Introduction
Globally, numerous patients succumb to lung cancer
(1). The use of cytotoxic
chemotherapy remains a major means of treating patients with
unresectable non-small cell lung cancer (NSCLC). Nevertheless, the
effectiveness of cytotoxic chemotherapy may be limited in certain
patients, with a response rate of 20–35% and a median survival time
of 10–12 months (2,3). For treating such patients, gefitinib and
erlotinib, the first generation orally administered epidermal
growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs)
were developed. At first, the effect of EGFR-TKIs was limited due
to treating unselected patients with NSCLC (4–7). However,
previous studies have revealed that the presence of EGFR mutation
may be associated with increased responsiveness to EGFR-TKIs in
patients with NSCLC (8–11). In previous studies, the response rate
was 62.1–83.0%, with a median progression-free survival (PFS) and
overall survival (OS) of 9.2–13.1 months and 19.3–30.5 months,
respectively (8–12). The toxicities of EGFR-TKIs are
decreased compared with those of cytotoxic drugs and patients can
achieve a good quality of life while using them (8,12). In
patients treated with first generation EGFR-TKIs, brain, bone and
liver metastasis and pleural effusion (PE) predicted a poorer
prognosis compared with patients without these metastasis (13–17).
However, few reports concern the association between the site of
metastasis and prognosis (18,19).
Understanding which metastatic organ sites influence the prognosis
of patients treated with EGFR-TKIs and the prognostic significance
of the number of metastatic organ sites is crucial in explaining
the condition to patients and aiding them in tolerating the
treatment.
Therefore, the present study was a retrospective
cohort study conducted to analyze the association between the site
and number of metastases, and the prognosis of EGFR-TKI-treated
EGFR mutation-positive patients with NSCLC.
Materials and methods
Patients
Pathology reports from the National Hospital
Organization Kinki-chuo Chest Medical Center (Osaka, Japan) were
retrospectively reviewed between January 2009 and December 2014 and
533 patients were identified as having EGFR
mutation-positive NSCLC. Patients with stage IA-IIIA disease, based
on the 7th TNM staging system (20),
and SCLC were excluded. All participants provided written informed
consent for their data to be included. The study protocol was
approved by the Institutional Review Board (approval no. 561;
October 20, 2016) of the National Hospital Organization Kinki-chuo
Chest Medical Center. Research was conducted in accordance with the
1964 Declaration of Helsinki and its later amendments.
Data collection
Clinical data, including age, sex, type of
EGFR mutation, TNM stage, smoking status, treatment history,
PFS, OS and metastatic status were collected at the point of
first-line treatment. Clinical responses were defined according to
the Response Evaluation Criteria in Solid Tumors, version 1.1
(21). PFS was measured from the date
of the commencement of primary systemic therapy to the date of
disease progression or mortality from any cause. OS was measured
from the date of diagnosis to the date of death, loss to follow-up
or last follow-up, whichever occurred first. Patients were
followed-up for disease status until February 2016.
EGFR mutation identification
Lung cancer was pathologically confirmed using
tissue specimens obtained from bronchoscopy, computed
tomography-guided biopsy, PE cytology, or surgical procedures.
Mutational analysis of the EGFR gene was performed using
Scorpion technology in combination with the Amplified Refractory
Mutation System or polymerase chain reaction-Invader technique, as
previously described (22,23).
Statistical analysis
Statistical analysis was conducted using the JMP
statistical software program, version 11 (SAS Institute Inc., Cary,
NC, USA) to compare clinical outcomes according to the metastatic
status of the patients. Survival curves were estimated using the
Kaplan-Meier method and the differences between the groups were
compared using the log-rank test. Univariate and multivariate
analyses were performed using the Cox proportional hazards models.
Fisher's exact test was used to compare the non-parametric
variables. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Of the 533 EGFR mutation-positive patients
with NSCLC initially recruited to the study, 355 were excluded
based on the following criteria: Stage I–IIIA disease (n=228),
treated with chemotherapy (n=50), treated with EGFR-TKIs and
chemotherapy (n=31), received best supportive care only (n=25),
treated with chemoradiotherapy (n=8), treated with second
generation EGFR-TKIs (n=6), unknown TNM stage (n=3), small cell
carcinoma (n=3) and treated with radiotherapy (n=1). A total of 178
patients remained, who were treated with first generation EGFR-TKIs
as the first-line treatment (Fig. 1).
Of these patients, 127 were female and 51 were male. The median age
at the time of first-line treatment was 72 (range, 39–91) years. A
total of 168 patients had adenocarcinoma, 134 patients had stage IV
disease, 71 patients had a history of smoking and 156 patients were
treated with gefitinib (Table I).
