Introduction
Esophageal cancer (EC) is one of the leading causes
of mortality worldwide due to the majority of patients presenting
with the advanced stage of the disease at the time of diagnosis and
the high incidence of early recurrence following treatment
(1). Surgical resection is one of the
primary components of multimodality treatment, which unavoidably
reduces the quality of life, particularly due to complications,
including anastomotic leakage and stenosis (2). Even with the advancement in treatment
options, the 5-year survival rate of EC remains low (3,4). As a
result, there is a requirement for an effective biomarker for early
detection, the development of targeted anticancer therapies and
prognostication. Furthermore, a more detailed knowledge of the
signaling pathways associated with tumor malignancy would aid in
understanding tumor biology and individualizing treatment.
Depression is one of the common psychological
disorders observed in patients with cancer, with its prevalence
varying between 7.2 and 25.7% in patients with the advanced stage
(5,6).
Depression in EC not only reduces the quality of life, but also
shortens survival time and prolongs hospitalization (7,8). However,
in the majority of cases, depression remains undiagnosed and
untreated. The mechanism involved in the development of depression
remains unknown (9). Nowadays,
although several genes involved in the occurrence and prognosis of
depression are known, further information is warranted (10).
The p38 mitogen-activated protein kinase (MAPK)
signaling pathway can be activated by dual phosphorylation mediated
primarily by MAPK kinase 3 (MKK3) and MKK6 in response to a range
of cell stresses, and inflammatory cytokines (11). p38-MAPK signaling has been implicated
in the regulation of processes that lead to the development and
progression of a variety of cancer types (12,13).
Furthermore, in certain other tumor types, the inhibition of p38
enhances sensitivity to chemotherapy (14,15),
suggesting that p38 may serve as an oncogene in cancer progression.
However, the function of p38 in esophageal cancer remains unclear
(16,17). Zhong et al (18) reported that p38/p21 participates in
obatoclax-induced G1/G0 arrest in esophageal cancer in vitro
independent of extracellular regulated kinase 1/2. However, whether
or not p38 is able to regulate other biological effects remains
unknown. In the present study, the role of p38 MAPK in the
development and progression of esophageal cancer was investigated
in vitro in order to determine its clinical
significance.
Several studies have demonstrated that indoleamine
2,3-dioxygenase (IDO) (19,20), a well-known depression-associated
gene, can be upregulated by interferons and lipopolysaccharides
(LPS) via the p38 MAPK signaling pathway (21–23). In
the present study, whether or not p38 MAPK can increase the
expression of IDO directly and aggravate clinical depression in
esophageal cancer, was also examined.
Materials and methods
Tissue samples
A total of 228 surgically resected, unifocal,
primary human esophageal cancer tumor samples (age range, 37–78;
177 male and 51 female) were prospectively collected from The
Oncology Surgery Department of The First Affiliated Hospital of
Xi'an Jiaotong University (Xi'an, China) between January 2005 and
August 2009. The present study was approved by the Ethical Conduct
in Human Research Committee of the First Affiliated Hospital of
Xi'an Jiaotong University, and all 228 patients signed written
informed consent forms. All 228 tissue samples with matched distant
noncancerous esophageal tissue samples were histologically
diagnosed. Samples were snap-frozen in liquid nitrogen following
resection and stored in liquid nitrogen until use. Tumor stages and
histological grades were recorded using the classification
guidelines of the American Joint Committee on Cancer 7 (24). Among these 228 patients, 214 patients
with esophageal cancer were surgically treated with the same
therapeutic strategy; that is, complete tumor resection with
negative margins (R0 resection) and extensive lymphadenectomy. The
remaining 14 cases were at stage IV of the disease and underwent
palliative surgery. Furthermore, following surgery all patients
received postoperative chemotherapy with 5-fluorouracil (5-FU; 425
mg/m2 5-FU daily on days 1–5, cycled every 28 days),
leucovorin (20 mg/m2 of leucovorin on days 1–5), and
45–50 Gy of radiotherapy (1.8 Gy/day). Patients with only
palliative surgery or recurrent disease were treated with ECF
adjunctive treatment (50 mg/m2 of Epirubicin on day 1,
60 mg/m2 of cisplatin on day 1, 200 mg/m2 of
5-FU continuous infusion over 24 h daily on days 1–21 for three
cycles). All patients were followed up on a regular basis for
>60 months or until mortality. The last follow-up was conducted
in March 2015.
Immunohistochemistry
All tissue specimens were fixed in 10% buffered
formalin at 37°C for 12 h and embedded in paraffin for preparation
of consecutive 4-µm thick sections. For immunohistochemistry,
tissue sections were first deparaffinized and rehydrated, subjected
to an antigen retrieval treatment with a pressure cooker, and
incubated with 10% normal goat serum (Qcbio S&T Co., Ltd.,
Shanghai, China) at room temperature for 30 min to block potential
non-specific binding of the secondary antibody. Sections were then
incubated with a primary mouse anti-human anti-phosphorylated
(p)-p38 antibody (cat. no. H00001432-M01, 1:2,500; Abnova, Walnut,
CA, USA) at 4°C overnight. On the following day, the sections were
washed with PBS and further incubated with a secondary goat
anti-mouse IgG at a dilution of 1:200 (cat. no. A-11029; Pierce;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 30 min at
room temperature and with 3,3′-diaminobenzidine for 15 min at room
temperature as a chromogen. The sections were finally
counterstained with hematoxylin solution for 5 min at room
temperature, dehydrated and covered with coverslips. All stained
sections were independently evaluated by two investigators and
agreement was reached following careful discussion, if
discrepancies occurred. P-p38 was scored by the multiplication of
the percentage of positive tumor cells and staining intensity as
described in our previous study (25). The percentage of positive cells was
scored as: 0, 0–15%; 1+, 16–30%; 2+, 31–60%; and 3+, 61–100%.
