Introduction
Small cell lung cancer (SCLC) is an aggressive
disease that accounts for ~14% of all lung cancers, and there are
~31,000 patients who are diagnosed with SCLC annually in the USA
(1). Due to the fast tumor doubling
time and early hematogenous spread exhibited by SCLC, the 5-year
survival rate remains <5% and the median survival rate is
between 7 and 12 months (2,3). Unlike non-small cell lung cancer, in
which major advances have been made using targeted therapies, there
are no approved targeted drugs for SCLC (1). Therefore, the identification of
effective targeted drugs is required.
One potential therapeutic target for lung cancer is
the Wnt signaling pathway (4–7). Wnt signaling regulates cell
proliferation, survival, and differentiation, and serves key
functions in embryonic development and tumorigenesis (8–11). In the
canonical Wnt signaling pathway, glycogen synthase kinase-3, in
complex with axin and adenomatous polyposis coli, constitutively
phosphorylates β-catenin, maintaining it at a decreased level
(12). Poly-ADP-ribose polymerase
(PARP) enzymes regulate the canonical Wnt activity: Tankyrase
(TNKS) 1 and TNKS2. These two enzymes poly-ADP-ribosylate and
destabilize axin, a key component of the β-catenin phosphorylation
complex (12–14). TNKS1 has been identified to be
upregulated in a variety of types of cancer, including plasma cell
leukemia, high-grade non-Hodgkin's lymphoma, breast, colon and
bladder cancer (15–20).
XAV939 is a small molecule TNKS inhibitor and is
synthetized using a chemical genetics approach (21). Waaler et al (22) and Bilir et al (23) revealed that XAV939 suppressed the
viability of colon cancer cells and triple-negative breast cancer
cells by inhibiting Wnt signaling. However, the association between
SCLC and the Wnt signaling pathway remains unknown. To the best of
our knowledge, it has not been identified whether XAV939 exhibits
an effect on SCLC cells, and it is hypothesized that the underlying
molecular mechanism may contribute to establishing SCLC targeted
therapy.
In the present study, the Wnt pathway inhibitor
XAV939 was investigated in the effective treatment of SCLC cells
and the inhibitory effect of XAV939 on the viability of SCLC cells
was identified. In addition, the effect of XAV939 on the cell cycle
and cell apoptosis was determined. The results of the present study
revealed that XAV939 may inhibit the viability of SCLC via the
repression of TNKS1, and TNKS1 may be a target for eliminating SCLC
cells.
Materials and methods
Chemicals and reagents
XAV939, MTT and dimethyl sulfoxide (DMSO) were
purchased from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). The
cisplatin injection was purchased from Hospira Australia Pty, Ltd.
(Melbourne, Australia). The cell cycle detection kit and annexin
V/fluorescein isothiocyanate (FITC) apoptosis detection kit was
purchased from Nanjing KeyGEN Biotech. Co. Ltd. (Nanjing,
China).
Cell culture
The NCI-H446 human SCLC cell line was purchased from
the Institute of Biochemistry and Cell Biology (Shanghai Institutes
for Biological Sciences, Chinese Academy of Science, Shanghai,
China). Cells were cultured in RPMI-1640 medium, supplemented with
10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U/ml
penicillin and 100 mg/ml streptomycin (all purchased from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) under standard
cell culture conditions (37°C, 100% relative humidity, atmosphere
containing 5% CO2).
MTT cell viability assays
To investigate the effectiveness of XAV939 targeting
the Wnt signaling pathway in SCLC cells, the inhibitory effects
XAV939 on the viability of H446 cells was determined. Cell
viability was measured using an MTT colorimetric dye reduction
assay, as previously described (24).
The assays were divided into three groups and each group received
various drug concentrations, as follows: XAV939 group (2, 4, 8, 16
and 32 µM XAV939), cisplatin group (1, 2, 4, 8 and 10 mg/l
cisplatin) and combination group (2.0 mg/l cisplatin combined with
2, 4, 8, 16 or 32 µM XAV939). Each experiment was performed in
96-well plates and repeated three times. The concentration range
for treatment with each inhibitor was determined on the basis of
previous studies (12,25). A total of 1×105 NCI-H446
cells/well were seeded in 96-well plates and treated with the three
groups drugs following incubation for 24 h. Cells treated with the
drugs were exposed for 24 or 48 h at 37°C, following which the drug
was removed. A total of 100 µl MTT was added to each well and
incubated for 4 h. Subsequently, the medium was removed and 100 µl
DMSO was added to dissolve the solid formazan for 15 min. The
absorbance at a wavelength of 570 nm was then determined.
Apoptosis analysis
Apoptosis was determined using an annexin V/FITC
apoptosis detection kit, according to the manufacturer's protocol.
