Introduction
Gastric cancer is the most common digestive tract
malignancy that affects ~1 million people per year worldwide
(1), and >90% of these cases are
gastric adenocarcinoma (GA). At present, surgery remains the
primary method for treating patients with GA. Lymph node metastasis
(LNM) usually indicates a poor prognosis of resected GA (RGA), as
it is closely associated with widespread metastasis that will
affect the survival of the patients (2,3). A reduced
risk of metastasis and recurrence and improved survival time are
the primary goals of cancer therapy. Therefore, it would be useful
to identify a biomarker associated with LNM that may assist in
predicting prognosis.
Cyclin-dependent kinase inhibitor 1A (CDKN1A; also
known as p21), a cell cycle regulator which is encoded by the
CDKN1A gene at chromosome 6p21.2, is primarily involved in
mediating cell growth, proliferation, differentiation, DNA repair
and apoptosis (4–6). Data from a number of studies have
demonstrated that it assists in regulating tumor development by
inducing cell cycle arrest, leading to a reduction in cancer cell
growth rate (7,8). Aberrant expression of CDKN1A has been
frequently observed in different types of cancer tissues (9–11). In the
present study, in order to understand the association between
CDKN1A expression and prognosis in patients with RGA, the
correlation between the expression of CDKN1A and
clinicopathological factors, LNM, recurrence rate, and survival in
patients with RGA was evaluated by various statistical
analyses.
Materials and methods
Patients
GA tissue samples for immunohistochemistry (IHC)
were retrospectively collected from 217 patients who were diagnosed
pathologically with primary GA and then underwent surgical
resection between January 2003 and November 2008. Information was
obtained from the medical records of each patient and follow-up
records subsequent to treatment were monitored until March 2013.
Survival data were obtained via telephone contact and the Social
Security Death Index. Recurrence was diagnosed according to the
criteria described in our previous study (12). Fluoropyrimidine-based regimens
(fluoropyrimidine or fluoropyrimidine plus platinum) were used as
first-line chemotherapy to treat all of the patients following
resection of GA. The standards described in the guidelines of the
International Union Against Cancer (UICC; 5th Edition) and the
National Comprehensive Cancer Network's (NCCN) Clinical Practice
Guidelines in Oncology (v.1.2011) (13,14) were
used to determine the tumor-node-metastasis (TNM) stage and
histological grade for each of the tissue samples used in the
present study.
For comparison of the results of western blotting
and IHC, GA tissue samples from 52 cases were collected from
patients with primary GA who underwent surgical resection between
March 2014 and December 2015.
The study was approved by the Ethics Committee of
Fuzhou General Hospital (Fuzhou, China) and all patients provided
informed consent.
IHC
IHC was used to determine the levels of CDKN1A in
paraffin-embedded RGA (217 cases) and normal gastric tissues (30
cases). The anti-CDKN1A antibody was obtained from ZSGB-BIO (cat.
no. TA808128; dilution, 1:150; Beijing, China). IHC was performed
as described previously (12,15). The expression intensity of CDKN1A was
scored from 0 to 3 according to the standards established by Kawata
et al (16). Samples with a
staining intensity of 0 or 1 were considered as low expression, and
with staining intensity of 2 or 3 as high expression.
Hematoxylin and eosin (H&E)
staining
H&E staining of paraffin-embedded normal gastric
tissues and cancer tissues in patients with RGA were performed by a
pathologist using an established methodology. The steps of H&E
staining are briefly described as follows: i) Sections of tissue
samples were dewaxed with xylene and washed with various
concentrations of ethanol (100, 100, 95 and 80%); ii) stained with
0.5% hematoxylin for 5 min, washed with water for 10 min and then
distilled water for 5 sec; iii) soaked in hydrochloric acid ethanol
for 30 sec and then water for 15 min; iv) 0.5% eosinstaining for 2
min; v) conventional dehydration, transparency and neutral resins
mount; vi) the results were observed using light microscopy
(magnification, ×200). All steps were performed at room temperature
(25°C).
Western blotting
As described previously (17), total protein from cancer tissues was
extracted using RIPA lysis buffer (cat. no. P0013B) and quantified
using the BCA Protein Assay kit (cat. no. P0012S), and western
blotting was performed using a SDS-PAGE Gel preparation reagent kit
[cat. no. P0012A: P0012A-1, 30% Acr-Bis (29:1); P0012A-2, 1M
Tris-HCl, pH 8.8; P0012A-3, 10% SDS; P0012A-4, ammonium persulfate;
P0012A-5, TEMED; P0012A-6, 1M Tris-HCl, pH 6.8; SDS-PAGE Sample
Loading Buffer (5X), cat. no. P0015], blocking buffer (cat. no.
P0023B) and enhanced chemiluminescence reagents (ECL; cat. no.
P0018) (all of the above reagents were from Beyotime Institute of
Biotechnology, Haimen, China). Briefly, the proteins (30 µg per
lane) were subjected to SDS-PAGE gel electrophoresis (concentrated
gel, 5%; separation gel, 12%) and then transferred to
polyvinylidene membranes. Following incubation with blocking buffer
at room temperature for 30 min, the membranes were incubated with
primary polyclonal rabbit anti-human CDKN1A antibody (cat. no.
SC-397; dilution, 1:300; ZSGB-BIO) or primary polyclonal rabbit
anti-human GAPDH antibody (cat. no. 2118S; dilution, 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA) overnight at 4°C.
Following three washes with TBST the membranes were incubated with
secondary goat anti-rabbit antibody IgG-horseradish peroxidase
(cat. no. M21002M; dilution, 1:2,000; abMart Company, Shanghai,
China) for 4 h at room temperature. Finally, the bands were
visualized using ECL and analyzed with a chemiluminescence imager
system (Image Quant LAS4000 mini system; GE Healthcare, Chicago,
IL, USA).
Statistical analysis
SPSS 17.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used to conduct data analysis, including the
correlation between CDKN1A expression and the clinicopathological
characteristics and prognosis of patients. The methods used in this
analysis were the χ2 test, and Kaplan-Meier and log-rank
analyses of recurrence-free survival (RFS) and overall survival
(OS) times in months. Additionally, univariate and multivariate Cox
models were used to assess the prognostic ability of known
unfavorable pathological factors, and the correlation between
CDKN1A expression profiles and recurrence or survival. The
agreement between CDKN1A expression detected by western blot and
immunohistochemical analyses was evaluated by Spearman's rank
correlation analysis. All tests were two-sided, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics of
patients
The clinicopathological characteristics of the 217
RGA patients are summarized in Table
I. All patients had received fluoropyrimidine or
fluoropyrimidine plus platinum agents as first-line chemotherapy
for an average of 4 cycles following resection. The patients
comprised 153 males and 64 females; 135 patients were <60 years
old, and 82 patients were ≥60 years old, the median age was 60
years. At the time of resection, 147 patients (67.7%) presented
with LNM. In 145 cases (66.8%), the tumors were moderately or
well-differentiated, while the remaining 72 cases (33.2%) exhibited
poorly differentiated histology. By the end of follow-up, 126 had
developed recurrent disease, and 116 patients had succumbed to the
disease. The median times to recurrence and mortality were 41 and
54 months, respectively. The median time of observation was 51
months.
 | Table I.Patient characteristics (n=217). |
Table I.
