Introduction
Angiogenesis is a complex process that includes
adhesion, migration, invasion, proliferation and differentiation in
endothelial cells (1).
Neovascularization is necessary for tumor growth by providing
oxygen anxd nutrients (2). The tumor
and the surrounding microenvironment, including cancer cells,
endothelial cells, fibroblasts and immune cells, are associated and
interact constantly (3,4). Tumors may influence the microenvironment
by releasing extracellular signals, including vascular endothelial
growth factor, tumor necrosis factor α, fibroblast growth factor 2
and interleukin 6 into the extracellular matrix (ECM), and
promoting tumor-associated angiogenesis (5,6).
Collagen triple helix repeat containing 1 (Cthrc1)
was identified to encode a secreted protein that serves a role in
the cellular response to arterial injury through vascular
remodeling (7). It was demonstrated
that Cthrc1 is related to vascular remodeling by inhibiting
collagen production and fibrogenesis, and by promoting cell
migration (8). The majority of Cthrc1
studies have focused on its effects and underlying molecular
mechanism, in promoting tumor cell invasion and metastasis
(9–14). In the present study, it was
demonstrated that increased expression of Cthrc1 protein in
gastrointestinal stromal tumor (GIST) is associated with increased
microvascular density (MVD) in a tissue microarray; however, to the
best of our knowledge, no previous study has demonstrated the
effect of Cthrc1 in endothelial angiogenesis.
Materials and methods
Immunohistochemical (IHC) staining and
evaluation
Two continuous tissue microarrays (no.
HDgS-GIST060CS-01) including surgical tissues from 60 GISTs were
purchased from Shanghai Outdo Biotech Co., Ltd. (Shanghai, China).
The 60 patients included 33 females and 27 males, with a median age
of 53.4±13.70 years. The location of the samples included 5 cases
in the stomach, 21 cases in the small intestine, 7 cases in the
colon, 3 cases in the peritoneum and 2 cases in the mesentery.
Sections were dewaxed in dimethylbenzene, hydrated in ethanol,
incubated with 3% oxydol to inactivate endogenous peroxidase and
incubated with a citrate solution for 30 min at 95°C for antigen
retrieval. Sections were blocked with goat serum for 30 min and
incubated with Anti-Cthrc1 (dilution, 1:200; cat. no. AP8778a;
Abgent, San Diego, CA, USA) or anti-CD31 (dilution, 1:200;
11265-1-AP; ProteinTech, Chicago, IL, USA) at 4°C overnight.
Sections were then incubated with a horseradish peroxidase
(HRP)-labeled goat anti-mouse/rabbit antibody (dilution, ready to
use: cat. no. D-3004; Shanghai Long Island Biotec. Co., Ltd.,
Shanghai, China) at 30°C for 30 min and DAB for 30 sec (Fuzhou
Maixin Biotechnology Development Co., Ltd., Fuzhou, China) at room
temperature, followed by hematoxylin staining and mounting. Images
were captured using a microscope (CX31-LV320; Olympus Corporation,
Tokyo, Japan). CD31 staining results were evaluated by the amount
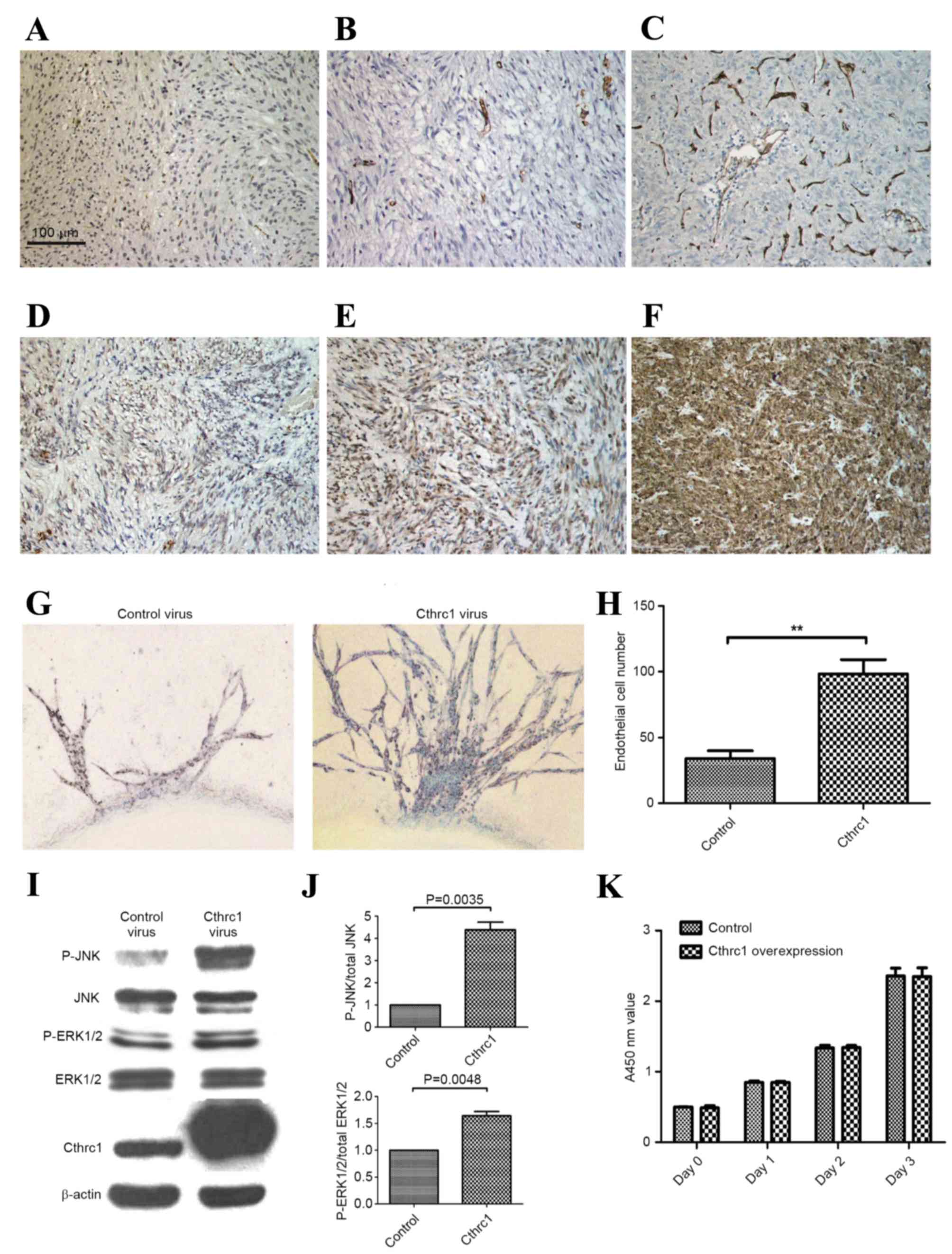
of vessels per ×200 fields (Fig.
1A-C). Cthrc1 staining results were evaluated as follows: -, no
tumor cells stained; +, <25% tumor cells stained (Fig. 1D); ++, 25–50% tumor cells stained