NCI-H446 cells (1×106 cells/ml) were seeded into 6-well
plates and subsequently treated with PBS (control) and XAV939 (8,
16 and 32 µM) at 37°C for 24 h. Cells were re-suspended in 500 µl
1X binding buffer and incubated with Annexin V/FITC and propidium
iodide (PI) for 20 min at room temperature in the dark. Cells were
analyzed using fluorescence-activated cell sorting (FACSCalibur; BD
Biosciences, San Jose, CA, USA) and FlowJo software (version 7.6;
Tree Star Inc., Ashland, OR, USA).
Cell cycle analysis
Cell cycle was determined using a cell cycle
detection kit (Nanjing KeyGEN Biotech. Co. Ltd.), according to the
manufacturer's protocol. The assays were divided into two groups:
PBS control group and XAV939 group. NCI-H446 cells with a
concentration of 1×106/ml were seeded into 6-well plates
and treated with PBS (control), XAV939 (8, 16 and 32 µM) at 37°C
for 24 h. Cells were harvested and fixed with 70% ethanol at 4°C
for 12 h and subsequently incubated at 37°C for 30 min with 100 µl
RNase A. A total of 400 µl PI was added to the cells at 4°C in the
dark for 30 min. Cell cycle distribution was analyzed for 10,000
selected cells using the Aria II flow cytometer (BD Biosciences) at
488 nm wavelength. The resulting DNA distributions were analyzed
using FlowJo software (version 7.6; Tree Star Inc.) to determine
the proportion of cells in G0/G1, S and
G2/M phases of the cell cycle.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 17.0; SPSS, Inc., Chicago, IL, USA). Multiple
group comparisons were made using one-way analysis of variance and
the post-hoc tests were performed using LSD test and Dunnett
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
XAV939 effectively inhibits the
viability of NCI-H446 cells
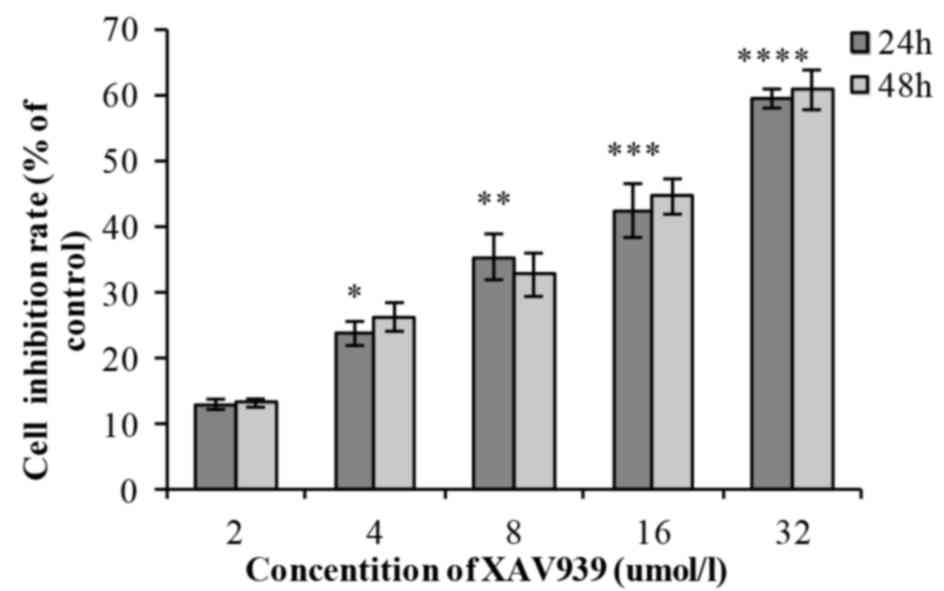
In the XAV939 group, with the increase of drug
concentration (2, 4, 8, 16 and 32 µM), the cell inhibition rate
increased gradually and the difference was statistically
significant (P<0.01; Table I). As
incubation time (24 or 48 h) increased, the cell inhibition rate of
each drug concentration increased gradually; however, there was no
statistically significant difference between the 24 and 48 h
(P>0.05; Table I). The
half-maximal inhibitory concentration, IC50, was 20.02
µmol/l. XAV939 induced an inhibitory effect on the viability of
NCI-H446 cells in a dose-dependent manner (Fig. 1). Each experiment was performed three
times.
 | Table I.NCI-H446 cell viability following
treatment with XAV939. |
Table I.
NCI-H446 cell viability following
treatment with XAV939.