Patient characteristics (n=217).
|
| Cases |
|---|
|
|
|
|---|
| Characteristics | n | % |
|---|
| Age, years |
|
|
|
<60 | 135 | 62.2 |
| ≥60 | 82 | 37.8 |
| Sex |
|
|
|
Male | 153 | 70.5 |
|
Female | 64 | 29.5 |
| Histological
grade |
|
|
|
Well/moderately
differentiated | 145 | 66.8 |
| Poorly
differentiated | 72 | 33.2 |
| Gross findings |
|
|
|
Apophysis | 17 | 7.8 |
|
Invasion | 200 | 92.2 |
| Tumor site |
|
|
|
Proximal | 45 | 20.7 |
|
Mid/distal | 172 | 79.3 |
| Lymph node
metastasis |
|
|
| No
metastasis | 70 | 32.3 |
|
Metastasis | 147 | 67.7 |
| T stage |
|
|
|
T1/T2 | 101 | 46.5 |
|
T3/T4 | 116 | 53.5 |
| Postoperative
chemotherapy |
|
|
|
Fluoropyrimidines | 62 | 28.6 |
|
Fluoropyrimidines +
platinum | 155 | 71.4 |
| CDKN1A
expression |
|
|
|
Low | 99 | 45.6 |
|
High | 118 | 54.4 |
| Survival |
|
|
|
Alive |
101 | 46.5 |
|
Succumbed | 116 | 53.5 |
| Recurrence |
|
|
| No | 91 | 41.9 |
|
Yes | 126 | 58.1 |
CDKN1A expression and correlation
between CDKN1A expression and clinicopathological
characteristics
CDKN1A exhibited differential expression patterns in
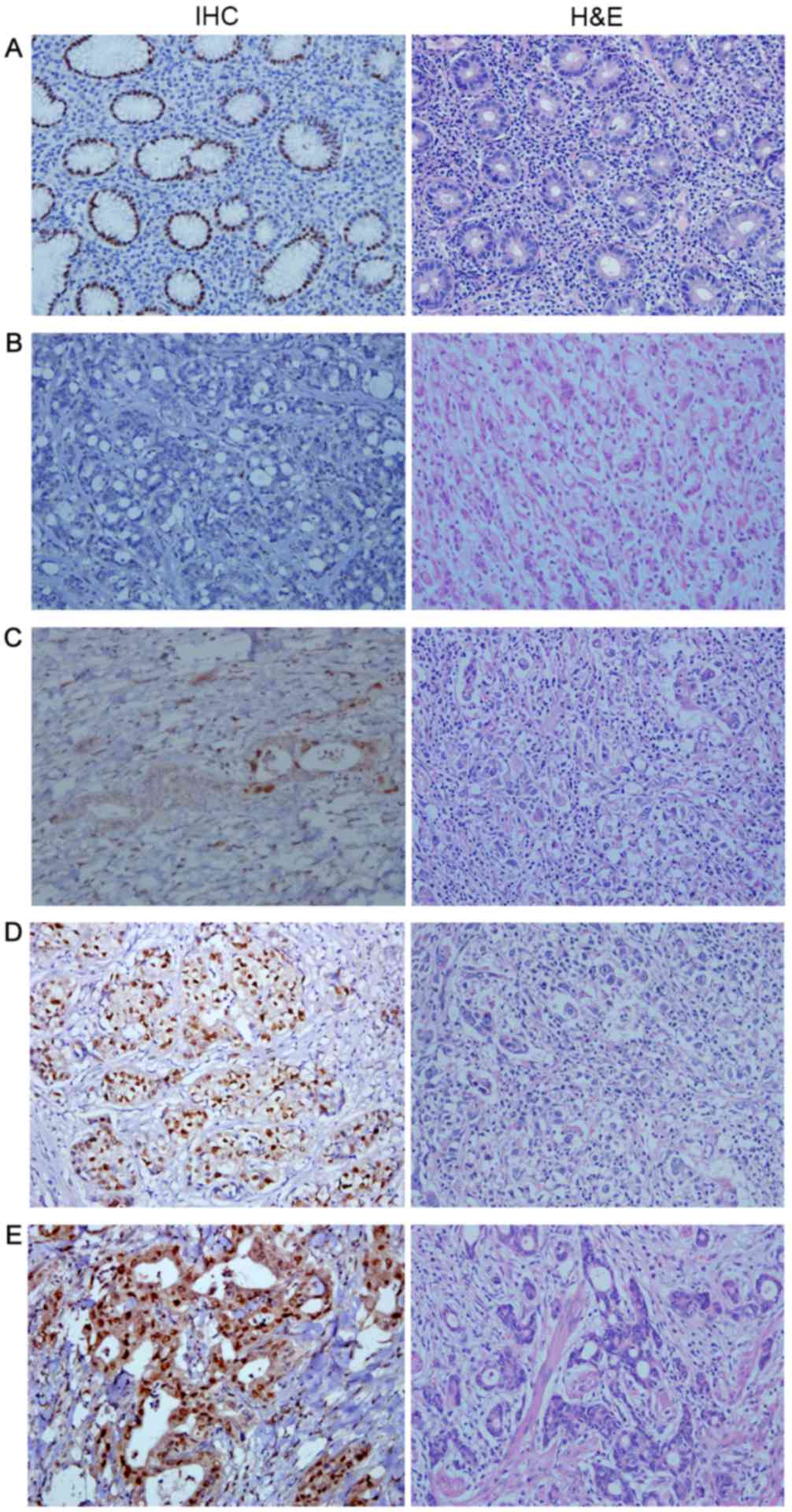
RGA and normal gastric tissues. In normal gastric tissues, CDKN1A
protein was located mainly in the cell nuclei, whereas CDKN1A
protein exhibited nuclear and cytoplasmic expression in RGA
tissues. Representative IHC results of the different intensities of
CDKN1A expression in RGA tissues and in normal gastric tissues are
presented in Fig. 1, along with their
corresponding H&E staining results. Representative western blot
results are shown in Fig. 2. Resected
tumor tissues from 118 cases (54.4%) of RGA exhibited high CDKN1A
expression, whereas those from 99 patients (45.6%) demonstrated low
CDKN1A expression (Table I). As
summarized in Table II, CDKN1A
expression was significantly associated with LNM (P=0.001),
recurrence (P<0.001), and survival (P<0.001), as determined
by χ2 test. No significant associations between CDKN1A
expression and other clinicopathological characteristics, including
age, gender, histological grade, gross pathology, tumor site, and
postoperative chemotherapy, were observed (Table II).