(Fig. 1E); +++, >50% tumor cells
stained (Fig. 1F).
Cell culture
Human umbilical vein endothelial cells (HUVECs) were
purchased from China Center for Type Culture Collection (Beijing,
China). HUVECs were cultured in Dulbecco's modified Eagle's medium
(DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; ScienCell Research
Laboratories, Inc., Carlsbad, CA, USA) at 37°C in a humidified
atmosphere of 5% CO2 for 72 h prior to use. U0126
(Selleck Chemicals, Houston, TX, USA) and SP600125 (Selleck
Chemicals) were used to inhibit extracellular-signal-regulated
kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK)
phosphorylation, respectively. U0126 and SP600125 were dissolved in
Dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany), so DMSO was used as a phosphorylation control.
The final concentration of U0126 and SP600125 was 10 µM in the
culture medium for the experiments.
Cthrc1-expressing adenoviral
vector
An adenoviral vector expressing the human Cthrc1
coding sequence under the control of a mouse cytomegalovirus
promoter was constructed as described previously (15). A control adenoviral vector expressing
green fluorescent protein was prepared in parallel (15).
Small interfering RNA (siRNA)
experiments
Cthrc1 was knocked down using siRNA (siCTHRC1) and
the synthetic duplex oligomers 5′-CCCAUUGAAGCUAUAAUUU-3′ and
5′-AAAUUAUAGCUUCAAUGGG-3′, purchased from Shanghai GenePharma Co.,
Ltd. (Shanghai, China). HUVECs were transiently transfected with
siRNAs using GenMute™ reagent (SignaGen Laboratories, Rockville,
MD, USA), according to the manufacturer's protocol.
Aortic ring assay
A total of 6 male Sprague-Dawley rats (8-weeks old)
weighing 320–345 g were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. The mice were sacrificed on the day of purchase.
Chloral hydrate (10%) was injected in to the abdominal cavity and
the mice were sacrificed via the cervical dislocation method under
the influence of the anesthetic. All animal experiments were
approved by the Institutional Animal Care and Use Committee of
Renji Hospital, School of Medicine, Shanghai Jiao Tong University
(Shanghai, China). Aortas were harvested and cut into aortic rings
of ~1 mm thickness. A total of ½ were immersed in DMEM with
Cthrc1-expressing adenovirus (2×107 pfu/ml), and the
other ½ were immersed in DMEM with the control adenovirus
(2×107 pfu/ml) at 37°C. Following a total of 4 h, the
rings were placed in 96-well culture plates and coated with liquid
Matrigel™ (BD Biosciences, Franklin Lakes, NJ, USA). Following
solidification of the Matrigel, 200 µl Opti-MEM (Thermo Fisher
Scientific, Inc.) with 2% FBS was added. The aortic rings were
incubated at 37°C, and medium was changed every two days. Images of
the aortic rings (3 fields per ring) were captured (original
magnification, ×200) on the eighth day with a microscope (Eclipse
Ti-U; Nikon Corporation, Tokyo, Japan), the images were captured
directly from the 96-well culture plates. Photoshop (version CS6;
Adobe, San Jose, CA, USA) was used to sharpen the images and count
the number of sprouting endothelia from the aortic ring for
statistical analysis.
