Introduction
Thyroid cancer, which primarily arises from thyroid
follicular epithelial cells, is the most common and deadly
endocrine malignancy. Well-differentiated thyroid carcinoma (DTC),
including papillary thyroid cancer (PTC) and follicular thyroid
cancer (FTC), comprise 80% of all cases of thyroid cancer and
frequently have a favorable prognosis; however, 20% of these
patients develop distant metastases or recurrences and eventually
succumb to the disease (1,2). Therefore, further studies are required
to identify novel prognostic factors for DTC. Once thyroid cancer
metastasizes to distant sites and is resistant to radioactive
iodine therapy, the expected survival time declines rapidly
(3); however, the underlying
molecular mechanisms of thyroid cancer metastasis remain
unclear.
Through activation by thyroid-stimulating hormone
(TSH), the TSH receptor (TSHR) serves a fundamental role in the
regulation of thyroid cell proliferation, differentiation and
function, in addition to the development of the thyroid gland
(4). It has been demonstrated that
TSHR signaling was required for thyroid carcinogenesis in a mouse
model (5). Additionally, TSH-TSHR
signaling has a dichotomous role in the development of thyroid
cancer; it can also suppress malignant transformation of thyroid
cells and therefore suppress the occurrence of thyroid cancer
(5,6).
However, the majority of these previous studies investigated the
underlying molecular mechanisms of thyroid cancer growth, and the
role of TSHR in the prognosis, migration, invasion and metastasis
of DTC remains unclear. Epithelial-mesenchymal transition (EMT), a
phenotypic plasticity conversion, which is essential in organ
morphogenesis, development and tissue remodeling, is typically
detected in neoplasias and during cancer progression (7,8).
Additionally, EMT is associated with tumor metastasis, and it is
thought that EMT endows invasive and stem cell-like features that
allow tumor cells to disseminate (9,10).
In the present study, the prognostic value of TSHR
for patients with thyroid cancer, and its role in the migration and
invasion of thyroid cancer were investigated. Low expression of
TSHR was identified to be associated with a high distant metastasis
rate and poor prognosis in patients with DTC, and TSHR inhibited
thyroid cancer cell migration and invasion.
Materials and methods
Cell culture
The primary normal human thyroid follicular
epithelial cell lines N1 and N2, which were isolated from primary
thyroid tissues of surgical resection of 2 healthy individuals
between August 2008 and April 2014. Written informed consent was
provided by all patients. The well-differentiated PTC cell lines
B-CPAP, PTC-1113A and PTC-uc3, and the poorly-differentiated
thyroid carcinoma cell lines FRO and WRO were cultured in RPMI-1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 0.1% mM non-essential amino acids, 1 mM sodium
pyruvate and 1% penicillin-streptomycin in a humidified incubator
with 5% CO2 at 37°C. The PTC cells were obtained from
the American Type Culture Collection (Manassas, VA, USA).
Patient information and tissue
specimens
A total of 172 formalin-fixed paraffin-embedded
thyroid cancer samples were collected at Sun Yat-Sen University
Cancer Center (Guangzhou, China) between January 1990 and December
2003. The follow-up time ranged between 120 and 183 months. The
clinical classification was performed according to The Chinese 1992
staging system (11).
All the cases included in the present study were
histopathologically and clinically diagnosed, and then verified.
Written informed consent was obtained from all patients and the
present study was approved by the Ethics Committee of Sun Yat-Sen
University Cancer Center and sample collection was performed in
accordance with the policies of the National Research Ethics
Committee of China. Clinical information is summarized in Table I.
 | Table I.Association between TSHR expression
and the clinicopathological features of 172 patients with DTC. |
Table I.
Association between TSHR expression
and the clinicopathological features of 172 patients with DTC.
|
| TSHR expression, no.