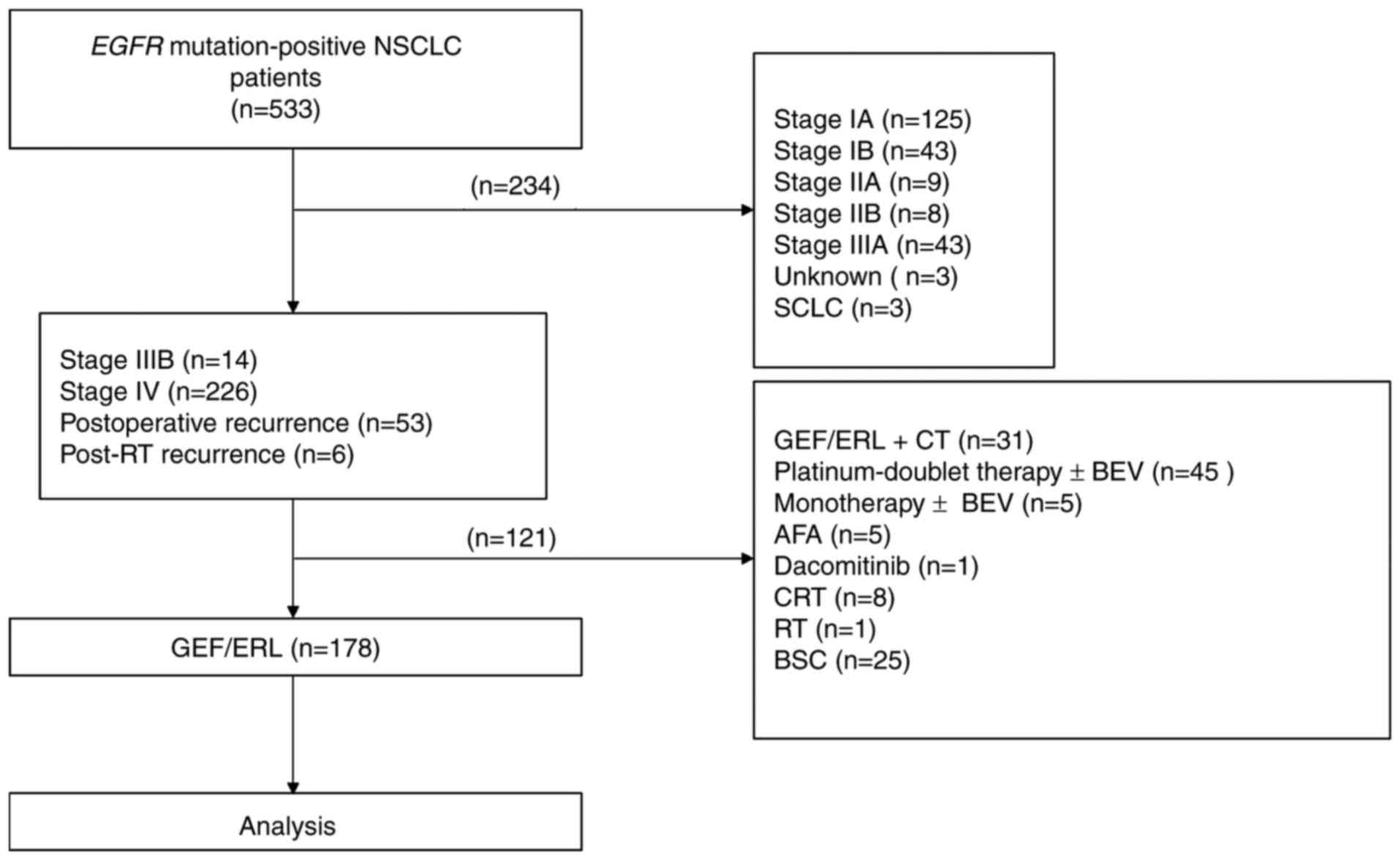 | Figure 1.Study flowchart. From 533 EGFR
mutation-positive patients with NSCLC, 178 patients treated with
GEF or ERL were enrolled in the present study. A total of 65
patients had brain metastases, 78 patients had bone metastases, 17
patients had liver metastases and 56 patients had pleural effusion
at the time of first-line treatment. EGFR, epithelial growth
factor receptor; NSCLC, non-small cell lung cancer; GEF, gefitinib;
ERL, erlotinib; RT, radiotherapy; CT, chemotherapy; BEV,
bevacizumab; AFA, afatinib; CRT, chemoradiotherapy; BSC, best
supportive care. |
 | Table I.Patient baseline characteristics. |
Table I.