|
| Cell viability
(%) | Comparison between
treatment durations |
|---|
|
|
|
|
|---|
| Treatment | 24 h | 48 h | F | P-value |
|---|
| XAV939, µM |
|
|
|
|
| 2 | 12.99±0.78 | 13.18±0.63 | 0.500 | 0.855 |
| 4 | 23.78±1.83 | 26.30±2.18 | 0.135 | 0.426 |
| 8 | 35.45±3.50 | 32.72±3.30 | 0.043 | 0.602 |
| 16 | 42.50±4.10 | 44.64±2.69 | 0.231 | 0.686 |
| 32 | 59.57±1.45 | 60.89±3.03 | 2.985 | 0.715 |
| Comparison between
treatment concentrations |
|
|
|
|
| F | 45.027 | 50.605 |
|
|
|
P-value |
<0.001a |
<0.001a |
|
|
Cisplatin inhibits the viability of
NCI-H446 cells
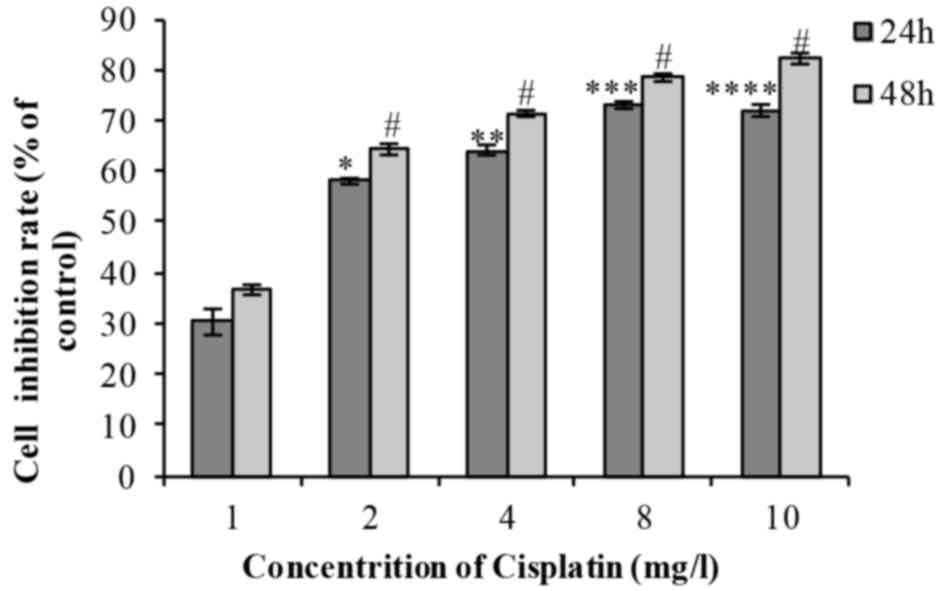
Following treatment of cells with 1, 2, 4, 8 or 10
mg/l cisplatin for 24 or 48 h, cell viability decreased in a time-
and dose-dependent manner (Fig. 2).
The difference was identified to be statistically significant
(Table II). The IC50 was
2.056 mg/l.
 | Table II.NCI-H446 cell viability following
treatment with cisplatin. |
Table II.
NCI-H446 cell viability following
treatment with cisplatin.
|
| Cell viability
(%) | Comparison between
treatment durations |
|---|
|
|
|
|
|---|
| Treatment | 24 h | 48 h | F | P-value |
|---|
| Cisplatin,
mg/l |
|
|
|
|
| 1 | 30.26±2.58 | 36.61±0.99 | 4.277 | 0.084 |
| 2 | 58.03±0.58 | 64.32±1.10 | 1.818 | 0.007b |