of patients |
|
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Negative | Positive | χ2 | P-value |
|---|
| Tissue |
|
|
|
|
| Normal
thyroid tissue | 15 | 6 |
|
|
| DTC
tissue | 79 | 93 | 6.856 | 0.009 |
| Gender |
|
|
|
|
| Male | 32 | 31 |
|
|
|
Female | 47 | 62 | 0.947 | 0.331 |
| Age, years |
|
|
|
|
|
<45 | 55 | 56 |
|
|
| ≥45 | 24 | 37 | 1.651 | 0.199 |
| Pathological
type |
|
|
|
|
|
Papillary | 68 | 83 |
|
|
|
Follicular | 11 | 10 | 0.401 | 0.527 |
| Pathological
stage |
|
|
|
|
|
I+II | 58 | 70 |
|
|
|
III+IV | 21 | 23 | 0.077 | 0.782 |
| T stage |
|
|
|
|
|
T1+T2 | 54 | 74 |
|
|
|
T3+T4 | 25 | 19 | 4.423 | 0.219 |
| N stage |
|
|
|
|
| N0 | 26 | 38 |
|
|
| N+ | 53 | 55 | 1.155 | 0.282 |
| Distant
metastasis |
|
|
|
|
| M0 | 68 | 89 |
|
|
| M1 | 11 | 4 | 4.969 | 0.026 |
| Recurrent laryngeal
nerve invasion |
|
|
|
|
|
Yes | 5 | 8 |
|
|
| No | 74 | 85 | 0.316 | 0.574 |
| Extracapsular
invasion |
|
|
|
|
|
Yes | 13 | 19 |
|
|
| No | 66 | 74 | 0.446 | 0.504 |
| Trachea
invasion |
|
|
|
|
|
Yes | 18 | 15 |
|
|
| No | 61 | 78 | 1.22 | 0.269 |
3D morphogenesis assay
Plates (24-well) were coated with Growth Factor
Reduced Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) and
covered with the RPMI plus 10% FBS growth medium supplemented with
2% Matrigel. Cells were trypsinized and then seeded at a density of
1×104 cells/well, cultured for 3–4 days. Images were
captured with a phase-contrast microscope at 2-day intervals for
2–3 weeks.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent (Thermo
Fisher Scientific, Inc.), and cDNA was produced using PrimeScript
RT reagent kit with gDNA Eraser from 1 µg cDNA (Takara Bio, Inc.,
Otsu, Japan). RT-qPCR was performed using SYBR-Green PCR Master Mix
(Takara Bio, Inc.) on a 7500 Fast Real time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). For the PCR reaction,
the thermocycler condition was as follows: 50°C for 2 min, and then
95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C
for 1 min. β-actin was used as an endogenous control for gene
normalization. Expression data was calculated as 2−ΔΔCq
(12).
Western blotting
Cells were lyzed with radioimmunoprecipitation
buffer (Beyotime Institute of Biotechnology, Haimen, China)
(2) and the protein concentration was
determined using a BCA Protein Assay kit (Pierce; Thermo Fisher
Scientific, Inc.) (2). Western
blotting was performed using standard methods as described
previously (2). Briefly, 40 ug
protein were applied to 12% polyacrylamide SDS gels, separated by
SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF)
membranes (GE Healthcare Life Sciences, Chalfont, UK). The
membranes were blocked with 5% no-fat milk at room temperature for
1 h, and incubated with anti-TSHR (dilution, 1:1,000; cat. no.
ab27974; Abcam, Cambridge, UK), anti-Vimentin (dilution, 1:1,000;
cat. no. 5741; Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-N-Cadherin (dilution, 1:1,000; cat. no. 13116; Cell Signaling
Technology, Inc.) or anti-β-actin (dilution, 1:5,000; cat. no.
A5441; Sigma-Aldrich) primary antibodies at 4°C overnight.
Subsequently, the secondary antibodies for Anti-mouse IgG
HRP-linked Antibody (dilution, 1:5,000; cat. no. 7056; Cell
Signaling Technology, Inc.) and Anti-rabbit IgG, HRP-linked
Antibody (dilution, 1:5,000; cat. no. 7074; Cell Signaling
Technology, Inc.) were used to incubated with the membranes at room
temperature for 2 h. Then an enhanced chemiluminescence Amersham
ECL Primer kit (GE Healthcare Life Sciences) was used to develop
the blots according to the manufacturer's protocol. β-actin was
used as the loading control.
Wound healing and invasion assays
A total of 1×105 cells were seeded and
cultured to confluence in 6-well plate, streaks were created in the
monolayer with a 200 µl pipette tip, migration progression was
observed and photographed at indicated time points. For invasion
assays, 1×105 cells were placed on Matrigel-coated
24-well Boyden chambers (Corning Incorporated, NY, USA). Following
24 h, the non-invading cells were gently removed with a soft cotton
swab. The cells that invaded to the bottom chamber were fixed with
methanol and glacial acetic acid (3:1) for 30 min, and stained with
0.1% crystal violet. Images for the scratch wound and invasion
assays were captured using a phase-contrast microscope.
RNA interference
A short interfering (si)RNA directed against TSHR
(cat. no. stB0005212A-1-5) was synthesized by Guangzhou RiboBio
Co., Ltd. (Guangzhou, China) and the control siRNA (cat. no.
siN0581522147-1-5) was purchased from RiboBio. The siRNA
transfection was performed in 6-well plates using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol.