Cell proliferation assay
HUVECs were seeded in 96-well plates at a density of
2,000 cells/well and were incubated at 37°C overnight. Culture
medium containing Cthrc1-expressing adenovirus or control
adenovirus (1×107 pfu/ml) was added into various wells,
and the culture medium was changed after 2 h. The optical density
value of each group (six wells/group) was determined using the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) on days 0, 1, 2 and 3, following the
manufacturer's protocol.
Western blotting
Proteins were extracted from tissue samples using
total protein extraction kits (cat. no. C510003; Sangon Biotech
Co., Ltd., Shanghai, China). The protein concentration was
determined using a BCA Protein Assay kit (cat. no. 23252; Thermo
Fisher Scientific, Inc.). Proteins (40 µg) were loaded and
electrophoresed on a 10% SDS-PAGE gel. Proteins were transferred to
a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA),
which was blocked using 5% milk for 2 h at room temperature, and
then incubated with the primary antibodies at 4°C overnight. An
HRP-labeled secondary antibody (dilution, 1:5,000; cat. no. 7074;
Cell Signaling Technology, Inc.) was added and incubated at room
temperature for 1 h. The immunoreactive signals were detected using
Super Signal West Femto Maximum Sensitivity Substrate (Thermo
Fisher Scientific, Inc.). The primary antibodies were anti-Cthrc1
(dilution, 1:1,000; cat. no. AP8778a; Abgent), anti-ERK1/2
(dilution, 1:1,000; cat. no. 9102; Cell Signaling Technology,
Inc.), anti-phospho (P)-ERK1/2 (dilution, 1:1,000; cat. no. 4370;
Cell Signaling Technology, Inc.), anti-JNK (dilution, 1:1,000; cat.
no. 9252; Cell Signaling Technology, Inc.), anti-P-JNK (dilution,
1:1,000; cat. no. 4668; Cell Signaling Technology, Inc.) and
anti-β-actin (dilution, 1:4,000; cat. no. 8457; Cell Signaling
Technology, Inc.). Western blots were analyzed densitometrically
using ImageJ software version 1.45S (National Institutes of Health,
Bethesda, MD, USA).
Tubule formation assay
Matrigel was pipetted into 48-well plates and
allowed to polymerize for 30 min at 37°C. HUVECs (6×104)
were seeded on Matrigel in 200 µl culture medium. The plates were
incubated at 37°C for 6–8 h, prior to the capture of images using
an Eclipse Ti-U microscope (magnification, ×100). ImageJ was used
to measure the tube length in µm. The sum of tube length was
determined for statistical analysis.
Scratch wound assay
HUVECs were cultured to full confluence in 6-well
culture plates. The cell layer was scratched using a sterile
pipette tip and the scratched area was imaged with an Eclipse Ti-U
microscope (original magnification, ×200). Following incubation at
37°C for 24 h, the scratched area was imaged again. ImageJ was used
to measure the scratched area in µm2. The healing area
was evaluated for statistical analysis.
Cell invasion assay
A Transwell chamber (24-well insert; pore size 8 µm;
EMD Millipore) was coated with Matrigel for 6 h prior to the
invasion assay. A total of 2×105 HUVECs were plated in
the Transwell chamber with 200 µl FBS-free DMEM. DMEM (600 µl)
containing 20% FBS was added to the lower chambers of the 24-well
plates. After 36 h of incubation at 37°C, the cells that had
invaded through the pores were fixed using 4% paraformaldehyde (25
min) and stained using 0.5% crystal violet (30 min) at room
temperature, and the number of cells was counted under a ×200
Eclipse Ti-U light microscope.
Statistical analysis
Each individual experiment was conducted with three
or five replicates. All data are presented as the mean ± standard
error, and were analyzed using the Student's t-test or Mann-Whitney
U test with SPSS (version 18.0; SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
MVD is associated with the tumor site
and Cthrc1 protein expression level
Quantification of the IHC staining demonstrated that
there was a significant decrease in MVD in tumors located in the
stomach compared with non-stomach-located tumors (8.19±6.97 vs.
16.48±17.04, respectively; P=0.003; Table
I). GIST with increased expression of Cthrc1 protein exhibited
increased MVD compared with GIST with decreased expression of
Cthrc1 (14.44±15.35 vs. 7.67±7.05, respectively; P=0.021; Table I). Statistical analysis indicated that
MVD was not significantly associated with sex, mitotic index, tumor
size or National Institutes of Health risk grade (Table I).
 | Table I.Characteristics of patients with
gastrointestinal stromal tumors. |
Table I.