| 4 | 64.18±1.02 | 71.40±0.62 | 1.837 | 0.004b |
| 8 | 73.05±0.71 | 78.47±0.76 | 0.028 | 0.007b |
| 10 | 71.98±1.19 | 82.23±1.09 | 0.007 | 0.003b |
| Comparison between
treatment concentrations |
|
|
|
|
| F | 151.939 | 107.277 |
|
|
|
P-value | <0.001a |
<0.001a |
|
|
Combination treatment inhibits the
viability of NCI-H446 cells
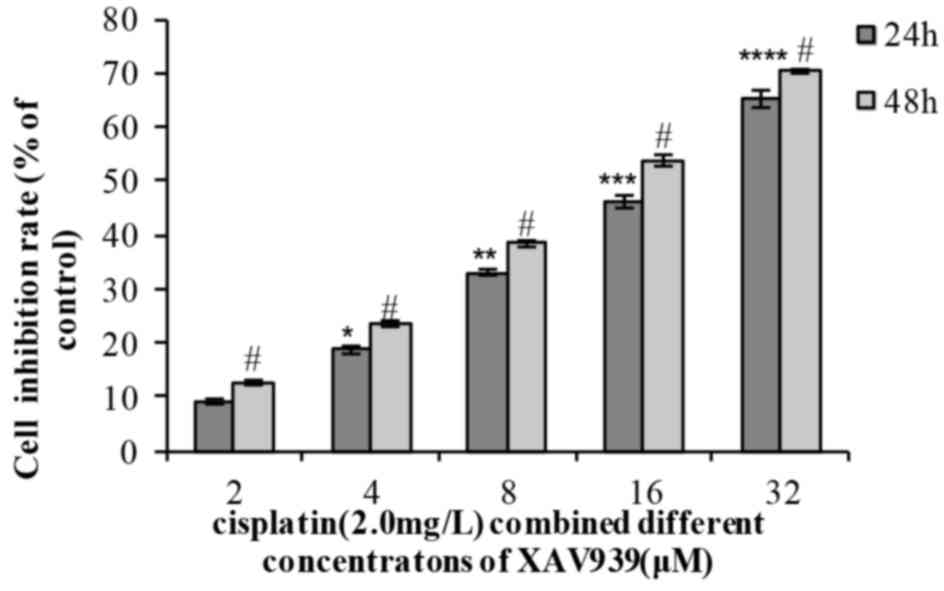
In the combination group, the cell inhibition rate
increased with the extension of time (24 or 48 h) and with XAV939
concentration (2, 4, 8, 16 or 32 µM). The results of the present
study indicated that a combination of the two drugs effectively
inhibited NCI-H446 cell viability in a dose- and time-dependent
manner (Table III; Fig. 3). When distinct concentrations of
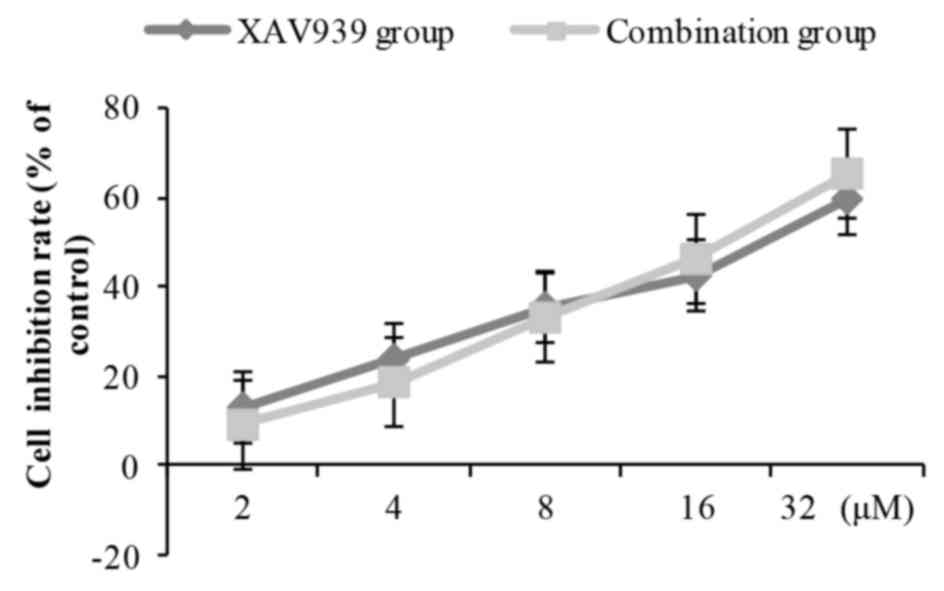
XAV939 were combined with cisplatin (2.0 mg/l), the inhibition of
NCI-H446 cell viability was slightly decreased at low doses
compared with XAV939 treatment alone, and was slightly increased at
high doses compared with XAV939 treatment alone (Fig. 4). However, there was no statistically
significant difference between combination group and the XAV939
group at any concentration of XAV939.
 | Table III.Cell viability of NCI-H446 cells