Immunofluorescence
Cells were seeded onto coverslips 1 day prior to
fixation. For immunofluorescence analysis, the cells were probed
with the antibodies against N-Cadherin and Vimentin that were also
used in the western blot overnight at 4°C. Following several
washes, Alexa Fluor® 594-conjugated secondary antibodies
(1:500, cat. no. Z25007; Invitrogen; Thermo Fisher Scientific,
Inc.) were used for staining at room temperature for 1 h. All cells
were counterstained with DAPI and imaged by confocal laser-scanning
microscopy (LSM710; Zeiss GmbH, Jena, Germany).
Immunohistochemistical staining and
analysis
Immunohistochemistry (IHC) was performed according
to previous methods (5): 4 µm
paraffin-embedded thyroid cancer tissue sections using monoclonal
antibodies directed against TSHR (1:100, cat. no. ab219322; Abcam)
at room temperature for 1 h. The secondary antibody Goat Anti-Mouse
IgG H&L (HRP) (cat. no. ab205719; Abcam) was incubated at room
temperature for 15 min. Tissue sections were observed under an
AX10-Imager A1 (Zeiss GmbH), and all images were captured using
AxioVision microscopy software (version 4.7; Zeiss). The score was
evaluated by estimating the percentage and intensity of tumor cell
staining. The scores of positively stained tumor cells were graded
as: 0 (no positive tumor), 1 (<10%), 2 (10–50%) and 3 (>50%).
The intensity of tumor cell staining was determined as: 0 (no
staining), 1 (light yellow), 2 (yellow brown), 3 (brown). The
staining index was calculated as the product of staining intensity
× percentage of positive tumor cells, resulting in scores of 0, 1,
2, 3, 4, 6 and 9.
Statistical analysis
All statistical analyses were performed using SPSS
software (version 19.0; IBM SPSS, Armonk, NY, USA). The
χ2 test was used to analyze the association between TSHR
expression and the clinicopathologic characteristics of patients.
Logistic regression was used to analyze the factors associated with
distant metastasis in patients with DTC. Survival curves were
plotted using the Kaplan-Meier estimator method and compared using
the log-rank test. Survival data were evaluated using multivariate
Cox regression analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
TSHR is downregulated in
poorly-differentiated thyroid cancer cell lines and tissues
