wu Y, Pan J, Wang
Y and Ceng J: A new human acute monocytic leukemia cell line SHI-1
with t (6;11) (q27;q23), p53 gene alterations and high
tumorigenicity in nude mice. Haematologica. 90:766–775.
2005.PubMed/NCBI
|
|
25
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferrara F and Del Vecchio L: Acute myeloid
leukemia with t(8;21)/AML1/ETO: A distinct biological and clinical
entity. Haematologica. 87:306–319. 2002.PubMed/NCBI
|
|
28
|
Murray AW: Recycling the cell cycle:
Cyclins revisited. Cell. 116:221–234. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Santo L, Siu KT and Raje N: Targeting
cyclin-dependent kinases and cell cycle progression in human
cancers. Semin Oncol. 42:788–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stark GR and Taylor WR: Control of the
G2/M transition. Mol Biotechnol. 32:227–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bosman MC, Schuringa JJ and Vellenga E:
Constitutive NF-κB activation in AML: Causes and treatment
strategies. Crit Rev Oncol Hematol. 98:35–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guzman ML, Neering SJ, Upchurch D, Grimes
B, Howard DS, Rizzieri DA, Luger SM and Jordan CT: Nuclear
factor-kappaB is constitutively activated in primitive human acute
myelogenous leukemia cells. Blood. 98:2301–2307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baumeister W, Walz J, Zühl F and Seemüller
E: The proteasome: Paradigm of a self-compartmentalizing protease.
Cell. 92:367–380. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ciechanover A: The ubiquitin-proteasome
pathway: On protein death and cell life. EMBO J. 17:7151–7160.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Adams J: The proteasome: A suitable
antineoplastic target. Nat Rev Cancer. 4:349–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen D, Frezza M, Schmitt S, Kanwar J and
Dou QP: Bortezomib as the first proteasome inhibitor anticancer
drug: Current status and future perspectives. Curr Cancer Drug
Targets. 11:239–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schwartz R and Davidson T: Pharmacology,
pharmacokinetics and practical applications of bortezomib. Oncology
(Williston Park). 18 14 Suppl 11:S14–S21. 2004.
|
|
38
|
Chen D and Dou QP: The
ubiquitin-proteasome system as a prospective molecular target for
cancer treatment and prevention. Curr Protein Pept Sci. 11:459–470.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen W, Lee J, Cho SY and Fine HA:
Proteasome-mediated destruction of the cyclin a/cyclin-dependent
kinase 2 complex suppresses tumor cell growth in vitro and in vivo.
Cancer Res. 64:3949–3957. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Diehl JA, Zindy F and Sherr CJ: Inhibition
of cyclin D1 phosphorylation on threonine-286 prevents its rapid
degradation via the ubiquitin-proteasome pathway. Genes Dev.
11:957–972. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Blagosklonny MV: P53: An ubiquitous target
of anticancer drugs. Int J Cancer. 98:161–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kalejta RF and Shenk T:
Proteasome-dependent, ubiquitin-independent degradation of the Rb
family of tumor suppressors by the human cytomegalovirus pp71
protein. Proc Natl Acad Sci USA. 100:pp. 3263–3268. 2003,
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li B and Dou QP: Bax degradation by the
ubiquitin/proteasome-dependent pathway: Involvement in tumor
survival and progression. Proc Natl Acad Sci USA. 97:pp. 3850–3855.
2000, View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pagano M, Tam SW, Theodoras AM,
Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF and Rolfe M:
Role of the ubiquitin-proteasome pathway in regulating abundance of
the cyclin-dependent kinase inhibitor p27. Science. 269:682–685.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen ZJ: Ubiquitin signalling in the
NF-kappaB pathway. Nat Cell Biol. 7:758–765. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Martens JH, Brinkman AB, Simmer F,
Francoijs KJ, Nebbioso A, Ferrara F, Altucci L and Stunnenberg HG:
PML-RARalpha/RXR alters the epigenetic landscape in acute
promyelocytic leukemia. Cancer Cell. 17:173–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, Zhu H, Hu J, Mi J, Chen S, Chen Z
and Wang Z: Progress in the treatment of acute promyelocytic
leukemia: Optimization and obstruction. Int J Hematol. 100:38–50.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Roussel MJ and Lanotte M: Maturation
sensitive and resistant t (15;17) NB4 cell lines as tools for APL
physiopathology: Nomenclature of cells and repertory of their known
genetic alterations and phenotypes. Oncogene. 20:7287–7291. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Špaček M: Small molecules in the treatment
of chronic lymphocytic leukemia in 2015 and in the near future.
Klin Onkol. 28 Suppl 3:3S45–3S49. 2015.(In Czech). View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang Y, Wu JZ, Li JY and Xu W: Know the
enemy as well as the weapons in hand: The aberrant death pathways
and therapeutic agents in chronic lymphocytic leukemia. Am J Cancer
Res. 5:2361–2375. 2015.PubMed/NCBI
|
|
51
|
Dighiero G and Hamblin TJ: Chronic
lymphocytic leukaemia. Lancet. 371:1017–1029. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Buggins AG and Pepper CJ: The role of
Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk Res.
34:837–842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Loeder S, Zenz T, Schnaiter A, Mertens D,
Winkler D, Döhner H, Debatin KM, Stilgenbauer S and Fulda S: A
novel paradigm to trigger apoptosis in chronic lymphocytic
leukemia. Cancer Res. 69:8977–8986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pepper C, Hoy T and Bentley DP: Bcl-2/Bax
ratios in chronic lymphocytic leukaemia and their correlation with
in vitro apoptosis and clinical resistance. Br J Cancer.
76:935–938. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pepper C, Thomas A, Hoy T and Bentley P:
Chlorambucil resistance in B-cell chronic lymphocytic leukaemia is
mediated through failed Bax induction and selection of high
Bcl-2-expressing subclones. Br J Haematol. 104:581–588. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Molica S, Dattilo A, Giulino C, Levato D
and Levato L: Increased bcl-2/bax ratio in B-cell chronic
lymphocytic leukemia is associated with a progressive pattern of
disease. Haematologica. 83:1122–1124. 1998.PubMed/NCBI
|
|
57
|
Pepper C, Lin TT, Pratt G, Hewamana S,
Brennan P, Hiller L, Hills R, Ward R, Starczynski J, Austen B, et
al: Mcl-1 expression has in vitro and in vivo significance in
chronic lymphocytic leukemia and is associated with other poor
prognostic markers. Blood. 112:3807–3817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Anderson MA, Huang D and Roberts A:
Targeting BCL2 for the treatment of lymphoid malignancies. Semin
Hematol. 51:219–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yu EM, Kittai A and Tabbara IA: Chronic
lymphocytic leukemia: Current concepts. Anticancer Res.
35:5149–5165. 2015.PubMed/NCBI
|
|
60
|
Hallek M: Chronic lymphocytic leukemia:
2015 Update on diagnosis, risk stratification, and treatment. Am J
Hematol. 90:446–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|