Characteristics of patients with
gastrointestinal stromal tumors.
| Characteristic | MVD (number of
vessels/field) | P-value |
|---|
| Sex |
| 0.899 |
| Male
(n=33) | 12.45±13.70 |
|
| Female
(n=27) | 13.11±14.62 |
|
| Mitosis per 50
HPFs |
| 0.947 |
| <5
(n=32) | 12.91±13.68 |
|
| ≥5
(n=28) | 12.57±14.62 |
|
| Tumor size, cm |
| 0.169 |
| <10
(n=44) | 11.57±12.13 |
|
| ≥10
(n=16) | 16.00±18.28 |
|
| NIH risk grade |
| 0.111 |
| Low and
intermediate (n=41) | 11.73±12.50 |
|
| High
(n=19) | 14.95±16.99 |
|
| Tumor site |
| 0.003a |
| Stomach
(n=27) | 8.19±6.97 |
|
|
Non-stomach (n=33) | 16.48±17.04 |
|
| Cthrc1 protein
expression |
| 0.021a |
| −, +
(n=15) | 7.67±7.05 |
|
| ++, +++
(n=45) | 14.44±15.35 |
|
Effect of Cthrc1 expression on aortic
ring sprouting
To investigate the effect of Cthrc1 on angiogenesis,
an aortic ring assay was performed. The aortic ring assay
demonstrated that Cthrc1-overexpressing aortic rings developed a
significantly increased number of sprouting endothelial cells
compared with the control (P<0.001), indicating that Cthrc1
serves an important role in angiogenesis (Fig. 1G and H).
Effect of Cthrc1 overexpression on
HUVEC proliferation, tubule formation, migration and invasion