Patient baseline characteristics.
|
|
| Metastasis |
|
|---|
|
|
|
|
|
|---|
| Characteristic | All patients | Brain | Bone | Liver | Pleural
effusion |
|---|
| Total, n | 178 | 65 | 78 | 17 | 56 |
| Sex, n |
|
|
|
|
|
|
Male | 51 | 15 | 27 | 5 | 16 |
|
Female | 127 | 50 | 51 | 12 | 40 |
| Age in years,
median (range) | 72 (39–91) | 71 (50–89) | 71 (42–89) | 71 (50–89) | 73 (39–91) |
| Histopathological
subtype, n |
|
|
|
|
|
|
Adenocarcinoma | 168 | 62 | 74 | 16 | 54 |
|
Squamous cell carcinoma | 1 | 0 | 0 | 0 | 0 |
| Not
otherwise specified | 9 | 3 | 4 | 1 | 2 |
| Tumor node
metastasis stage, n |
|
|
|
|
|
| Stage
IIIB | 1 | 0 | 0 | 0 | 0 |
| Stage
IV | 134 | 55 | 69 | 17 | 48 |
|
Postoperative recurrence | 39 | 10 | 8 | 0 | 6 |
|
Post-radiotherapy
recurrence | 4 | 0 | 1 | 0 | 2 |
| Smoking status,
n |
|
|
|
|
|
|
Smoker | 71 | 19 | 35 | 4 | 21 |
|
Non-smoker | 102 | 45 | 41 | 12 | 33 |
| EGFR
mutation type, n |
|
|
|
|
|
| Exon 19
deletion | 80 | 34 | 32 | 8 | 20 |
|
p.L858R | 78 | 23 | 33 | 6 | 29 |
|
Other | 20 | 8 | 13 | 3 | 7 |
| EGFR-tyrosine
kinase inhibitor therapy |
|
|
|
|
|
|
Gefitinib | 156 | 52 | 66 | 15 | 48 |
|
Erlotinib | 22 | 13 | 12 | 2 | 8 |
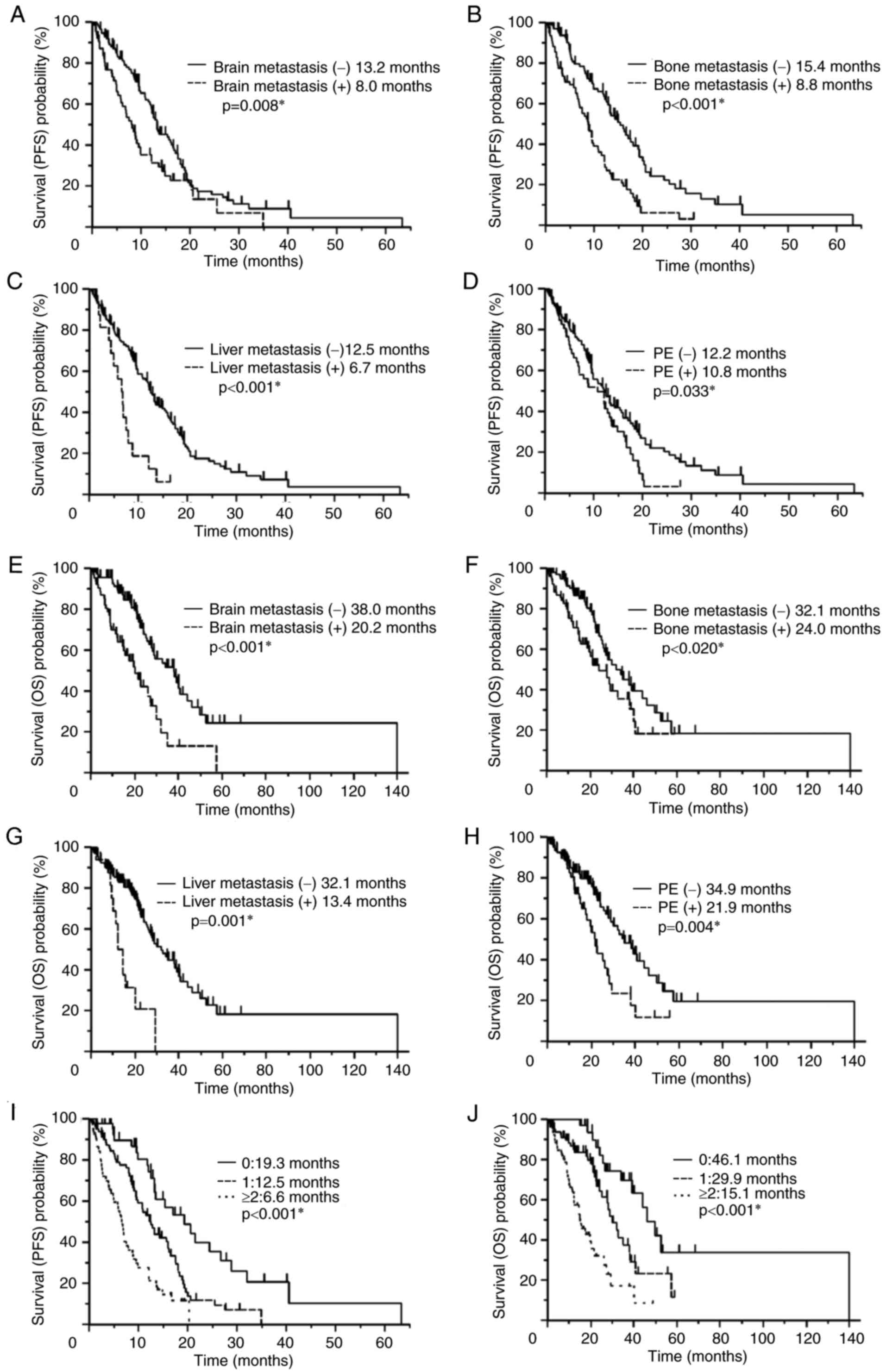
Survival analysis
The Kaplan-Meier method was used to assess patient
survival (Fig. 2). Patients with
brain metastases (8.0 vs. 13.2 months, P=0.008; Fig. 2A), bone metastases (8.8 vs. 15.4
months, P<0.001; Fig. 2B), liver
metastases (6.7 vs. 12.5 months, P<0.001; Fig. 2C) and PE (10.8 vs. 12.2 months,
P=0.033; Fig. 2D) at the time of
first-line treatment were associated with significantly poorer PFS
compared with patients without each of these metastases. Patients
with brain metastases (20.2 vs. 38.0 months, P<0.001l Fig. 2E), bone metastases (24.0 vs. 32.1
months, P=0.020; Fig. 2F), liver
metastases (13.4 vs. 32.1 months, P<0.001; Fig. 2G), and PE (21.9 vs. 34.9 months,
P=0.004; Fig. 2H) at the time of
first-line treatment also exhibited significantly poorer OS times
compared with patients without each of these metastases.
Response rate analysis
There were no significant differences in the rates
of response between patients with brain metastases (58.5 vs. 60.2%,
P=0.875), bone metastases (62.8 vs. 57.0%, P=0.446), liver
metastases (64.7 vs. 59.0%, P=0.797) and PE (60.7 vs. 59.0%,
P=0.871) at the time of first-line treatment and patients without
each of these metastases (Table
II).
 | Table II.Response rates to erlotinib
treatment. |