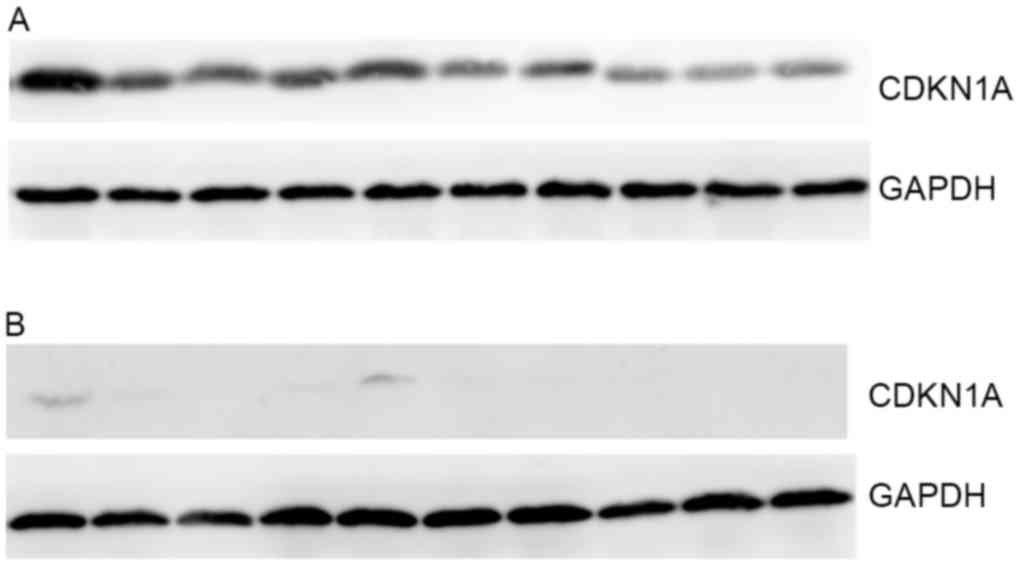 | Figure 2.Representative western blotting
results. (A) High expression of CDKN1A. From left to right, the
lanes represent the tissue sample number 3, 8, 12, 17, 18, 32, 43,
50, 55 and 131. (B) Low expression of CDKN1A. From left to right,
the lanes represent the tissue sample number 13, 41, 45, 51, 52,
69, 71, 80, 161 and 182. CDKN1A, cyclin-dependent kinase inhibitor
1A. |
 | Table II.Association between CDKN1A expression
and characteristics of patients with resected gastric
adenocarcinoma (n=217). |
Table II.
Association between CDKN1A expression
and characteristics of patients with resected gastric
adenocarcinoma (n=217).
|
| CDKN1A expression,
n (%) |
|
|---|
|
|
|
|
|---|
|
Characteristics | Low | High | P-value |
|---|
| Age, years |
|
| 0.072 |
|
<60 | 68 (50.4) | 67 (49.6) |
|
|
≥60 | 31 (37.8) | 51 (62.2) |
|
| Sex |
|
| 0.720 |
|
Male | 71 (46.4) | 82 (53.6) |
|
|
Female | 28 (43.8) | 36 (56.2) |
|
| Histological
grade |
|
| 0.593 |
|
Well/moderately
differentiated | 68 (46.9) | 77 (53.1) |
|
| Poorly
differentiated | 31 (43.1) | 41 (56.9) |
|
| Gross findings |
|
| 0.701 |
|
Apophysis | 7 (41.2) | 10 (58.8) |
|
|
Invasion | 92 (46.0) | 108 (54.0) |
|
| Tumor site |
|
| 0.621 |
|
Proximal | 22 (48.9) | 23 (51.1) |
|
|
Mid/distal | 77 (44.8) | 95 (55.2) |
|
| Lymph node
metastasis |
|
| 0.001a |
| No
metastasis | 20 (28.6) | 50 (71.4) |
|
|
Metastasis | 79 (53.7) | 68 (46.3) |
|
| T stage |
|
| 0.053 |
|
T1/T2 | 39 (38.6) | 62 (61.4) |
|
|
T3/T4 | 60 (51.7) | 56 (48.3) |
|
| Postoperative
chemotherapy |
|
| 0.085 |
|
Fluoropyrimidines | 34 (54.8) | 28 (45.2) |
|
|
Fluoropyrimidines +
platinum | 65 (41.9) | 90 (58.1) |
|
| Survival |
|
|
<0.001a |
|
Alive | 25 (24.8) | 76 (75.2) |
|
|
Succumbed | 74 (63.8) | 42 (36.2) |
|
| Recurrence |
|
|
<0.001a |
| No | 20 (22.0) | 71 (78.0) |
|
|
Yes | 79 (62.7) | 47 (37.3) |
|
Identification of prognostic factors
associated with OS
The 5-year OS rate of the entire patient group was
46.5% (Table I). Data from the
χ2 and log-rank tests indicated that patients with low
CDKN1A expression exhibited a significantly poorer survival rate
(25.3%; χ2 P<0.001) and lower median OS time
(log-rank P<0.001; Tables II and
III) compared with patients with
high CDKN1A expression. As demonstrated in Table III, other factors identified by
χ2 and log-rank tests to be associated with poor
survival included LNM (P<0.001 and P<0.001, respectively),
advanced T stage (P<0.001 and P<0.001, respectively), and
postoperative chemotherapy with fluoropyrimidine only (P=0.008 and