Densitometric analysis of western blots (Fig. 1I) demonstrated significantly increased
ratios of P-JNK/total JNK (P=0.004) and P-ERK1/2/total ERK1/2
(P=0.005) in HUVECs transfected with Cthrc1-expressing adenovirus
compared with HUVECs transfected with control adenovirus (Fig. 1J). CCK-8 results demonstrated that
HUVEC proliferation was not significantly affected by
Cthrc1-expressing adenovirus (Fig.
1K), indicating that Cthrc1 promotes aortic ring sprouting
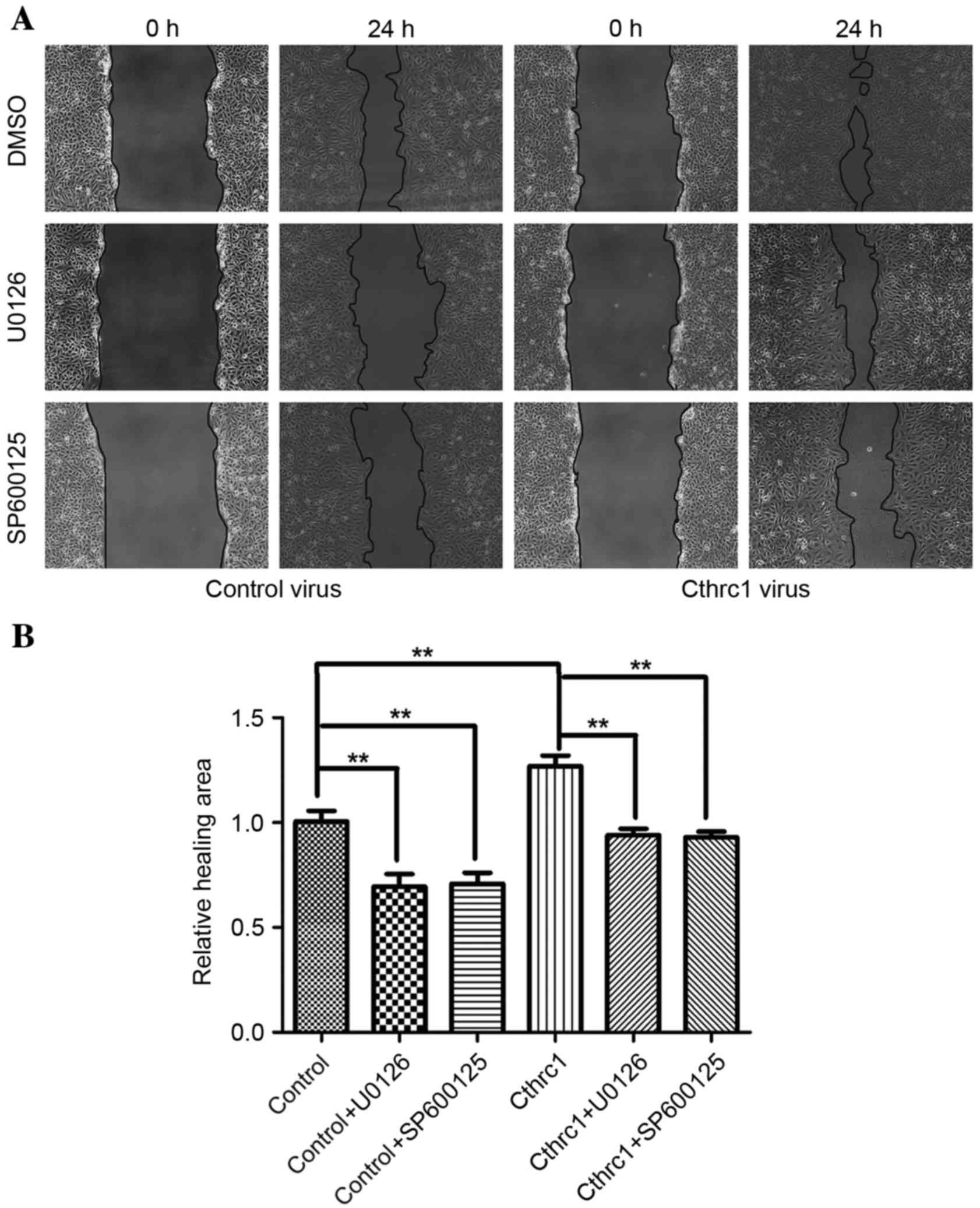
without promoting HUVEC proliferation. The scratch wound assay and
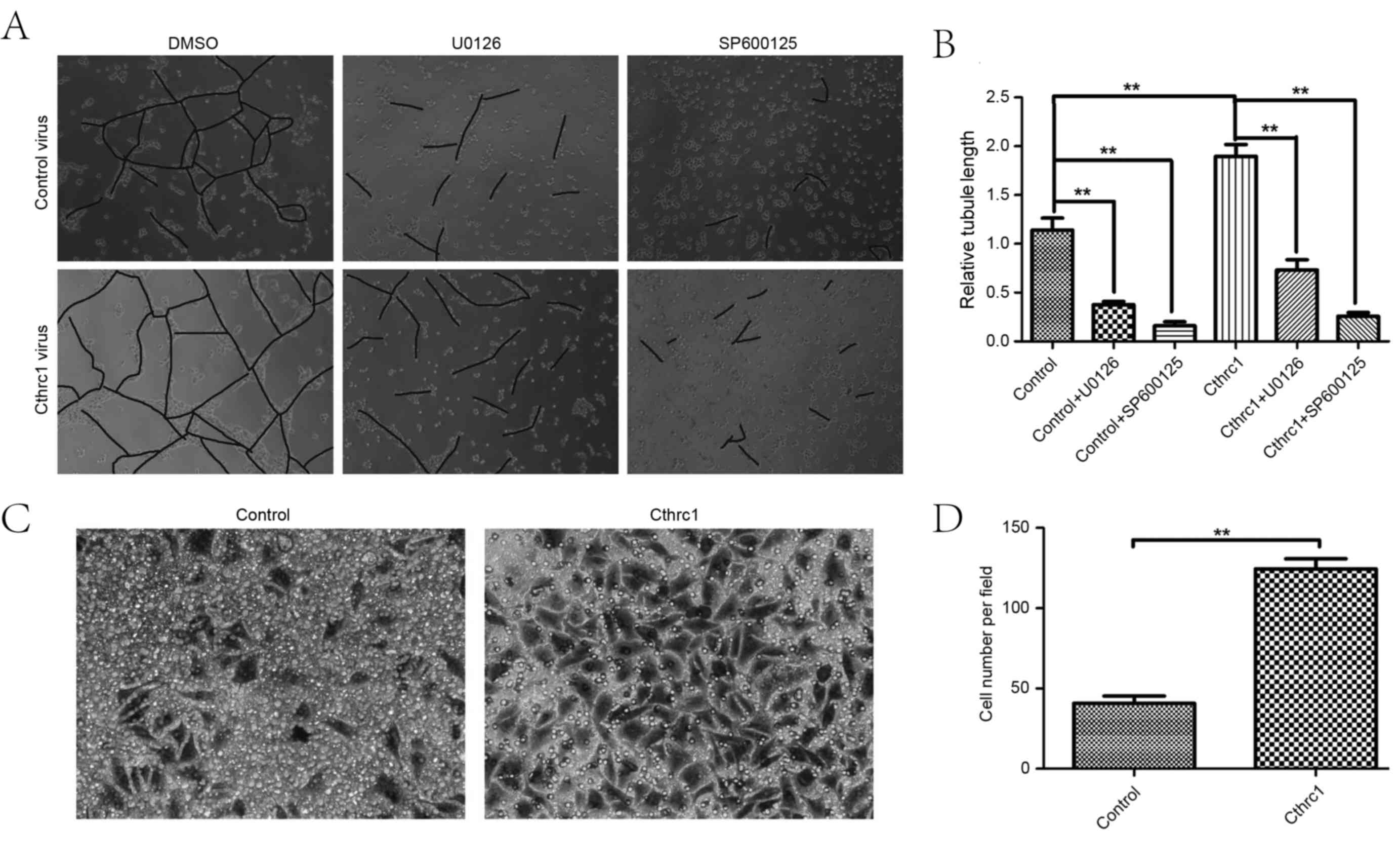
the tubule formation assay revealed that Cthrc1 protein
overexpression was able to increase the migratory function of
HUVECs (Fig. 2A and B), and increase
HUVEC tubule formation (Fig. 3A and
B). The cell invasion assay demonstrated that Cthrc1 protein
overexpression was able to increase HUVEC invasion (Fig. 3C and D). Decreased HUVEC migration and
tubule formation were observed following treatment with the kinase
inhibitors U0126 and SP600125 (Figs. 2A
and B, 3A and B), suggesting that
ERK1/2 and JNK phosphorylation may be the underlying molecular
mechanism of promotion of angiogenesis by Cthrc1.