Table II.
Response rates to erlotinib
treatment.
|
|
| Metastasis |
|
|---|
|
|
|
|
|
|---|
| Characteristic | All patients | Brain | Bone | Liver | PE |
|---|
| Total | 178 | 65 | 78 | 17 | 56 |
| CR/PR, n | 106 | 38 | 49 | 11 | 34 |
| RR, % |
59.6 |
58.5 |
62.8 |
64.7 |
60.7 |
| P-value | – | 0.875 | 0.446 | 0.797 | 0.871 |
Multivariate analysis of prognostic
factors
In the multivariate analysis, bone metastasis was
significantly associated with a poorer PFS time [hazard ratio (HR),
2.11; 95% confidence interval (CI), 1.44–3.09; P<0.001] and
brain metastasis exhibited a trend towards a poorer PFS time,
although it was not significant (HR, 2.11; 95% CI, 0.99–2.15;
P=0.051; Table III). In addition,
brain metastasis was significantly associated with a shorter OS
time (HR, 2.41; 95% CI, 1.46–3.95; P<0.001; Table IV).
 | Table III.Cox proportional hazards model
analysis of factors associated with progression-free survival. |
Table III.
Cox proportional hazards model
analysis of factors associated with progression-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Brain
metastasis | 1.63 | 1.13–2.33 | 0.010a | 1.48 | 0.99–2.15 | 0.051 |
| Bone
metastasis | 2.22 | 1.55–3.20 | ≤0.001a | 2.11 | 1.44–3.09 | ≤0.001a |
| Liver
metastasis | 2.73 | 1.51–4.62 | 0.002a | 1.42 | 0.74–2.56 | 0.280 |
| Pleural
effusion | 1.49 | 1.02–2.17 | 0.039a | 1.48 | 0.99–2.16 | 0.053 |
 | Table IV.Cox proportional hazards model of
factors associated with overall survival. |
Table IV.
Cox proportional hazards model of
factors associated with overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Brain
metastasis | 2.60 | 1.64–4.10 | ≤0.001a | 2.41 | 1.46–3.95 | ≤0.001a |
| Bone
metastasis | 1.69 | 1.08–2.64 | 0.022a | 1.59 | 0.97–2.58 | 0.066 |
| Liver
metastasis | 3.81 | 1.96–6.90 | ≤0.001a | 1.84 | 0.88–3.68 | 0.104 |
| Pleural
effusion | 1.94 | 1.21–3.05 | 0.006a | 1.62 | 0.99–2.60 | 0.052 |
Effect of metastatic site number on
prognosis
The number of metastatic organ sites per patient in
the brain, bone, liver, and pleura, (0 in 43 patients, 1 in 81
patients, and ≥2 in 54 patients) was significantly associated with
a reduced PFS (19.3 vs. 12.5 vs. 6.6 months, P<0.001) and OS
(46.1 vs. 29.9 vs. 15.1 months, P<0.001; Fig. 2I and J) time. No significant
differences in PFS (6.2 vs. 7.5 vs. 5.9 months, P=0.545) or OS
(14.9 vs. 19.6 vs. 13.4 months, P=0.497) time were observed between
patients with 2 (n=33), 3 (n=15) or 4 (n=6) metastatic organ
sites.