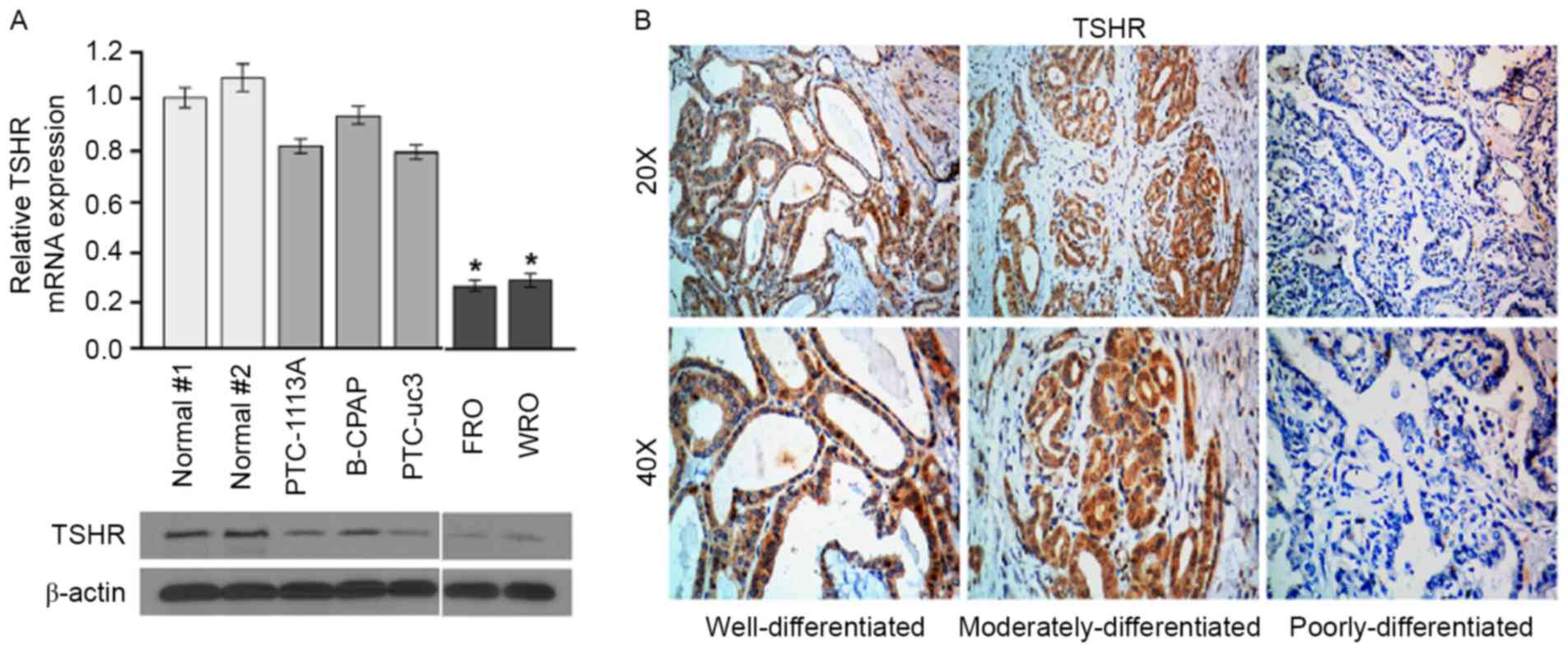
To investigate the role of TSHR in thyroid cancer,
TSHR levels in normal thyroid cells and thyroid cancer cells with
different differentiation stages were determined using western
blotting and RT-qPCR analysis. All differentiated and
poorly-differentiated thyroid cancer cell lines studied expressed
TSHR mRNA and protein, and expression levels of TSHR mRNA were
significantly higher in the well-differentiated cell lines
(PTC-113A, B-CPAP and PTC-uc3) compared with the
poorly-differentiated cell lines (FRO and WRO) (Fig. 1A). To confirm this result, the
expression of TSHR in thyroid cancer tissues was determined using
immunohistochemical analysis. The well-differentiated thyroid
cancer tissues exhibited higher TSHR expression compared with
moderately- and poorly-differentiated thyroid cancer tissues
(Fig. 1B). These results demonstrate
that TSHR is downregulated in poorly differentiated DTC cell lines
and clinical tissues.
Downregulation of TSHR promotes
thyroid cancer cell invasion and metastasis
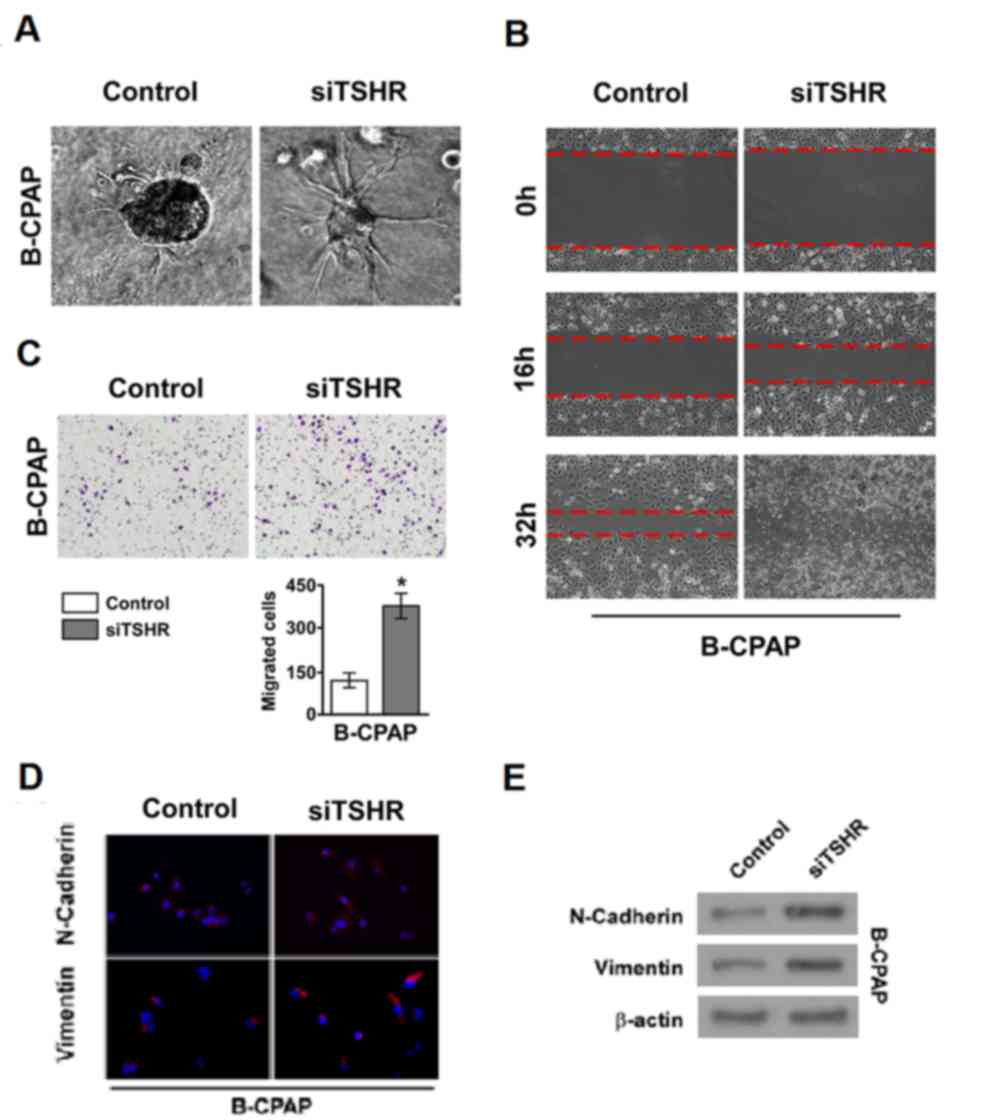
The importance of TSHR in thyroid cancer cell
metastasis in the well-differentiated cancer cell line B-CPAP was
investigated. The 3D culture model revealed that the invasive and
metastatic ability of cells was markedly increased when TSHR
expression was silenced in B-CPAP cell lines compared with the
control siRNA group (Fig. 2A). In
addition, similar results were obtained from the wound healing and
invasion assays (Fig. 2B and C,
respectively). These results indicate that the downregulation of
TSHR promotes the invasion and metastasis of DTC. EMT is associated
with the invasion and metastasis of tumors, so immunofluorescence
and western blotting was performed to examine the expression of
markers of EMT in thyroid cancer cells. In the well-differentiated
thyroid cancer cell line B-CPAP, high expression of TSHR inhibited
the expression of two classical mesenchymal cell markers,
N-cadherin and vimentin (Fig. 2D and
E). When TSHR was silenced, the expression of N-cadherin and
vimentin was recovered (Fig. 2D and
E). These results suggest that TSHR suppresses thyroid cancer
cell invasion and metastasis by inhibiting EMT.
Low expression of TSHR is associated
with a high distant metastasis rate in patients with DTC