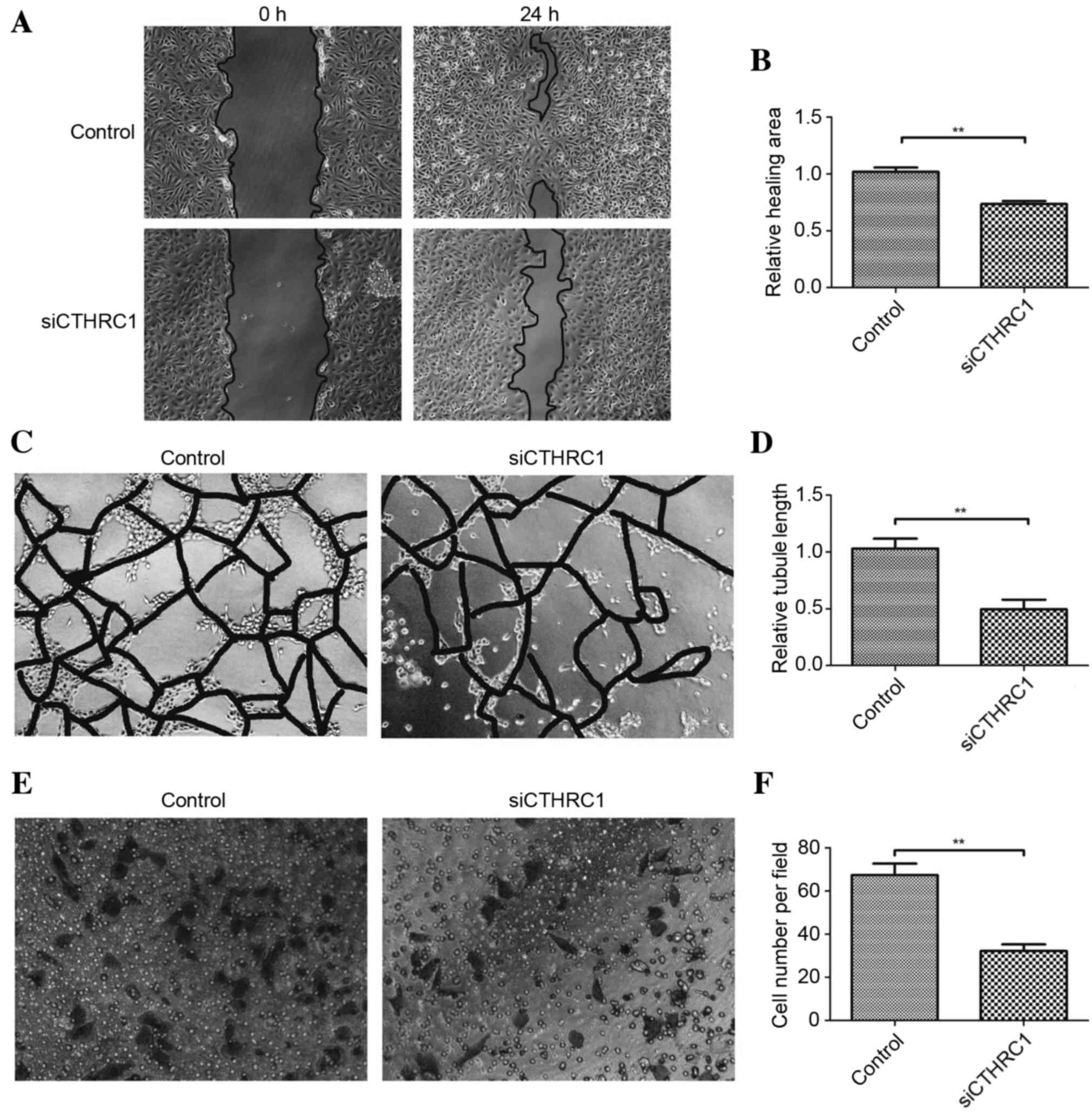
Effect of Cthrc1 knockdown on HUVEC
tubule formation, migration and invasion
Following Cthrc1 knockdown using siRNA in HUVECs,
the ability of HUVECs to form tubules, migrate and invade was
significantly decreased (Fig. 4),
indicating that inhibition of Cthrc1 protein expression may be a
promising method of inhibiting GIST angiogenesis.