Effect of EGFR exon 19 deletion and
p.L858R mutations on prognosis
Patients with major EGFR mutations (including
exon 19 deletion and p.L858R; n=158) were also evaluated. In a
multivariate analysis, bone metastasis was identified to be
significantly associated with a poorer PFS time. In addition, brain
and liver metastases were significantly associated with a poorer OS
time (Tables V and VI).
 | Table V.Cox proportional hazards model
analysis of factors associated with progression-free survival in
patients with epithelial growth factor receptor exon 19 deletion
and p.L858R mutations. |
Table V.
Cox proportional hazards model
analysis of factors associated with progression-free survival in
patients with epithelial growth factor receptor exon 19 deletion
and p.L858R mutations.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Brain
metastasis | 1.69 | 1.12–2.52 | 0.012a | 1.45 | 0.93–2.22 | 0.102 |
| Bone
metastasis | 1.97 | 1.33–2.91 | ≤0.001a | 1.78 | 1.16–2.65 | 0.008a |
| Liver
metastasis | 3.25 | 1.66–5.85 | 0.001a | 1.95 | 0.94–3.84 | 0.101 |
| Pleural
effusion | 1.44 | 0.95–2.16 | 0.083 | – | – | – |
 | Table VI.Cox proportional hazards model
analysis of factors associated with overall survival in patients
with epithelial growth factor receptor exon 19 deletion and p.L858R
mutations. |
Table VI.
Cox proportional hazards model
analysis of factors associated with overall survival in patients
with epithelial growth factor receptor exon 19 deletion and p.L858R
mutations.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Brain
metastasis | 2.55 | 1.51–4.26 | ≤0.001a | 2.06 | 1.16–3.58 |
0.014a |
| Bone
metastasis | 1.43 | 0.86–2.36 | 0.165 | – | – | – |
| Liver
metastasis | 5.00 | 2.29–10.05 | ≤0.001a | 3.05 | 1.34–6.51 |
0.009a |
| Pleural
effusion | 1.89 | 1.12–3.16 | 0.018a | 1.66 | 0.97–2.81 | 0.066 |
Discussion
In the univariate analysis of patients treated with
first generation EGFR-TKIs, brain metastasis, bone metastasis,
liver metastasis and PE were all associated with poorer PFS and OS
times. Furthermore, in the multivariate analysis, bone metastasis
was associated with a poorer PFS time and brain metastasis was
associated with a poorer OS time. The number of metastatic organ
sites was associated with a poorer PFS and OS time.
Between 30 and 40% of patients with lung cancer
develop bone metastases during the course of their disease
(24). To the best of our knowledge,
this is the first study to assess the association between bone
metastasis and a poorer PFS time in EGFR mutation-positive
NSCLC patients. In a previous report, Fujimoto et al
(15) revealed that bone metastasis
was a significant independent negative predictive factor for OS
time in EGFR mutation-positive patients. By contrast, in the
present study, bone metastasis was not associated with OS time. One
possible explanation for the association between bone metastasis
and a poorer prognosis is the tumor-bone interaction that is
reported to increase the malignant behavior of cancer cells. In the
development of bone metastases, there is an exchange of factors
from the bone matrix that are released during bone resorption, the
most notable of which is transforming growth factor-β, which has
been demonstrated to enhance tumor growth and the
epithelial-mesenchymal transition (25,26).
Brain metastases are a frequent complication of
NSCLC, with 25–40% of patients developing brain metastases during
the course of their disease, often within the first 2 years
following the diagnosis of the primary tumor (24,27). The
risk of brain metastasis was increased in EGFR-mutated
tumors at the time of diagnosis, as well as during the
postoperative course of the disease. Compared with patients with
wild-type tumors, patients with EGFR-mutated tumors
exhibited more widespread brain lesions (28). The clinical activity of EGFR-TKIs
against intracranial disease has previously been described
(29–33). Furthermore, erlotinib may exhibit a
superior control of intracranial disease due to the higher central
nervous system penetration and drug concentrations achieved
relative to gefitinib (34). However,
in the present study, 80% of patients were treated with gefitinib.
In patients treated with EGFR-TKIs, brain metastasis has been
reported as a risk factor for poorer PFS and OS (13,35).
Similarly, in the present study, brain metastasis was the only
negative prognostic factor identified by multivariate analysis for
OS time.