To further investigate the role of TSHR in thyroid
cancer progression, immunohistochemical staining of TSHR levels was
statistically analyzed to determine their association with the
clinical features of patients with DTC (Table I). TSH expression was detected in
93/172 DTC cases (54.1%). Distant metastasis occurred in 15/172
cases (8.72%), including lung metastasis in 14 cases and liver
metastasis in 1 case. The distant metastasis rates in the
TSHR-negative and -positive groups were 13.92 and 4.30%,
respectively (P=0.026). Logistic regression was performed to
analyze the factors associated with distant metastasis in patients
with DTC (Table II). The results
revealed that TSHR expression was a significant independent factor
affecting distant metastasis in patients with DTC (P=0.035).
Distant metastasis status was also associated with age,
pathological type, T stage, and primary treatment; however, it was
not associated with N stage, recurrent laryngeal nerve invasion,
extracapsular invasion or trachea invasion (data not shown).
 | Table II.Logistic regression results of the
clinicopathological factors associated with distant metastasis in
patients with DTC. |
Table II.
Logistic regression results of the
clinicopathological factors associated with distant metastasis in
patients with DTC.
|
|
|
|
|
|
| Exp (B) 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
factor | B | SE | Wals | P-value | Exp (B) | Lower | Upper |
|---|
| Age | 1.522 | 0.771 | 3.898 | 0.048 | 4.583 | 1.011 | 20.771 |
| TSHR
expression | −1.469 | 0.696 | 4.450 | 0.035 | 0.230 | 0.059 | 0.901 |
| Pathological
type | 3.044 | 0.877 | 12.051 | 0.001 | 20.987 | 3.763 | 117.035 |
| T stage | 0.615 | 0.314 | 3.830 | 0.050 | 1.849 | 0.999 | 3.423 |
| N stage | 1.408 | 0.844 | 2.780 | 0.095 | 4.086 | 0.781 | 21.374 |
| Primary
treatment | 1.372 | 0.667 | 4.236 | 0.040 | 3.943 | 1.068 | 14.560 |
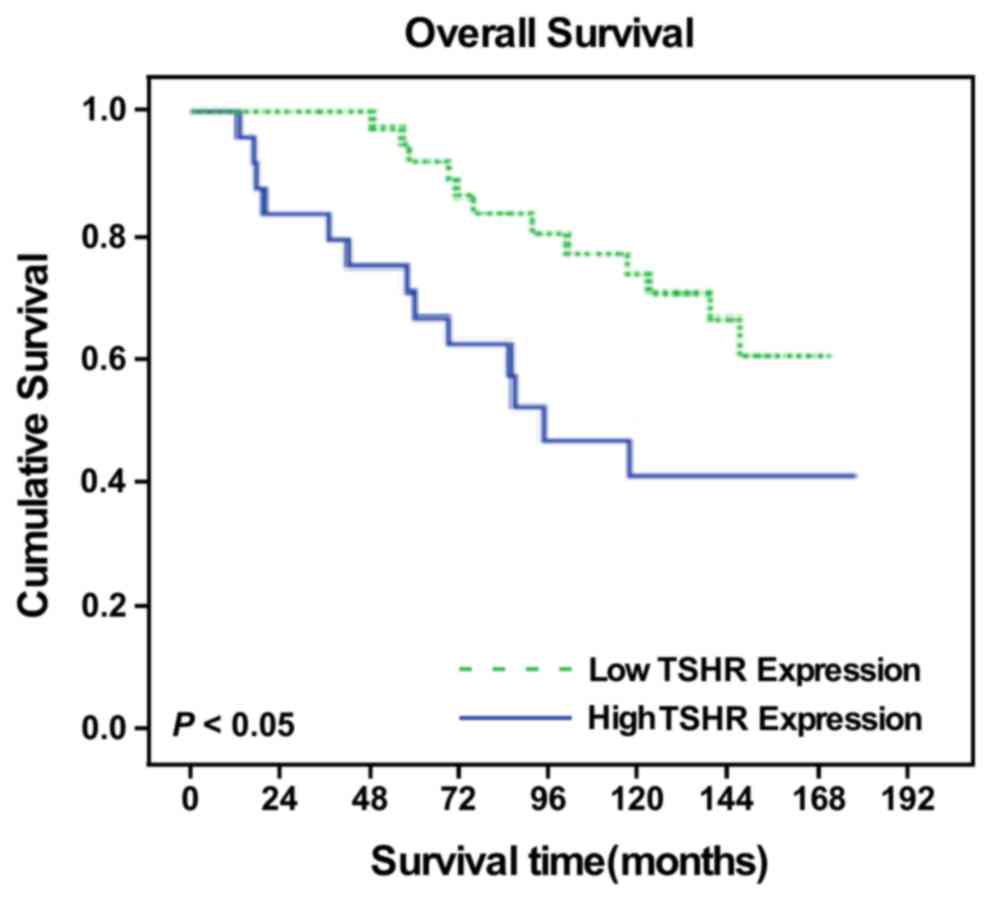
TSHR expression is an independent
prognostic factor for patients with DTC
As age is the most important independent prognostic
factor for DTC (13), the present
study demonstrated that the 10-year overall survival (OS) rates
were 98.2 and 61.2% in the <45 and ≥45-year-old groups,
respectively (x2=43.335; P<0.001). Therefore, a