Discussion
Previous studies have demonstrated that patients
with increased expression of Cthrc1 protein have a poorer prognosis
compared with those with decreased expression, a phenomenon that
has been observed in numerous types of tumor (9–12,16). Tumor MVD is negatively associated with
tumor prognosis. The present study demonstrated that Cthrc1 protein
expression is associated with GIST MVD. The aortic ring assay
demonstrated that Cthrc1 was able to promote aortic ring sprouting.
The wound healing assay, cell invasion assay and tubule formation
assay demonstrated that Cthrc1 was able to promote HUVEC migration,
invasion and tubule formation. The results of the present study
indicated that Cthrc1 serves an important role in GIST angiogenesis
and may explain why patients with increased expression of Cthrc1
protein have a poorer prognosis.
Cthrc1 has been demonstrated to promote the
phosphorylation of ERK and JNK in colon cancer (12) and GIST (9), respectively. Previous studies have
revealed that the increase in phosphorylation of ERK and JNK may
promote HUVEC migration and tubule formation (17–19). In
the present study, it was demonstrated that Cthrc1 was able to
promote ERK and JNK phosphorylation in HUVECs, and inhibition of
ERK and JNK phosphorylation may decrease HUVEC migration and tubule
formation. These results indicate that the pro-angiogenic effect of
Cthrc1 is associated with the phosphorylation of ERK and JNK.
The planar cell polarity (PCP) signaling pathway is
a highly conserved signaling cascade that coordinates epithelial
and axonal morphogenic movements during organ development by
regulating angiogenesis (20).
Inhibition of the PCP signaling pathway disrupts endothelial cell
growth, polarity and migration (20–22).
Cthrc1 is a Wnt co-factor protein that selectively activates the
Wnt/PCP signaling pathway by stabilizing ligand-receptor
interactions (9,23,24).
Cthrc1 may activate the PCP signaling pathway; however, it was
demonstrated to suppress the canonical Wnt signaling pathway in
human embryonic kidney-293T and GIST cells (9,23).
Therefore, Cthrc1 may activate the PCP signaling pathway in HUVECs
and promote tumor angiogenesis.
Collagen matrix deposition in ECM is an important
process in the inhibition of tumor invasion and angiogenesis
(25,26). Cthrc1 may reduce collagen type I mRNA
and protein levels in fibroblasts, resulting in decreased collagen
synthesis and contributing to vascular remodeling (27). Matrix metalloproteinase 9 (MMP9) is an
important factor in collagen degradation (28). Cthrc1 has been demonstrated to promote
MMP9 secretion in colon cancer (12).
Therefore, inhibiting Cthrc1 may increase collagen matrix
deposition and decrease tumor invasion and angiogenesis.
Although the present study has revealed certain
underlying molecular mechanisms regarding the pro-angiogenic effect
of Cthrc1, further studies in vivo and in vitro are
required to validate these experimental findings.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81270474). The
authors of the present study thank Professor Xiong Ma (Shanghai
Jiao-Tong University, Shanghai, China) for providing the
Cthrc1-expressing adenovirus and the control adenovirus.
References
|
1
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Eng J Med. 285:1182–1186. 1971. View Article : Google Scholar
|
|
3
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pyagay P, Heroult M, Wang Q, Lehnert W,
Belden J, Liaw L, Friesel RE and Lindner V: Collagen triple helix
repeat containing 1, a novel secreted protein in injured and
diseased arteries, inhibits collagen expression and promotes cell
migration. Circ Res. 96:261–268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Durmus T, LeClair RJ, Park KS, Terzic A,
Yoon JK and Lindner V: Expression analysis of the novel gene
collagen triple helix repeat containing-1 (Cthrc1). Gene Expr
Patterns. 6:935–940. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma MZ, Zhuang C, Yang XM, Zhang ZZ, Ma H,
Zhang WM, You H, Qin W, Gu J, Yang S, et al: CTHRC1 acts as a
prognostic factor and promotes invasiveness of gastrointestinal
stromal tumors by activating Wnt/PCP-Rho signaling. Neoplasia.
16(265–278): e1–13. 2014.
|
|
10
|
Hou M, Cheng Z, Shen H, He S, Li Y, Pan Y,
Feng C, Chen X, Zhang Y, Lin M, et al: High expression of CTHRC1
promotes EMT of epithelial ovarian cancer (EOC) and is associated
with poor prognosis. Oncotarget. 6:35813–35829. 2015.PubMed/NCBI
|
|
11
|
Ke Z, He W, Lai Y, Guo X, Chen S, Li S,
Wang Y and Wang L: Overexpression of collagen triple helix repeat
containing 1 (CTHRC1) is associated with tumour aggressiveness and
poor prognosis in human non-small cell lung cancer. Oncotarget.