Wu et al (17)
reported that lung adenocarcinoma patients with Stage IV disease
and malignant PE at the time of diagnosis have poorer OS times than
patients who develop malignant PE following disease progression.
However, the difference was only statistically significant in
patients with distant metastases. For patients without distant
metastases, there was no significant difference. In the present
study, PE was not associated with a poorer PFS or OS by univariate
analysis. In addition, Wu et al (16) reported that EGFR
mutation-positive stage IV lung adenocarcinoma patients with liver
metastases treated with gefitinib as first-line treatment exhibited
significantly poorer PFS and OS times compared with patients who
did not have liver metastases. In the present study, liver
metastasis was not associated with a poorer PFS or OS by
multivariate analysis. However, the number of patients with liver
metastases was relatively small (n=17). This may explain why the
results of the present study differ from the Wu et al study.
Additionally, in patients with major EGFR mutations (exon 19
deletion and p.L858R), liver metastasis was significantly
associated with a poorer OS time in the multivariate analysis (HR,
3.05; 95% CI, 1.34–6.51; P<0.001; Table VI).
Higher numbers of metastatic organ sites were
associated with a poorer PFS and OS time, as previously reported
(18,19). It has been suggested that the
prognostic significance of the number of metastatic organ sites may
be due to resistance in patients with a larger tumor burden
(36). As aforementioned, patients
with bone or brain metastases and patients with ≥2 metastatic organ
sites do not respond effectively to treatment with first-generation
EGFR-TKIs. Therefore, more effective treatments are required. For
EGFR mutation-positive NSCLC patients, novel treatment
approaches have been proposed. Seto et al (37) reported that bevacizumab in addition to
erlotinib significantly improved PFS. Tamiya et al (38) reported that triplet chemotherapy with
gefitinib, carboplatin and tegafur/gimeracil/oteracil as a
first-line treatment was efficacious and well tolerated.
Furthermore, Kanda et al (39)
reported that the addition of cisplatin and docetaxel to gefitinib
treatment may have prevented the development of acquired resistance
to EGFR-TKIs in EGFR mutation-positive patients with
advanced NSCLC. Sugawara et al (40) reported that concurrent chemotherapy
with gefitinib and carboplatin or pemetrexed was efficacious as a
first-line treatment for EGFR mutation-positive NSCLC
patients. Furthermore, Park et al (41) reported that afatinib significantly
improved the outcome in treatment-naive patients with EGFR
mutation-positive NSCLC compared with gefitinib, with a manageable
tolerability profile. Such therapies may be beneficial for patients
with the poor prognostic factors identified in the present study;
however, no research has been conducted in a clinical setting and
further systemic and clinical research is therefore warranted.
Furthermore, for patients with brain metastasis, combining
EGFR-TKIs and radiotherapy has potential synergistic effects;
radiation permeabilizes the blood-brain barrier and TKIs exhibit
radio-sensitizing effects (42).
The present study has certain limitations. First,
the retrospective design means that undefined biases may have
existed, which could have influenced the patients' clinical
outcomes. Second, the data collection and analysis was performed at
a single tertiary academic center, thus imposing a possible
selection bias.
To conclude, bone metastasis was associated with
reduced PFS time and brain metastasis was associated with reduced
OS time in NSCLC patients with EGFR mutations treated with
first-generation EGFR-TKIs. The number of metastatic organ sites
was also associated with a poorer PFS and OS.
Acknowledgements
The authors declare the following potential
conflicts of interest: S. Atagi has received personal fees from
Chugai Pharmaceutical, AstraZeneca, Taiho Pharmaceutical, Hisamitsu
Pharmaceutical Co, Bristol-Myers Squibb, Eli Lilly, Boehringer
Ingelheim; and has received grants from Chugai Pharmaceutical,
AstraZeneca, Pfizer, Merck Serono Co., Ltd., Taiho Pharmaceutical,
Yakult Pharmaceutical Industry, Eli Lilly, Ono Pharmaceutical and
Boehringer Ingelheim. A. Tamiya has received personal fees from
Chugai Pharmaceutical, Ono Pharmaceutical, Eli Lilly and Boehringer
Ingelheim.
Glossary
Abbreviations
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
CI
|
confidence interval
|
|
HR
|
hazard ratio
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PE
|
pleural effusion
|
|
PFS
|
progression-free survival
|
|
TKI
|
tyrosine kinase inhibitor
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nakagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm Cooperative Study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fukuoka M, Yano S, Giaccone G, Tamura T,
Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S,
Rischin D, et al: Multi-institutional randomized phase II trial of
gefitinib for previously treated patients with advanced
non-small-cell lung cancer (The IDEAL 1 Trial) [corrected]. J Clin
Oncol. 21:2237–2246. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kris MG, Natale RB, Herbst RS, Lynch TJ
Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H,
Sandler A, et al: Efficacy of gefitinib, an inhibitor of the
epidermal growth factor receptor tyrosine kinase, in symptomatic
patients with non-small cell lung cancer: A randomized trial. JAMA.