stratified prognostic analysis was performed according to age, and
patients who were ≥45 years old were selected for survival
analysis. Among patients with DTC who were >45 years old, the
survival time was significantly different between the patients with
low and high TSHR expression as measured by IHC scores
(x2=4.863; P=0.027; data not shown), and the
TSHR-positive group had a 10-year OS of 73.8%, whereas the OS in
the TSHR-negative group was only 41.0% (Fig. 3). In addition, a multivariate analysis
demonstrated that TSHR expression, pathological stage, distant
metastasis, N stage and primary treatment were significant
independent prognostic factors in patients with DTC (all P<0.05;
Table III).
 | Table III.Multivariate analysis results of the
clinicopathological factors affecting the prognosis of patients
with DTC that were ≥45 years old. |
Table III.
Multivariate analysis results of the
clinicopathological factors affecting the prognosis of patients
with DTC that were ≥45 years old.
|
|
|
|
|
|
| Exp (B) 95% CI |
|---|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
factor | B | SE | Wald | P-value | Exp (B) | Lower | Upper |
|---|
| TSHR
expression | −1.107 | 0.467 | 5.623 | 0.018 | 0.331 | 0.132 | 0.825 |
| N stage | 2.094 | 0.799 | 6.865 | 0.009 | 8.117 | 1.695 | 38.877 |
| Pathological
stage | −1.506 | 0.845 | 3.171 | 0.075 | 0.222 | 0.042 | 1.164 |
| Distant
metastasis | 1.957 | 0.468 | 17.450 | 0.000 | 7.076 | 2.825 | 17.720 |
| Primary
treatment | 0.991 | 0.440 | 5.070 | 0.024 | 2.695 | 1.137 | 6.386 |
Discussion
Thyroid cancer is a major cause of mortality and
morbidity. A number of studies have been performed to elucidate the
underlying molecular mechanisms that regulate thyroid cancer
proliferation and progression (1,8). However,
the underlying molecular mechanisms of the distant metastasis of
thyroid cancer remain unclear (14,15). It
has been demonstrated that several signaling pathways, including
the TSHR signaling pathway, contribute to the development of
thyroid cancer (16); however, the
association between the TSHR signaling pathway and metastasis has
not yet been reported, to the best of our knowledge. The present
study demonstrated that TSHR inhibits the invasion and metastasis
of the well-differentiated cancer cell line B-CPAP in
vitro.