following combined treatment of XAV939 of the indicated
concentrations and 2.0 mg/l cisplatin. |
Table III.
Cell viability of NCI-H446 cells
following combined treatment of XAV939 of the indicated
concentrations and 2.0 mg/l cisplatin.
|
| Cell viability
(%) | Comparison between
treatment durations |
|---|
|
|
|
|
|---|
| Treatment | 24 h | 48 h | F | P-value |
|---|
| XAV939, µM |
|
|
|
|
| 2 | 9.11±0.48 | 12.62±0.45 | 0.058 | 0.006b |
| 4 | 18.66±0.73 | 23.55±0.56 | 0.535 | 0.003b |
| 8 | 33.07±0.56 | 38.37±0.60 | 0.001 | 0.003b |
| 16 | 46.26±1.17 | 53.81±1.06 | 0.001 | 0.008b |
| 32 | 65.22±1.61 | 70.34±0.38 | 2.267 | 0.036b |
| Comparison between
treatment concentrations |
|
|
|
|
| F | 490.954 | 1228.98 |
|
|
|
P-value |
<0.001a | <0.001a |
|
|
XAV939 induces NCI-H446 cell
apoptosis
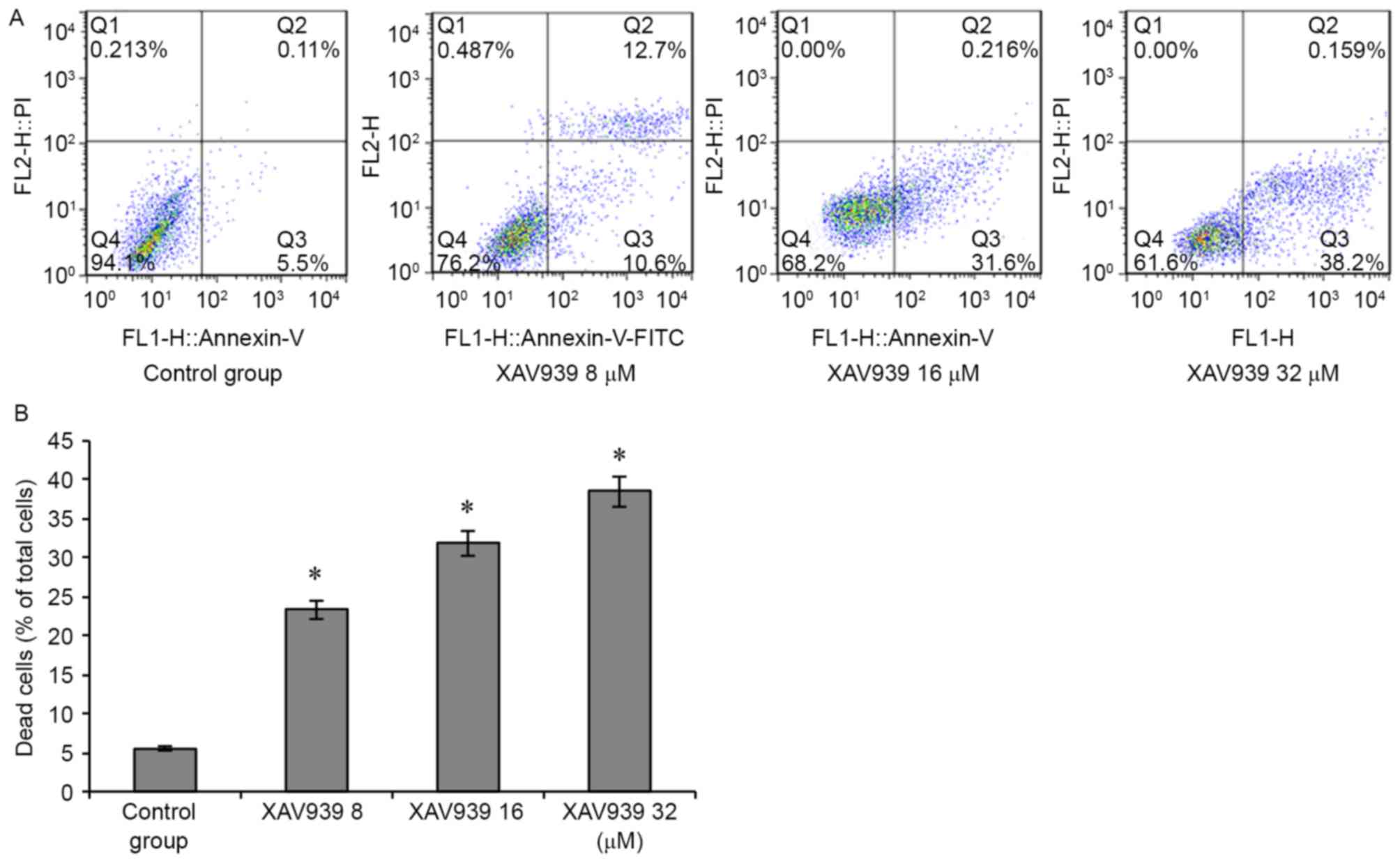
To determine whether the inhibitory effect of XAV939
on cell viability was associated with the induction of cell
apoptosis, NCI-H446 cells were treated with a variety of
concentrations (0, 8, 16 and 32 µM) of XAV939 for 24 h. The
proportion of apoptotic NCI-H446 cells increased with XAV939 dosage
(P<0.05; Fig. 5), suggesting that
the XAV939 may induce increased apoptosis at increased
concentrations.