290:2149–2158. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim ES, Hirsh V, Mok T, Socinski MA,
Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, et al:
Gefitinib versus docetaxel in previously treated non-small-cell
lung cancer (INTEREST): A randomised phase III trial. Lancet.
372:1809–1818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Maruyama R, Nishiwaki Y, Tamura T,
Yamamoto N, Tsuboi M, Nakagawa K, Shinkai T, Negoro S, Imamura F,
Eguchi K, et al: Phase III study, V-15-32, of gefitinib versus
docetaxel in previously treated Japanese patients with
non-small-cell lung cancer. J Clin Oncol. 26:4244–4252. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I,
et al: Gefitinib or chemotherapy for non-small-cell lung cancer
with mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, et
al: Gefitinib versus cisplatin plus docetaxel in patients with
non-small-cell lung cancer harbouring mutations of the epidermal
growth factor receptor (WJTOG3405): An open label, randomised phase
3 trial. Lancet Oncol. 11:121–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ,
Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Erlotinib versus
chemotherapy as first-line treatment for patients with advanced
EGFR mutation-positive non-small-cell lung cancer (OPTIMAL,
CTONG-0802): A multicentre, open-label, randomised, phase 3 study.
Lancet Oncol. 12:735–742. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R,
Pallares C, Sanchez JM, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): A multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin JJ, Cardarella S, Lydon CA, Dahlberg
SE, Jackman DM, Jänne PA and Johnson BE: Five-Year Survival in
EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs.
J Thorac Oncol. 11:556–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Noronha V, Joshi A, Gokarn A, Sharma V,
Patil V, Janu A, Purandare N, Chougule A, Jambhekar N and Prabhash
K: The Importance of Brain Metastasis in EGFR Mutation Positive
NSCLC Patients. Chemother Res Pract. 2014:8561562014.PubMed/NCBI
|
|
15
|
Fujimoto D, Ueda H, Shimizu R, Kato R,
Otoshi T, Kawamura T, Tamai K, Shibata Y, Matsumoto T, Nagata K, et
al: Features and prognostic impact of distant metastasis in
patients with stage IV lung adenocarcinoma harboring EGFR
mutations: importance of bone metastasis. Clin Exp Metastasis.
31:543–551. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu KL, Tsai MJ, Yang CJ, Chang WA, Hung
JY, Yen CJ, Shen CH, Kuo TY, Lee JY, Chou SH, et al: Liver
metastasis predicts poorer prognosis in stage IV lung
adenocarcinoma patients receiving first-line gefitinib. Lung
Cancer. 88:187–194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu SG, Yu CJ, Tsai MF, Liao WY, Yang CH,
Jan IS, Yang PC and Shih JY: Survival of lung adenocarcinoma
patients with malignant pleural effusion. Eur Respir J.
41:1409–1418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JH, Kim TM, Keam B, Jeon YK, Lee SH,
Kim DW, Chung DH, Kim YT, Kim YW and Heo DS: Tumor burden is
predictive of survival in patients with non-small-cell lung cancer
and with activating epidermal growth factor receptor mutations who
receive gefitinib. Clin Lung Cancer. 14:383–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee JY, Lim SH, Kim M, Jung HA, Chang WJ,
Choi MK, Hong JY, Lee SJ, Sun JM, Ahn JS, et al: Is there any
predictor for clinical outcome in EGFR mutant NSCLC patients
treated with EGFR TKIs? Cancer Chemother Pharmacol. 73:1063–1070.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Travis WD, Giroux DJ, Chansky K, Crowley
J, Asamura H, Brambilla E, Jett J, Kennedy C, Rami-Porta R, Rusch
VW, et al: The IASLC Lung Cancer Staging Project: proposals for the
inclusion of broncho-pulmonary carcinoid tumors in the forthcoming
(seventh) edition of the TNM Classification for Lung Cancer. J
Thorac Oncol. 3:1213–1223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kimura H, Fujiwara Y, Sone T, Kunitoh H,
Tamura T, Kasahara K and Nishio K: High sensitivity detection of
epidermal growth factor receptor mutations in the pleural effusion
of non-small cell lung cancer patients. Cancer Sci. 97:642–648.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mast A and de Arruda M: Invader assay for
single-nucleotide polymorphism genotyping and gene copy number
evaluation. Methods Mol Biol. 335:173–186. 2006.PubMed/NCBI
|
|
24
|
D'Antonio C, Passaro A, Gori B, Del
Signore E, Migliorino MR, Ricciardi S, Fulvi A and de Marinis F:
Bone and brain metastasis in lung cancer: Recent advances in
therapeutic strategies. Ther Adv Med Oncol. 6:101–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoneda T and Hiraga T: Crosstalk between
cancer cells and bone microenvironment in bone metastasis. Biochem
Biophys Res Commun. 328:679–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rahmathulla G, Toms SA and Weil RJ: The
molecular biology of brain metastasis. J Oncol. 2012:7235412012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shin DY, Na II, Kim CH, Park S, Baek H and
Yang SH: EGFR mutation and brain metastasis in pulmonary
adenocarcinomas. J Thorac Oncol. 9:195–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim JE, Lee DH, Choi Y, Yoon DH, Kim SW,
Suh C and Lee JS: Epidermal growth factor receptor tyrosine kinase
inhibitors as a first-line therapy for never-smokers with
adenocarcinoma of the lung having asymptomatic synchronous brain
metastasis. Lung Cancer. 65:351–354. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Porta R, Sanchez-Torres JM, Paz-Ares L,
Massutí B, Reguart N, Mayo C, Lianes P, Queralt C, Guillem V,
Salinas P, et al: Brain metastases from lung cancer responding to
erlotinib: The importance of EGFR mutation. Eur Respir J.