TSHR is typically considered to be an oncogene in
thyroid cell carcinogenesis and the expression of TSHR has been
correlated with poor patient outcomes (17); however, another study reported that
aberrant methylation of the TSHR gene in epithelial thyroid cancers
leads to TSHR expression silencing and malignant epithelial thyroid
tumors (5,17). The results from the present study
suggest that the expression of TSHR can suppress the invasive and
metastatic abilities of thyroid cancer cells in vitro.
Furthermore, analysis of the clinicopathologic characteristics of
patients with DTC indicated that the expression of TSHR is
associated with good prognosis.
Metastasis is an essential feature of tumors and
accounts for the majority of cancer-associated mortalities in
humans (18). EMT is a key
morphological change of cells that can facilitate the dissemination
of cells from the original organ to a distant site. EMT is a common
event, which is frequently observed during cancer development and
progression (8,9). The data from the present study suggests
that TSHR expression can suppress thyroid cancer cell metastasis by
inhibiting thyroid cancer cell EMT in vitro. This indicates
that TSHR expression could be a biomarker and independent
prognostic factor for thyroid cancer in the clinic. However, it is
not clear how TSHR suppresses EMT. Previous studies have
demonstrated that microRNA (miRNA/miR) serves an important role in
EMT; for example, miR-146b-5p induces EMT through the regulation of
Wnt/β-catenin signaling (19,20) and the miR-200 family inhibits polycomb
complex protein BMI1, and zinc finger E-box-binding homeobox 1 and
2, to suppress EMT (21–23). Further studies are required to
demonstrate whether TSHR regulates EMT through miRNAs.
Data from the present study demonstrated that a lack
of expression of TSHR was associated with distant metastasis and a
poor survival rate in patients with DTC. The distant metastasis
rate in the TSHR negative group was significantly higher compared
with that of the TSHR positive group (13.92 vs. 4.30%,
respectively) and the lung was the most common metastasis site.
TSHR expression was also a significant independent factor affecting
distant metastasis in patients with DTC. N stage was not associated
with distant metastasis; however, a study from Jeon et al
(24) demonstrated that the location
of associated lymph node (LN) metastasis categories is more useful
than the amount of associated LNs categories for estimating the
risk of distant metastasis in PTC. The use of molecular biomarkers,
including TSHR, combined with clinicopathological characteristics
may aid in the accurate prediction of the incidence of distant
metastases in patients with thyroid cancer.
Endocrine therapy has been used in clinical practice
for >30 years. Theoretically, the downregulation of TSH through
suppression of pituitary TSH feedback by oral thyroxine may inhibit
the proliferation of PTC cells, which may reduce the recurrence
rate of the tumor; however, the exact treatment effect is not clear
and there is a lack of strong clinical evidence (25–27). The
results from the current study regarding the role of TSHR on
distant metastases in DTC may provide some theoretical basis for
the application of endocrine treatment options in DTC. However,
prospective clinical trials are required to evaluate whether TSH
endocrinal treatment can significantly improve the survival rate of
patients by inhibiting distant metastasis.
In conclusion, the present study identified that a
lack of expression of TSHR was associated with distant metastases
and poor survival time in patients with DTC. In addition, the
expression of TSHR was revealed to reduce thyroid cancer cell
metastatic capability by inhibiting EMT. Further clinical studies
are required to elucidate the diagnostic and therapeutic
implications of the role or TSHR in thyroid tumors, and prospective
clinical trials are required to evaluate whether TSH endocrinal
treatment can significantly decrease the incidence of distant
metastasis in these patients.
Acknowledgements
The current study was supported by the National
Science Foundation of China (grant nos. 81272955 and 81302368) and
the Guangdong Province Natural Science Foundation (grant nos.
2014A020212100, S2011010003997 and 2016A020215082).
References
|
1
|
Nikiforov YE and Nikiforova MN: Molecular
genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol.
7:569–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cras A, Politis B, Balitrand N,
Darsin-Bettinger D, Boelle PY, Cassinat B, Toubert ME and Chomienne
C: Bexarotene via CBP/p300 induces suppression of NF-κB-dependent
cell growth and invasion in thyroid cancer. Clin Cancer Res.
18:442–453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shoup M, Stojadinovic A, Nissan A,
Ghossein RA, Freedman S, Brennan MF, Shah JP and Shaha AR:
Prognostic indicators of outcomes in patients with distant
metastases from differentiated thyroid carcinoma. J Am Coll Surg.