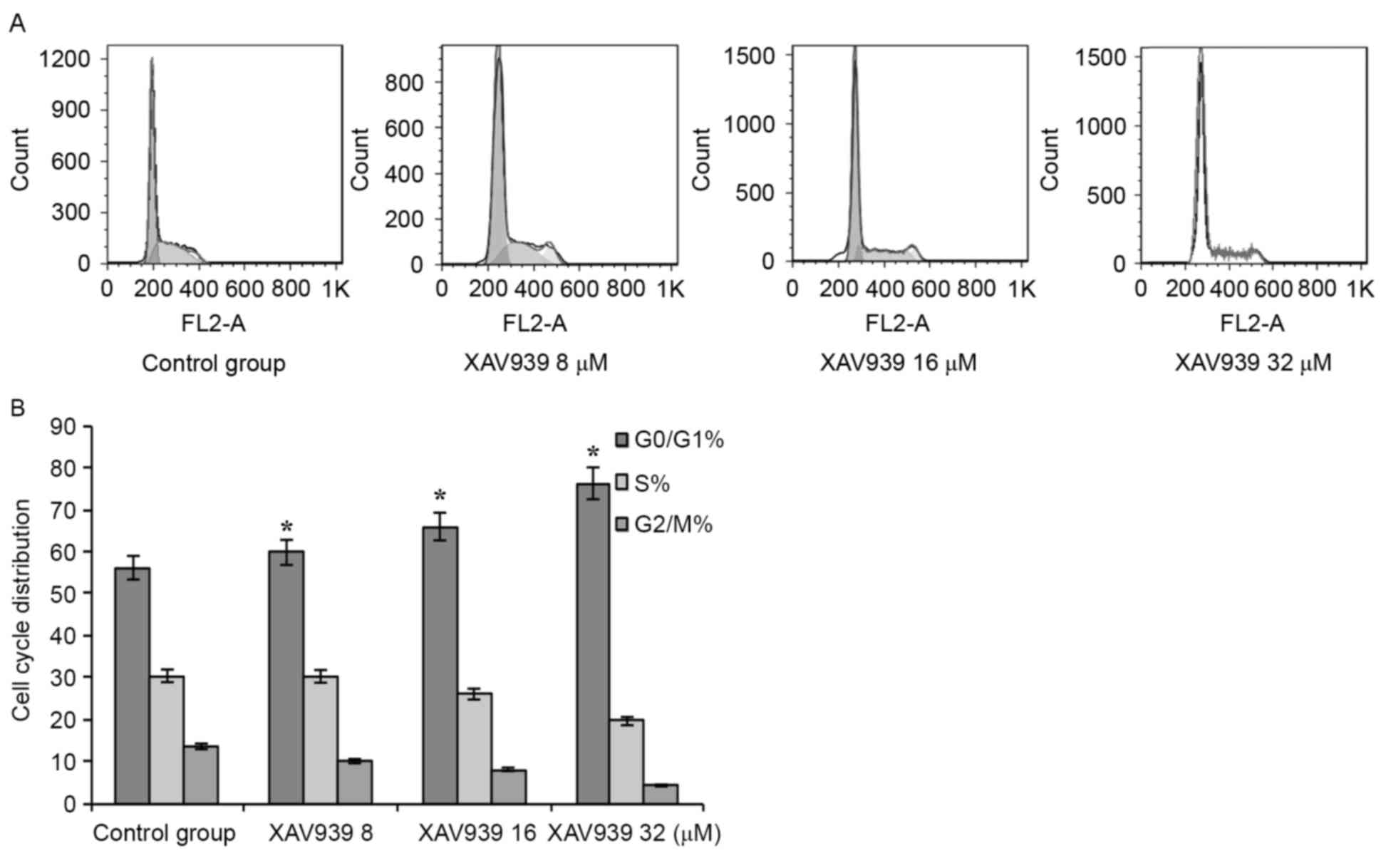
Cell cycle analysis
When the drug group was compared with the control
group, the G0/G1 phase cell proportion
increased significantly, and the S phase to G2/M ratio
markedly decreased. The difference exhibited a statistical
significance (P<0.05; Fig. 6).
Cell cycle analysis, using flow cytometry, revealed a
dose-dependent increase in the accumulation of cells in
G0/G1 phase (P<0.05; Fig. 6), indicating that XAV939 induced cell
apoptosis by cell cycle inhibition.
Discussion
The typical treatment for SCLC is systemic
chemotherapy, which exhibits a curative effect; however, the
recurrence rate is high, indicating a requirement to develop more
effective and targeted therapy options for patients with SCLC.
Aberrant activation of Wnt signaling, which serves a key function
in the regulation of cell viability, development and
differentiation, has been associated with a number of types of
cancer including colorectal, prostate, liver, and breast cancer
(10,11,26,27). Thus,
inhibition of Wnt signaling may be an effective approach to the
treatment of distinct types of cancer.
A number of signaling pathway inhibitors have been
used to suppress tumor growth. Dickkopf-related protein 1 (28) and secreted frizzled-related proteins
(29) are typical Wnt antagonists.
XAV939, a novel small molecule Wnt signaling pathway inhibitor, may
restrain the abnormal activation of Wnt/β-catenin and exhibit no
effect on the normal function of cells. XAV939 may inhibit the
activity of tankyrases and may, through poly-ADP-ribosylation,
stabilize axin and inhibit Wnt signaling pathways. Compared with
other Wnt pathway inhibitors, XAV939 exhibits a strong specificity
for the Wnt signaling pathway, without influencing NF-kB or TGF-B
signaling pathways.
Huang et al (13) and Chen et al (14) validated that XAV939 inhibited the
viability of colon cancer cells by suppressing Wnt signaling,
through binding to the catalytic PARP domain of TNKS. In addition,
Bilir et al (23) identified
that XAV939 inhibited the viability of triple-negative breast
cancer cells by suppressing Wnt signaling. Shao et al
(30) used XAV939 in combination with
nedaplatin on HeLa cells, indicating that XAV939 inhibits the
viability of cervical cancer cells. To determine whether XAV939 is
able to inhibit the viability of SCLC cells, an MTT assay was used.
Following treatment with XAV939, a marked inhibition of cell
viability in the SCLC cells was observed. When XAV939 was combined
with cisplatin, the inhibition of viability of NCI-H446 cells was
slightly weakened at a low dose and was slightly strengthened at a
high dose compared with XAV939 treatment alone. However, there was
no statistically significant difference between combination group
and the XAV939 alone group at any concentration of XAV939.