P=0.002, respectively). The results of the Kaplan-Meier analysis
demonstrated that patients with low CDKN1A expression exhibited a
significantly shorter OS time compared with those with high CDKN1A
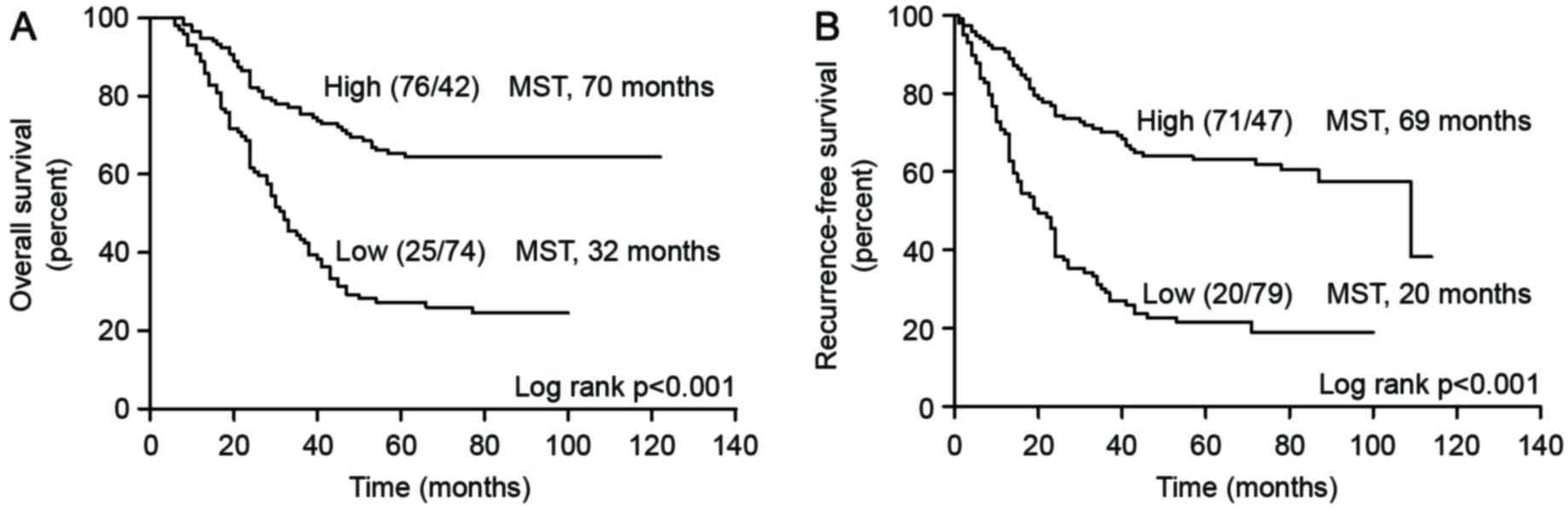
expression (median, 32 months vs. 70 months, P<0.001; Fig. 3A).
 | Table III.Association between OS and
characteristics of patients with resected gastric adenocarcinoma
(n=217). |
Table III.
Association between OS and
characteristics of patients with resected gastric adenocarcinoma
(n=217).
|
| Survival |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | Alive (%) | Deceased (%) |
P-valuea | Median OS time
(months) |
P-valueb |
|---|
| Age, years |
|
| 0.743 |
| 0.783 |
|
<60 | 64 (47.4) | 71 (52.6) |
| 57.0 |
|
|
≥60 | 37 (45.1) | 45 (54.9) |
| 48.0 |
|
| Sex |
|
| 0.718 |
| 0.611 |
|
Male | 70 (45.8) | 83 (54.2) |
| 51.0 |
|
|
Female | 31 (48.4) | 33 (51.6) |
| 53.0 |
|
| Histological
grade |
|
| 0.468 |
| 0.417 |
|
Well/moderately
differentiated | 70 (48.3) | 75 (51.7) |
| 54.0 |
|
| Poorly
differentiated | 31 (43.1) | 41 (56.9) |
| 41.0 |
|
| Gross findings |
|
| 0.290 |
| 0.229 |
|
Apophysis | 10 (58.8) | 7 (41.2) |
| 63.0 |
|
|
Invasion | 91 (45.5) | 109 (54.5) |
| 47.0 |
|
| Tumor site |
|
| 0.751 |
| 0.733 |
|
Proximal | 20 (44.4) | 25 (55.6) |
| 51.0 |
|
|
Mid/distal | 81 (47.1) | 91 (52.9) |
| 53.0 |
|
| Lymph node
metastasis |
|
|
<0.001c |
|
<0.001c |
| No
metastasis | 46 (65.7) | 24 (34.3) |
| 76.0 |
|
|
Metastasis | 55 (37.4) | 92 (62.6) |
| 39.0 |
|
| T stage |
|
|
<0.001c |
|
<0.001c |
|
T1/T2 | 63 (62.4) | 38 (37.6) |
| 69.0 |
|
|
T3/T4 | 38 (32.8) | 78 (67.2) |
| 35.0 |
|
| Postoperative
chemotherapy |
|
| 0.008c |
| 0.002c |
|
Fluoropyrimidines | 20 (32.3) | 42 (67.7) |
| 33.0 |
|
|
Fluoropyrimidines +
platinum | 81 (52.3) | 74 (47.7) |
| 63.0 |
|
| CDKN1A
expression |
|
|
<0.001c |
|
<0.001c |
|
Low | 25 (25.3) | 74 (74.7) |
| 32.0 |
|
|
High | 76 (64.4) | 42 (35.6) |
| 70.0 |
|
Results from the univariate and multivariate Cox
analyses additionally confirmed that positive LNM (P<0.001 and
P=0.006, respectively), advanced T stage (T3/T4) (P<0.001 and
P=0.001, respectively), postoperative chemotherapy with
fluoropyrimidine only (P=0.003 and P=0.003, respectively) and low
CDKN1A expression (P<0.001 and P<0.001, respectively) were
significantly associated with increased risk of mortality (Table IV). Taken together, these data
suggest that CDKN1A was a statistically significant independent
prognostic significance for OS in patients with RGA.
 | Table IV.Univariate and multivariate Cox
regression analyses of overall survival. |
Table IV.