197:191–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kimura T, Van Keymeulen A, Golstein J,
Fusco A, Dumont JE and Roger PP: Regulation of thyroid cell
proliferation by TSH and other factors: A critical evaluation of in
vitro models. Endocr Rev. 22:631–656. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu C, Zhao L, Ying H, Willingham MC and
Cheng SY: Growth activation alone is not sufficient to cause
metastatic thyroid cancer in a mouse model of follicular thyroid
carcinoma. Endocrinology. 151:1929–1939. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davies T, Marians R and Latif R: The TSH
receptor reveals itself. J Clin Invest. 110:161–164. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cannito S, Novo E, di Bonzo LV, Busletta
C, Colombatto S and Parola M: Epithelial-mesenchymal transition:
From molecular mechanisms, redox regulation to implications in
human health and disease. Antioxid Redox Signal. 12:1383–1430.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liao WT, Song LB, Zhang HZ, Zhang X, Zhang
L, Liu WL, Feng Y, Guo BH, Mai HQ, Cao SM, et al: Centromere
protein H is a novel prognostic marker for nasopharyngeal carcinoma
progression and overall patient survival. Clin Cancer Res.
13:508–514. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krook KA, Fedewa SA and Chen AY:
Prognostic indicators in well-differentiated thyroid carcinoma when
controlling for stage and treatment. Laryngoscope. 125:1021–1027.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gandolfi G, de Biase D, Sancisi V, Ragazzi
M, Acquaviva G, Pession A, Piana S, Tallini G and Ciarrocchi A:
Deep sequencing of KIT, MET, PIK3CA, and PTEN hotspots in papillary
thyroid carcinomas with distant metastases. Endocr Relat Cancer.
21:L23–L26. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gandolfi G, Ragazzi M, Frasoldati A, Piana
S, Ciarrocchi A and Sancisi V: TERT promoter mutations are
associated with distant metastases in papillary thyroid carcinoma.
Eur J Endocrinol. 172:403–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cancer Genome Atlas Research Network:
Integrated genomic characterization of papillary thyroid carcinoma.
Cell. 159:676–690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xing M, Usadel H, Cohen Y, Tokumaru Y, Guo
Z, Westra WB, Tong BC, Tallini G, Udelsman R, Califano JA, et al:
Methylation of the thyroid-stimulating hormone receptor gene in
epithelial thyroid tumors: A marker of malignancy and a cause of
gene silencing. Cancer Res. 63:2316–2321. 2003.PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Deng X, Wu B, Xiao K, Kang J, Xie J, Zhang
X and Fan Y: MiR-146b-5p promotes metastasis and induces
epithelial-mesenchymal transition in thyroid cancer by targeting
ZNRF3. Cell Physiol Biochem. 35:71–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hardin H, Guo Z, Shan W, Montemayor-Garcia
C, Asioli S, Yu XM, Harrison AD, Chen H and Lloyd RV: The roles of
the epithelial-mesenchymal transition marker PRRX1 and miR-146b-5p
in papillary thyroid carcinoma progression. Am J Pathol.
184:2342–2354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Braun J, Hoang-Vu C, Dralle H and
Hüttelmaier S: Downregulation of microRNAs directs the EMT and
invasive potential of anaplastic thyroid carcinomas. Oncogene.
29:4237–4244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Z, Liu ZB, Ren WM, Ye XG and Zhang
YY: The miR-200 family regulates the epithelial-mesenchymal
transition induced by EGF/EGFR in anaplastic thyroid cancer cells.
Int J Mol Med. 30:856–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng X, Wang Z, Fillmore R and Xi Y:
MiR-200, a new star miRNA in human cancer. Cancer Lett.
344:166–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeon MJ, Kim TY, Kim WG, Han JM, Jang EK,
Choi YM, Song DE, Yoon JH, Chung KW, Hong SJ, et al:
Differentiating the location of cervical lymph node metastasis is
very useful for estimating the risk of distant metastases in
papillary thyroid carcinoma. Clin Endocrinol (Oxf). 81:593–599.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Middendorp M and Grünwald F: Update on
recent developments in the therapy of differentiated thyroid
cancer. Semin Nucl Med. 40:145–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cooper DS, Specker B, Ho M, Sperling M,
Ladenson PW, Ross DS, Ain KB, Bigos ST, Brierley JD, Haugen BR, et
al: Thyrotropin suppression and disease progression in patients
with differentiated thyroid cancer: Results from the National
Thyroid Cancer Treatment Cooperative Registry. Thyroid. 8:737–744.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugitani I and Fujimoto Y: Does
postoperative thyrotropin suppression therapy truly decrease
recurrence in papillary thyroid carcinoma? A randomized controlled
trial. J Clin Endocrinol Metab. 95:4576–4583. 2010. View Article : Google Scholar : PubMed/NCBI
|