The effect of XAV939 on the apoptosis of SCLC cells
and the cell cycle was analyzed using flow cytometry. Flow
cytometry analysis indicated that XAV939 inhibited the viability of
NCI-H446 cells. The proportion of apoptotic NCI-H446 cells
increased with XAV939 dosage. In addition, cell cycle analysis
revealed a dose-dependent increased accumulation of cells in
G0/G1 phase. The underlying molecular
mechanism of apoptosis induction may be as follows: XAV939
decreases the level of β-catenin in the Wnt signaling pathway and
prevents it from binding to T cell factor/lymphoid enhancer-binding
factor, thus inhibiting the expression of downstream target gene
c-myc and Cyclin D1. C-myc gene, an oncogene, primarily functions
in the cell cycle and is the switch between
G0/G1 and S phase. When the Wnt signaling
pathway is activated, c-myc gene is expressed, which causes the
cells to transition from the quiescent period into the
proliferative phase, and promotes cancer cell proliferation,
invasion and metastasis (31,32). Cyclin D1 is a specific cyclin that
acts on the G1 phase, which is highly conserved, and
promotes dysregulated cell proliferation and malignancy by
regulating cyclin-dependent kinase to promote G1/S phase
conversion (33). XAV939 inhibited
the expression of c-myc gene and Cyclin D1 by blocking the signal
pathway. The cells were arrested in G0/G1
phase and induced apoptosis. Furthermore, Yang et al
(34) identified that XAV939 induced
G0/G1 phase inhibition in acute lymphoblastic
leukemia cells, which is consistent with this study.
The results of the present study preliminary
validate XAV939-induced inhibition of cell viability in SCLC, and
also provides a reference basis and theoretical support for SCLC
targeted therapy. Additional studies on the basis of targeting gene
expression to intervene in and inhibit tumor growth, are required.
Inhibition of SCLC-associated signaling pathways may provide an
effective method of treatment (35).
References
|
1
|
Byers LA and Rudin CM: Small cell lung
cancer: Where do we go from here? Cancer. 121:664–672. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Merrill RM, Henson DE and Barnes M:
Conditional survival among patients with carcinoma of the lung.
Chest. 116:697–703. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gustafsson BI, Kidd M, Chan A,
Malfertheiner MV and Modlin IM: Bronchopulmonary neuroendocrine
tumors. Cancer. 113:5–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Snow GE, Kasper AC, Busch AM, Schwarz E,
Ewings KE, Bee T, Spinella MJ, Dmitrovsky E and Freemantle SJ: Wnt
pathway reprogramming during human embryonal carcinoma
differentiation and potential for therapeutic targeting. BMC
Cancer. 9:3832009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
You L, He B, Xu Z, Uematsu K, Mazieres J,
Mikami I, Reguart N, Moody TW, Kitajewski J, McCormick F and
Jablons DM: Inhibition of Wnt-2-mediated signaling induces
programmed cell death in non-small-cell lung cancer cells.
Oncogene. 23:6170–6174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pacheco-Pinedo EC, Durham AC, Stewart KM,
Goss AM, Lu MM, Demayo FJ and Morrisey EE: Wnt/β-catenin signaling
accelerates mouse lung tumorigenesis by imposing an embryonic
distal progenitor phenotype on lung epithelium. J Clin Invest.
121:1935–1945. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen DX, Chiang AC, Zhang XH, Kim JY,
Kris MG, Ladanyi M, Gerald WL and Massagué J: WNT/TCF signaling
through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis.
Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Howe LR and Brown AM: Wnt signaling and
breast cancer. Cancer Biol Ther. 3:36–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khramtsov AI, Khramtsova GF, Tretiakova M,
Huo D, Olopade OI and Goss KH: Wnt/beta-catenin pathway activation
is enriched in basal-like breast cancers and predicts poor outcome.
Am J Pathol. 176:2911–2920. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Polakis P: Wnt signaling in cancer. Cold
Spring Harb Perspect Biol. 4:a0080522012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Busch AM, Johnson KC, Stan RV, Sanglikar
A, Ahmed Y, Dmitrovsky E and Freemantle SJ: Evidence for tankyrases
as antineoplastic targets in lung cancer. BMC Cancer. 13:2112013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang SM, Mishina YM, Liu S, Cheung A,
Stegmeier F, Michaud GA, Charlat O, Wiellette E, Zhang Y, Wiessner
S, et al: Tankyrase inhibition stabilizes axin and antagonizes Wnt
signalling. Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan
CW, Wei S, Hao W, Kilgore J, Williams NS, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
MacNamara B, Wang W, Chen Z, Hou M, Mazur
J, Gruber A and Porwit-MacDonald A: Telomerase activity in relation
to pro- and anti-apoptotic protein expression in high grade
non-Hodgkin's lymphomas. Haematologica. 86:386–393. 2001.PubMed/NCBI
|
|
16
|
Klapper W, Krams M, Qian W, Janssen D and
Parwaresch R: Telomerase activity in B-cell non-Hodgkin lymphomas
is regulated by hTERT transcription and correlated with
telomere-binding protein expression but uncoupled from
proliferation. Br J Cancer. 89:713–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gelmini S, Poggesi M, Distante V, Bianchi
S, Simi L, Luconi M, Raggi CC, Cataliotti L, Pazzagli M and Orlando
C: Tankyrase, a positive regulator of telomere elongation, is over
expressed in human breast cancer. Cancer Lett. 216:81–87. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gelmini S, Poggesi M, Pinzani P, Mannurita
SC, Cianchi F, Valanzano R and Orlando C: Distribution of
Tankyrase-1 mRNA expression in colon cancer and its prospective
correlation with progression stage. Oncol Rep. 16:1261–1266.