Univariate and multivariate Cox
regression analyses of overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
| 0.785 |
| 0.366 |
|
<60 | 1.00 |
| 1.00 |
|
|
≥60 | 1.05
(0.73–1.53) |
| 1.20
(0.81–1.78) |
|
| Sex |
| 0.614 |
| 0.188 |
|
Male | 1.00 |
| 1.00 |
|
|
Female | 0.90
(0.60–1.35) |
| 0.75
(0.49–1.15) |
|
| Histological
grade |
| 0.422 |
| 0.252 |
|
Well/moderately
differentiated | 1.00 |
| 1.00 |
|
| Poorly
differentiated | 1.17
(0.80–1.71) |
| 1.26
(0.85–1.89) |
|
| Gross findings |
| 0.237 |
| 0.441 |
|
Apophysis | 1.00 |
| 1.00 |
|
|
Invasion | 1.59
(0.74–3.41) |
| 1.36
(0.62–2.98) |
|
| Tumor site |
| 0.735 |
| 0.462 |
|
Proximal | 1.00 |
| 1.00 |
|
|
Mid/distal | 0.93
(0.60–1.44) |
| 0.84
(0.53–1.34) |
|
| Lymph node
metastasis |
|
<0.001a |
| 0.006a |
| No
metastasis | 1.00 |
| 1.00 |
|
|
Metastasis | 2.40
(1.53–3.76) |
| 1.94
(1.21–3.14) |
|
| T stage |
|
<0.001a |
| 0.001a |
|
T1/T2 | 1.00 |
| 1.00 |
|
|
T3/T4 | 2.38
(1.61–3.51) |
| 2.03
(1.36–3.03) |
|
| Postoperative
chemotherapy |
| 0.003a |
| 0.003a |
|
Fluoropyrimidines | 1.00 |
| 1.00 |
|
|
Fluoropyrimidines +
platinum | 0.56
(0.38–0.81) |
| 0.55
(0.37–0.82) |
|
| CDKN1A
expression |
|
<0.001a |
|
<0.001a |
|
Low | 1.00 |
| 1.00 |
|
|
High | 0.33
(0.22–0.48) |
| 0.39
(0.26–0.58) |
|
Identification of prognostic factors
associated with recurrence risk
The overall recurrence rate of the whole patient
cohort was 58.1% (Table I). Data from
the χ2 and log-rank tests indicated that patients with
low CDKN1A expression exhibited a significantly higher recurrence
rate (79.8%; χ2 P<0.001) and lower median
recurrence-free survival (log-rank P<0.001) compared with those
with high CDKN1A expression (Tables
II and V). As demonstrated in
Table V, other factors identified by
χ2 and log-rank tests to be associated with a higher
recurrence rate included LNM (P<0.001 and P<0.001,
respectively), and T3/T4 stage (P<0.001 and P<0.001,
respectively). The results of the Kaplan-Meier analysis indicated
that patients with low CDKN1A expression exhibited a significantly
shorter RFS time compared with those with high CDKN1A expression
(median, 20 vs. 69 months, P<0.001; Fig. 3B).
 | Table V.Association between recurrence and
characteristics of patients with resected gastric carcinoma
(n=217). |
Table V.
Association between recurrence and
characteristics of patients with resected gastric carcinoma
(n=217).
|
| Recurrence, n
(%) |
|
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | No | Yes |
P-valuea | Median RFS time
(months) |
P-valueb |
|---|
| Age, years |
|
| 0.647 |
| 0.461 |
|
<60 | 55 (40.7) | 80 (59.3) |
| 35.0 |
|
|
≥60 | 36 (43.9) | 46 (56.1) |
| 42.0 |
|
| Sex |
|
| 0.961 |
| 0.876 |
|
Male | 64 (41.8) | 89 (58.2) |
| 39.0 |
|
|
Female | 27 (42.2) | 37 (57.8) |
| 41.0 |
|
| Histological
grade |
|
| 0.727 |
| 0.684 |
|
Well/moderately
differentiated | 62 (42.8) | 83 (57.2) |
| 41.0 |
|
| Poorly
differentiated | 29 (40.3) | 43 (59.7) |
| 36.0 |
|
| Gross findings |
|
| 0.338 |
| 0.249 |
|
Apophysis | 9 (52.9) | 8 (47.1) |
| 63.0 |
|
|
Invasion | 82 (41.0) | 118 (59.0) |
| 36.0 |
|
| Tumor site |
|
| 0.330 |
| 0.428 |
|
Proximal | 16 (35.6) | 29 (64.4) |
| 24.0 |
|
|
Mid/distal | 75 (43.6) | 97 (56.4) |
| 40.0 |
|
| Lymph node
metastasis |
|
|
<0.001c |
|
<0.001c |
| No
metastasis | 43 (61.4) | 27 (38.6) |
| 70.5 |
|
|
Metastasis | 48 (32.7) | 99 (67.3) |
| 24.0 |
|
| T stage |
|
|
<0.001c |
|
<0.001c |
|
T1/T2 | 56 (55.4) | 45 (44.6) |
| 68.0 |
|
|
T3/T4 | 35 (30.2) | 81 (69.8) |
| 24.0 |
|
| Postoperative
chemotherapy |
|
| 0.068 |
| 0.035c |
|
Fluoropyrimidines | 20 (32.3) | 42 (67.7) |
| 24.0 |
|
|
Fluoropyrimidines +
platinum | 71 (45.8) | 84 (54.2) |
| 53.0 |
|
| CDKN1A
expression |
|
|
<0.001c |
|
<0.001c |
|
Low | 20 (20.2) | 79 (79.8) |
| 20.0 |
|
|
High | 71 (60.2) | 47 (39.8) |
| 69.0 |
|
Results from the univariate and multivariate Cox
analyses demonstrated that LNM (P<0.001 and P=0.001,
respectively), advanced T stage (P<0.001 and P=0.003,
respectively), postoperative chemotherapy with fluoropyrimidine
only (P=0.038 and P=0.007) and low CDKN1A expression (P<0.001
and P<0.001) were significantly associated with increased risk
of recurrence (Table VI). Taken
together, these data suggest that CDKN1A was a statistically
significant independent prognostic significance for recurrence risk
in patients with RGA.
 | Table VI.Univariate and multivariate Cox
regression analysis of recurrence. |
Table VI.