5:9410–9424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HC, Kim YS, Oh HW, Kim K, Oh SS, Kim
JT, Kim BY, Lee SJ, Choe YK, Kim DH, et al: Collagen triple helix
repeat containing 1 (CTHRC1) acts via ERK-dependent induction of
MMP9 to promote invasion of colorectal cancer cells. Oncotarget.
5:519–529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang P, Wang YC, Chen XY, Shen ZY, Cao H,
Zhang YJ, Yu J, Zhu JD, Lu YY and Fang JY: CTHRC1 is upregulated by
promoter demethylation and transforming growth factor-β1 and may be
associated with metastasis in human gastric cancer. Cancer Sci.
103:1327–1333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu X, Liu B, Cui Y, Wang F, Sun H and Lv
F: Collagen triple helix repeat containing 1 (Cthrc1) is an
independently prognostic biomarker of non-small cell lung cancers
with cigarette smoke. Tumour Biol. 35:11677–11683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bian Z, Miao Q, Zhong W, Zhang H, Wang Q,
Peng Y, Chen X, Guo C, Shen L, Yang F, et al: Treatment of
cholestatic fibrosis by altering gene expression of Cthrc1:
Implications for autoimmune and non-autoimmune liver disease. J
Autoimmun. 63:76–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gu L, Liu L, Zhong L, Bai Y, Sui H, Wei X,
Zhang W, Huang P, Gao D, Kong Y and Lou G: Cthrc1 overexpression is
an independent prognostic marker in gastric cancer. Hum Pathol.
45:1031–1038. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu F, Song H, Zhang Y, Zhang Y, Mu Q,
Jiang M, Wang F, Zhang W, Li L, Li H, et al: Irisin induces
angiogenesis in human umbilical vein endothelial cells in vitro and
in Zebrafish Embryos in vivo via activation of the ERK signaling
pathway. PLoS One. 10:e01346622015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee OH, Kim YM, Lee YM, Moon EJ, Lee DJ,
Kim JH, Kim KW and Kwon YG: Sphingosine 1-phosphate induces
angiogenesis: Its angiogenic action and signaling mechanism in
human umbilical vein endothelial cells. Biochem Biophys Res Commun.
264:743–750. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin YJ, Park I, Hong IK, Byun HJ, Choi J,
Kim YM and Lee H: Fibronectin and vitronectin induce AP-1-mediated
matrix metalloproteinase-9 expression through integrin
α(5)β(1)/α(v)β(3)-dependent Akt, ERK and JNK signaling pathways in
human umbilical vein endothelial cells. Cell Signal. 23:125–134.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cirone P, Lin S, Griesbach HL, Zhang Y,
Slusarski DC and Crews CM: A role for planar cell polarity
signaling in angiogenesis. Angiogenesis. 11:347–360. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ju R, Cirone P, Lin S, Griesbach H,
Slusarski DC and Crews CM: Activation of the planar cell polarity
formin DAAM1 leads to inhibition of endothelial cell proliferation,
migration, and angiogenesis. Proc Natl Acad Sci USA. 107:pp.
6906–6911. 2010, View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Descamps B, Sewduth R, Tojais Ferreira N,
Jaspard B, Reynaud A, Sohet F, Lacolley P, Allières C, Lamazière
JM, Moreau C, et al: Frizzled 4 regulates arterial network
organization through noncanonical Wnt/planar cell polarity
signaling. Circ Res. 110:47–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamamoto S, Nishimura O, Misaki K, Nishita
M, Minami Y, Yonemura S, Tarui H and Sasaki H: Cthrc1 selectively
activates the planar cell polarity pathway of Wnt signaling by
stabilizing the Wnt-receptor complex. Dev Cell. 15:23–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kelley MW: Leading Wnt down a PCP path:
Cthrc1 acts as a coreceptor in the Wnt-PCP pathway. Dev Cell.
15:7–8. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang C and Werb Z: The many faces of
metalloproteases: Cell growth, invasion, angiogenesis and
metastasis. Trends Cell Biol. 11:S37–S43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cox G and O'Byrne KJ: Matrix
metalloproteinases and cancer. Anticancer Res. 21:4207–4219.
2001.PubMed/NCBI
|
|
27
|
LeClair R and Lindner V: The role of
collagen triple helix repeat containing 1 in injured arteries,
collagen expression, and transforming growth factor beta signaling.
Trends Cardiovasc Med. 17:202–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Burg-Roderfeld M, Roderfeld M, Wagner S,
Henkel C, Grötzinger J and Roeb E: MMP-9-hemopexin domain hampers
adhesion and migration of colorectal cancer cells. Int J Oncol.
30:985–992. 2007.PubMed/NCBI
|