37:624–631. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jamal-Hanjani M and Spicer J: Epidermal
growth factor receptor tyrosine kinase inhibitors in the treatment
of epidermal growth factor receptor-mutant non-small cell lung
cancer metastatic to the brain. Clin Cancer Res. 18:938–944. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park SJ, Kim HT, Lee DH, Kim KP, Kim SW,
Suh C and Lee JS: Efficacy of epidermal growth factor receptor
tyrosine kinase inhibitors for brain metastasis in non-small cell
lung cancer patients harboring either exon 19 or 21 mutation. Lung
Cancer. 77:556–560. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu YL, Zhou C, Cheng Y, Lu S, Chen GY,
Huang C, Huang YS, Yan HH, Ren S, Liu Y and Yang JJ: Erlotinib as
second-line treatment in patients with advanced non-small-cell lung
cancer and asymptomatic brain metastases: A phase II study
(CTONG-0803). Ann Oncol. 24:993–999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Togashi Y, Masago K, Masuda S, Mizuno T,
Fukudo M, Ikemi Y, Sakamori Y, Nagai H, Kim YH, Katsura T and
Mishima M: Cerebrospinal fluid concentration of gefitinib and
erlotinib in patients with non-small cell lung cancer. Cancer
Chemother Pharmacol. 70:399–405. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lim SH, Lee JY, Sun JM, Ahn JS, Park K and
Ahn MJ: Comparison of clinical outcomes following gefitinib and
erlotinib treatment in non-small-cell lung cancer patients
harboring an epidermal growth factor receptor mutation in either
exon 19 or 21. J Thorac Oncol. 9:506–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldie JH and Coldman AJ: The genetic
origin of drug resistance in neoplasms: Implications for systemic
therapy. Cancer Res. 44:3643–3653. 1984.PubMed/NCBI
|
|
37
|
Seto T, Kato T, Nishio M, Goto K, Atagi S,
Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al:
Erlotinib alone or with bevacizumab as first-line therapy in
patients with advanced non-squamous non-small-cell lung cancer
harbouring EGFR mutations (JO25567): An open-label, randomised,
multicentre, phase 2 study. Lancet Oncol. 15:1236–1244. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tamiya A, Tamiya M, Shiroyama T, Saijo N,
Nakatani T, Minomo S, Tsuji T, Takeuchi N, Omachi N, Kurata K, et
al: Phase II trial of carboplatin, S-1, and gefitinib as first-line
triplet chemotherapy for advanced non-small cell lung cancer
patients with activating epidermal growth factor receptor
mutations. Med Oncol. 32:402015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kanda S, Horinouchi H, Fujiwara Y,
Nokihara H, Yamamoto N, Sekine I, Kunitoh H, Kubota K, Tamura T and
Ohe Y: Cytotoxic chemotherapy may overcome the development of
acquired resistance to epidermal growth factor receptor tyrosine
kinase inhibitors (EGFR-TKIs) therapy. Lung Cancer. 89:287–293.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sugawara S, Oizumi S, Minato K, Harada T,
Inoue A, Fujita Y, Maemondo M, Yoshizawa H, Ito K, Gemma A, et al:
Randomized phase II study of concurrent versus sequential
alternating gefitinib and chemotherapy in previously untreated
non-small cell lung cancer with sensitive EGFR mutations:
NEJ005/TCOG0902. Ann Oncol. 26:888–894. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Park K, Tan EH, O'Byrne K, et al: Afatinib
versus gefitinib as first-line treatment of patients with EGFR
mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase
2B, open-label, randomised controlled trial. Lancet Oncol.
17:577–589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dempke WC, Edvardsen K, Lu S, Reinmuth N,
Reck M and Inoue A: Brain Metastases in NSCLC-are TKIs changing the
treatment strategy? Anticancer Res. 35:5797–5806. 2015.PubMed/NCBI
|