Univariate and multivariate Cox
regression analysis of recurrence.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
| 0.466 |
| 0.733 |
|
<60 | 1.00 |
| 1.00 |
|
|
≥60 | 0.87
(0.61–1.26) |
| 0.94
(0.64–1.37) |
|
| Sex |
| 0.877 |
| 0.165 |
|
Male | 1.00 |
| 1.00 |
|
|
Female | 0.97
(0.66–1.42) |
| 0.75
(0.50–1.13) |
|
| Histological
grade |
| 0.687 |
| 0.507 |
|
Well/moderately
differentiated | 1.00 |
| 1.00 |
|
| Poorly
differentiated | 1.08
(0.75–1.56) |
| 1.14
(0.77–1.68) |
|
| Gross findings |
| 0.257 |
| 0.470 |
|
Apophysis | 1.00 |
| 1.00 |
|
|
Invasion | 1.51
(0.74–3.10) |
| 1.31
(0.63–2.75) |
|
| Tumor site |
| 0.434 |
| 0.315 |
|
Proximal | 1.00 |
| 1.00 |
|
|
Mid/distal | 0.85
(0.56–1.28) |
| 0.80
(0.51–1.24) |
|
| Lymph node
metastasis |
|
<0.001a |
| 0.001a |
| No
metastasis | 1.00 |
| 1.00 |
|
|
Metastasis | 2.54
(1.66–3.90) |
| 2.09
(1.33–3.27) |
|
| T stage |
|
<0.001a |
| 0.003a |
|
T1/T2 | 1.00 |
| 1.00 |
|
|
T3/T4 | 2.16
(1.50–3.13) |
| 1.77
(1.21–2.58) |
|
| Postoperative
chemotherapy |
| 0.038a |
| 0.007a |
|
Fluoropyrimidines | 1.00 |
| 1.00 |
|
|
Fluoropyrimidines +
platinum | 0.67
(0.46–0.98) |
| 0.58
(0.39–0.87) |
|
| CDKN1A
expression |
|
<0.001a |
|
<0.001a |
|
Low | 1.00 |
| 1.00 |
|
|
High | 0.31
(0.22–0.45) |
| 0.38
(0.26–0.56) |
|
Identification of risk factors
associated with LNM
At the time of surgical resection, 79 of 99 patients
(79.8%) with low CDKN1A expression presented with LNM, compared
with 68 of 118 patients (57.6%) with high CDKN1A expression, a
difference that was identified to be significant (P=0.001 by
χ2 test; Tables II and
VII). Univariate and multivariate
logistic regression analyses additionally demonstrated that low
CDKN1A expression was significantly associated with increased risk
of LNM (P=0.001 and P=0.001, respectively). Other factors,
including gross pathology (P=0.02 and P=0.035 from univariate and
multivariate analyses, respectively) and advanced T stage (T3/T4;
P=0.001 and P=0.005, from univariate and multivariate analyses,
respectively) were also significantly associated with LNM (Table VII). Taken together, these data
suggest that CDKN1A was a statistically significant independent
risk factor for LNM in patients with RGA.
 | Table VII.Univariate and multivariate analyses
of variables correlated with LNM. |
Table VII.
Univariate and multivariate analyses
of variables correlated with LNM.
|
| LNM, n (%) | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
|
Characteristics | No | Yes | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age, years |
|
|
| 0.643 |
| 0.206 |
|
<60 | 42 (31.1) | 93 (68.9) | 1.00 |
| 1.00 |
|
|
≥60 | 28 (34.1) | 54 (65.9) | 0.87
(0.49–1.56) | 0.99
(0.52–1.91) |
|
|
| Sex |
|
|
| 0.247 |
| 0.991 |
|
Male | 53 (34.6) | 100 (65.4) | 1.00 |
| 1.00 |
|
|
Female | 17 (26.6) | 47 (73.4) | 1.47
(0.77–2.80) | 1.58
(0.78–3.19) |
|
|
| Histological
grade |
|
|
| 0.584 |
| 0.363 |
|
Well/moderately
differentiated | 45 (31.0) | 100 (69.0) | 1.00 |
| 1.00 |
|
| Poorly
differentiated | 25 (34.7) | 47 (65.3) | 0.85
(0.47–1.54) | 0.73
(0.37–1.43) |
|
|
| Gross findings |
|
|
| 0.020a |
| 0.035a |
|
Apophysis | 10 (58.8) | 7 (41.2) | 1.00 |
| 1.00 |
|
|
Invasion | 60 (30.0) | 140 (70.0) | 3.33
(1.21–9.17) |
| 3.31
(1.09–10.02) |
|
| Tumor site |
|
|
| 0.587 |
| 0.736 |
|
Proximal | 13 (28.9) | 32 (71.1) | 1.00 |
| 1.00 |
|
|
Mid/distal | 57 (33.1) | 115 (66.9) | 0.82
(0.40–1.68) |
| 0.87
(0.40–1.92) |
|
| T stage |
|
|
| 0.001a |
| 0.005a |
|
T1/T2 | 44 (43.6) | 57 (56.4) | 1.00 |
| 1.00 |
|
|
T3/T4 | 26 (22.4) | 90 (77.6) | 2.67
(1.49–4.81) |
| 2.44
(1.31–4.53) |
|
| Postoperative
chemotherapy |
|
|
| 0.200 |
| 0.102 |
|
Fluoropyrimidines | 24 (38.7) | 38 (61.3) | 1.00 |
| 1.00 |
|
|
Fluoropyrimidines +
platinum | 46 (29.7) | 109 (70.3) | 1.50
(0.81–2.77) |
| 1.78
(0.89–3.55) |
|
| CDKN1A
expression |
|
|
| 0.001a |
| 0.001a |
|
Low | 20 (20.2) | 79 (79.8) | 1.00 |
| 1.00 |
|
|
High | 50 (42.4) | 68 (57.6) | 0.34
(0.19–0.64) |
| 0.33
(0.17–0.64) |
|
Correlation between western blot and
IHC analyses for detection of CDKN1A
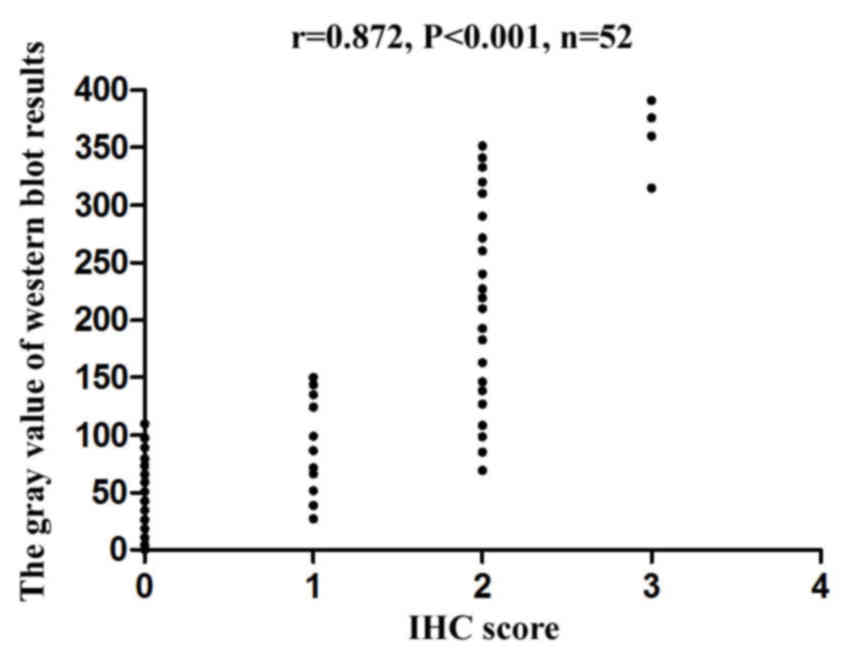
As presented in the representative results of
Fig. 2, CDKN1A protein levels in
tissues from 52 patients with RGA detected by western blotting and
IHC exhibited a good correlation (r=0.872, P<0.001), which was
obtained by scanning the western-blot bands with the gel analysis
system to obtain the gray value, and then performing Spearman's
rank correlation analysis to analyze the correlation between the
gray values and their corresponding IHC scores so as to plot the
scatter plot (Fig. 4). These data
suggest that CDKN1A expression in RGA tissues may be determined by
either western blotting or immunohistochemical analysis.