2006.PubMed/NCBI
|
|
19
|
Gelmini S, Quattrone S, Malentacchi F,
Villari D, Travaglini F, Giannarini G, Della Melina A, Pazzagli M,
Nicita G, Selli C and Orlando C: Tankyrase-1 mRNA expression in
bladder cancer and paired urine sediment: Preliminary experience.
Clin Chem Lab Med. 45:862–866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shervington A, Patel R, Lu C, Cruickshanks
N, Lea R, Roberts G, Dawson T and Shervington L: Telomerase
subunits expression variation between biopsy samples and cell lines
derived from malignant glioma. Brain Res. 1134:45–52. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian X, Hou W, Bai S, Fan J, Tong H and Xu
H: XAV939 inhibits the stemness and migration of neuroblastoma
cancer stem cells via repression of tankyrase 1. Int J Oncol.
45:121–128. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waaler J, Machon O, Tumova L, Dinh H,
Korinek V, Wilson SR, Paulsen JE, Pedersen NM, Eide TJ, Machonova
O, et al: A novel tankyrase inhibitor decreases canonical Wnt
signaling in colon carcinoma cells and reduces tumor growth in
conditional APC mutant mice. Cancer Res. 72:2822–2832. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bilir B, Kucuk O and Moreno CS: Wnt
signaling blockage inhibits cell proliferation and migration, and
induces apoptosis in triple-negative breast cancer cells. J Transl
Med. 11:2802013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jahromi Zare M, Ranjbarian P and Shiravi
S: Cytotoxicity evaluation of Iranian propolis and calcium
hydroxide on dental pulp fibroblasts. J Dent Res Dent Clin Dent
Prospects. 8:130–133. 2014.PubMed/NCBI
|
|
25
|
Fong JT, Jacobs RJ, Moravec DN, Uppada SB,
Botting GM, Nlend M and Puri N: Alternative signaling pathways as
potential therapeutic targets for overcoming EGFR and c-Met
inhibitor resistance in non-small cell lung cancer. PLoS One.
8:e783982013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moon RT: Wnt/β-catenin pathway. Science's
STKE. 2005:cm12005.
|
|
27
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salim H, Zong D, Hååg P, Novak M, Mörk B,
Lewensohn R, Lundholm L and Viktorsson K: DKK1 is a potential novel
mediator of cisplatin-refractoriness in non-small cell lung cancer
cell lines. BMC Cancer. 15:6282015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Y, He B, You L and Jablons DM: Roles
of secreted frizzled-related proteins in cancer. Acta Pharmacol
Sin. 28:1499–1504. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao W and Wang W: Experimental and basic
research affect telomerase inhibitor XAV939 joint nedaplatin on
HeLa cell proliferation and apoptosis. Maternal and Child Health
Care of China. 19:1001–4411. 2014.
|
|
31
|
Partanen JI, Nieminen AI, Mäkelä TP and
Klefstrom J: Suppression of oncogenic properties of c-Myc by
LKB1-controlled epithelial organization. Proc Natl Acad Sci USA.
104:pp. 14694–14699. 2007, View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Guessous F, Johnson EB, Eberhart CG,
Li XN, Shu Q, Fan S, Lal B, Laterra J, Schiff D, et al: Functional
and molecular interactions between the HGF/c-Met pathway and c-Myc
in large-cell medulloblastoma. Lab Invest. 88:98–111. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jirawatnotai S, Hu Y, Livingston DM and
Sicinski P: Proteomic identification of a direct role for cyclin d1
in DNA damage repair. Cancer Res. 72:4289–4293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang Y, Mallampati S, Sun B, Zhang J, Kim
SB, Lee JS, Gong Y, Cai Z and Sun X: Wnt pathway contributes to the
protection by bone marrow stromal cells of acute lymphoblastic
leukemia cells and is a potential therapeutic target. Cancer Lett.
333:9–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dihlmann S and von Knebel Doeberitz M:
Wnt/beta-catenin-pathway as a molecular target for future
anti-cancer therapeutics. Int J Cancer. 113:515–524. 2005.
View Article : Google Scholar : PubMed/NCBI
|