Discussion
The results of the present study demonstrated that
low CDKN1A expression in GA tissues was associated with LNM and
poor prognoses, as indicated by the shorter survival times and
increased recurrence risks in these patients. Thus, CDKN1A appears
to have independent prognostic significance in patients with
RGA.
Previously published data have demonstrated that
CDKN1A is a tumor suppressor due to its ability to induce cell
cycle arrest (18–21). Confirming this role, downregulation of
CDKN1A has been identified in different cancer tissues, and has
been associated with poor prognoses in various types of human
cancer, such as pancreatic and colon cancer, primary hepatocellular
carcinoma, early-stage human papillomavirus-associated lung cancer
and ovarian cancer (4,22–27).
Khalili et al (28) revealed
that low CDKN1A expression level may be associated with gastric
cancer initiation and progression. However, there have been
conflicting results in terms of the CDKN1A expression profiles in
different cancer tissues. Certain studies have identified that
CDKN1A overexpression in cancer tissues is associated with tumor
aggressiveness and poor survival: Dai et al (29) suggested that CDKN1A expression in
breast cancer tissues was correlated with LNM and poor survival of
patients with breast cancer, and increased MDA-MB231 breast cancer
cell migration and invasion via transforming growth factor
β-mediated mothers against decapentaplegic homolog 3 acetylation.
In addition to the aforementioned studies (4,22–27), there have been other investigations
indicating that low expression of CDKN1A in various cancer tissues
is associated with poor prognosis (30,31). The
molecular mechanism by which different CDKN1A expression levels
affect prognoses in patients with different types of cancer remains
unclear and requires additional investigation. The present study
identified that low expression of CDKN1A in RGA tissues was
associated with poor prognosis in patients with gastric cancer.
As CDKN1A is a tumor suppressor, we hypothesize that
epigenetic changes, such as promoter methylation, may be one of the
mechanisms underlying the low CDKN1A expression in RGA tissues;
promoter methylation is capable of inhibiting gene transcript and
protein expression, as identified by Watanabe et al
(32), who suggested that abnormal
genomic methylation may be involved in the reduced expression of
CDKN1A in adult T-cell leukemia/lymphoma (32). A study conducted by Nie et al
(33) indicated that long noncoding
RNAs (lncRNA) may also serve a role in regulation of CDKN1A
transcription; the authors demonstrated that the lncRNA antisense
noncoding RNA in the INK4 locus was upregulated in non-small cell
lung cancer (NSCLC) tissues, and that this lncRNA promoted NSCLC
cell proliferation and inhibited NSCLC cell apoptosis through the
inhibition of Krüppel-like factor 2 and CDKN1A transcription
(33). These studies additionally
demonstrate that CDKN1A is a key regulator of the cell cycle, and
may be a target for cancer treatment.
In conclusion, the present study indicated, via
multiple statistical analyses, that low expression level of CDKN1A
in RGA tissues was significantly associated with LNM, a shorter
survival time and a high recurrence rate and risk of mortality.
CDKN1A demonstrated important prognostic significance, and may be
used as an independent prognostic factor for patients with RGA.
Acknowledgements
The present study was supported by grants from the
National NaturalScience Foundation of China (grant no. 81372788),
the Medical Scientific Research Key Foundation of Nanjing Command
(grant no. 11Z032), and the Natural Science Foundation of Fujian
Province Grant (grant no. 2014J01427), all awarded to Professor
Qiaojia Huang; the Medical Scientific Innovation Foundation of
Nanjing Command (grant no. 2013-MS122), awarded to Professor
Yinghao Yu; and the National Natural Science Foundation of China
(grant no. 81274002), awarded to Professor Xuenong Ouyang.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu C, Lu P, Lu Y, Xu H, Wang S and Chen
J: Clinical implications of metastatic lymph node ratio in gastric
cancer. BMC Cancer. 7:2002007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohta K, Hoshino H, Wang J, Ono S, Iida Y,
Hata K, Huang SK, Colquhoun S and Hoon DS: MicroRNA-93 activates
c-Met/PI3K/Akt pathway activity in hepatocellular carcinoma by
directly inhibiting PTEN and CDKN1A. Oncotarget. 6:3211–3324. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cazzalini O, Scovassi AI, Savio M, Stivala
LA and Prosperi E: Multiple roles of the cell cycle inhibitor p21
(CDKN1A) in the DNA damage response. Mutat Res. 704:12–20. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Prives C and Gottifredi V: The p21 and
PCNA partnership: A new twist for an old plot. Cell Cycle.
7:3840–3846. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Andries V, Vandepoele K, Staes K, Berx G,
Bogaert P, Van Isterdael G, Ginneberge D, Parthoens E,
Vandenbussche J, Gevaert K, et al: NBPF1, a tumor suppressor
candidate in neuroblastoma, exerts growth inhibitory effects by
inducing a G1 cell cycle arrest. BMC Cancer. 15:3912015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park C, Jeong NY, Kim GY, Han MH, Chung
IM, Kim WJ, Yoo YH and Choi YH: Momilactone B induces apoptosis and
G1 arrest of the cell cycle in human monocytic leukemia U937 cells
through downregulation of pRB phosphorylation and induction of the
cyclin-dependent kinase inhibitor p21Waf1/Cip1. Oncol Rep.
31:1653–1660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei CY, Tan QX, Zhu X, Qin QH, Zhu FB, Mo
QG and Yang WP: Expression of CDKN1A/p21 and TGFBR2 in breast
cancer and their prognostic significance. Int J Clin Exp Pathol.
8:14619–14629. 2015.PubMed/NCBI
|
|
10
|
Li S, Wang C, Yu X, Wu H, Hu J, Wang S and
Ye Z: miR-3619-5p inhibits prostate cancer cell growth by
activating CDKN1A expression. Oncol Rep. 37:241–248. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang H, Wu Y, Liu M, Qin Y, Wang H, Wang
L, Li S, Zhu H, He Z, Luo J, et al: SEMA3B improves the survival of
patients with esophageal squamous cell carcinoma by upregulating
p53 and p21. Oncol Rep. 36:900–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Lin Y, Lan F, Yu Y, Ouyang X, Wang
X, Huang Q, Wang L, Tan J and Zheng F: A GG allele of 3′-side AKT1
SNP is associated with decreased AKT1 activation and better
prognosis of gastric cancer. J Cancer Res Clin Oncol.
140:1399–1411. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoo CH, Noh SH, Kim YI and Min JS:
Comparison of prognostic significance of nodal staging between old
(4th edition) and new (5th edition) UICC TNM classification for
gastric carcinoma. International union against cancer. World J
Surg. 23:492–497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ward JH: NCCN Guidelines and the
International community. J Natl Compr Canc Netw. 9:133–134. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Lin Y, Lan F, Yu Y, Ouyang X, Liu
W, Xie F, Wang X and Huang Q: BAX and CDKN1A polymorphisms
correlated with clinical outcomes of gastric cancer patients
treated with postoperative chemotherapy. Med Oncol. 31:2492014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kawata N, Tsuchiya N, Horikawa Y, Inoue T,
Tsuruta H, Maita S, Satoh S, Mitobe Y, Narita S and Habuchi T: Two
surviving polymorphisms are cooperatively associated with bladder
cancer susceptibility. Int J Cancer. 129:1872–1880. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang Q, Huang Q, Chen W, Wang L, Lin W,
Lin J and Lin X: Identification of transgelin as a potential novel
biomarker for gastric adenocarcinoma based on proteomics
technology. J Cancer Res Clin Oncol. 134:1219–1227. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galeano F, Rossetti C, Tomaselli S,
Cifaldi L, Lezzerini M, Pezzullo M, Boldrini R, Massimi L, Di Rocco
CM, Locatelli F, et al: ADAR2-editing activity inhibits
glioblastoma growth through the modulation of the
CDC14B/Skp2/p21/p27 axis. Oncogene. 32:998–1009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morton JP, Jamieson NB, Karim SA, Athineos
D, Ridgway RA, Nixon C, McKay CJ, Carter R, Brunton VG, Frame MC,
et al: LKB1 haploinsufficiency cooperates with Kras to promote
pancreatic cancer through suppression of p21-dependent growth
arrest. Gastroenterology. 139:586–597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brown SG, Knowell AE, Hunt A, Patel D,
Bhosle S and Chaudhary J: Interferon inducible antiviral MxA is
inversely associated with prostate cancer and regulates cell cycle,
invasion and Docetaxel induced apoptosis. Prostate. 75:266–279.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Koh DI, Han D, Ryu H, Choi WI, Jeon BN,
Kim MK, Kim Y, Kim JY, Parry L, Clarke AR, et al: KAISO, a critical
regulator of p53-mediated transcription of CDKN1A and apoptotic
genes. Proc Natl Acad Sci USA. 111:pp. 15078–15083. 2014,
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Yang S, Sun N and Chen J:
Differential expression of STAT1 and p21 proteins predicts
pancreatic cancer progression and prognosis. Pancreas. 43:619–623.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Belt EJ, Brosens RP, Delis-van Diemen PM,
Bril H, Tijssen M, van Essen DF, Heymans MW, Beliën JA, Stockmann
HB, Meijer S, et al: Cell cycle proteins predict recurrence in
stage II and III colon cancer. Ann Surg Oncol. 19 Suppl
3:S682–S692. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mitomi H, Ohkura Y, Fukui N, Kanazawa H,
Kishimoto I, Nakamura T, Yokoyama K, Sada M, Kobayashi K, Tanabe S,
et al: P21WAF1/CIP1 expression in colorectal carcinomas is related
to Kras mutations and prognosis. Eur J Gastroenterol Hepatol.
19:883–889. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu DW, Liu WS, Wang J, Chen CY, Cheng YW
and Lee H: Reduced p21 (WAF1/CIP1) via alteration of p53-DDX3
pathway is associated with poor relapse-free survival in
early-stage human papillomavirus-associated lung cancer. Clin
Cancer Res. 17:1895–1905. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Young TW, Rosen DG, Mei FC, Li N, Liu J,
Wang XF and Cheng X: Up-regulation of tumor susceptibility gene 101
conveys poor prognosis through suppression of p21 expression in
ovarian cancer. Clin Cancer Res. 13:3848–3854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buchynska LG, Nesina IP, Yurchenko NP,
Bilyk OO, Grinkevych VN and Svintitsky VS: Expression of p53,
p21WAF1/CIP1, p16INK4A and Ki-67 proteins in serous ovarian tumors.
Exp Oncol. 29:49–53. 2007.PubMed/NCBI
|
|
28
|
Khalili M, Vasei M, Khalili D,
Alimoghaddam K, Sadeghizadeh M and Mowla SJ: Downregulation of the
genes involved in reprogramming (SOX2, c-MYC, miR-302, miR-145, and
P21) in gastric adenocarcinoma. J Gastrointest Cancer. 46:251–258.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dai M, Al-Odaini AA, Arakelian A, Rabbani
SA, Ali S and Lebrun JJ: A novel function for p21Cip1 and
acetyltransferase p/CAF as critical transcriptional regulators of
TGF β-mediated breast cancer cell migration and invasion. Breast
Cancer Res. 14:R1272012. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang H, Zhang X, Ji S, Hao C, Mu Y, Sun J
and Hao J: Sohlh2 inhibits ovarian cancer cell proliferation by
upregulation of p21 and downregulation of cyclin D1.
Carcinogenesis. 35:1863–1871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Hu Y, Hu W, Xie X, Bella Ela A, Fu
J and Rao D: Expression and prognostic relevance of p21WAF1 in
stage III esophageal squamous cell carcinoma. Dis Esophagus.
25:67–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watanabe M, Nakahata S, Hamasaki M, Saito
Y, Kawano Y, Hidaka T, Yamashita K, Umeki K, Taki T, Taniwaki M, et
al: Downregulation of CDKN1A in adult T-cell leukemia/lymphoma
despite overexpression of CDKN1A in human T-lymphotropic virus
1-infected cell lines. J Virol. 84:6966–6977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nie FQ, Sun M, Yang JS, Xie M, Xu TP, Xia
R, Liu YW, Liu XH, Zhang EB, Lu KH, et al: Long noncoding RNA ANRIL
promotes non-small cell lung cancer cell proliferation and inhibits
apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther.
14:268–277. 2015. View Article : Google Scholar : PubMed/NCBI
|