Intensity of staining was graded as follows: Grade 0, negative; 1+,
weak positive; 2+, moderate positive; and 3+, strong positive
(24). According to these
immunohistochemical scores, p-p38 expression was then divided into
two groups: Negative or weak p-p38 expression (score ≤3); and
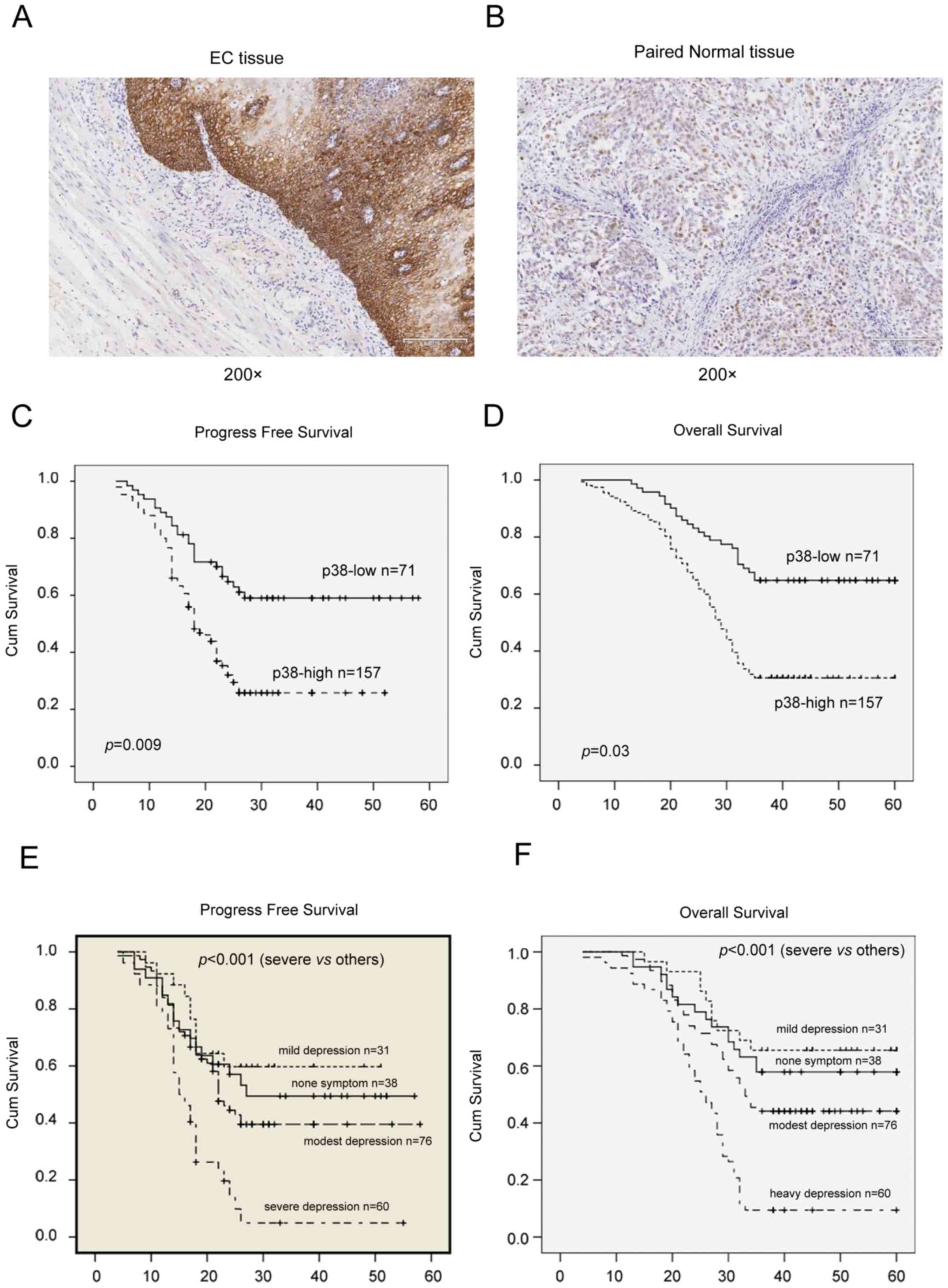
positive or strong p-p38 expression (score ≥4; Fig. 1). Images of the stained sections were
obtained using a light microscope (BX51) equipped with a digital
camera (PD71; Olympus Corporation, Tokyo, Japan).
Cell lines, culture, gene transfection
and treatment
Human esophageal cancer Eca-109 and TE-1 cell lines
were obtained from The Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% heat-inactivated fetal bovine serum (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml of
penicillin and 100 mg/ml of streptomycin (both from Gibco; Thermo
Fisher Scientific, Inc.) at 37°C in a 5% CO2 incubator. In order to
manipulate gene expression in cells, plasmids carrying p-p38 cDNA
were separately transfected into these two esophageal cancer cell
lines using X-treme HP (Roche Applied Science, Mannheim, Germany),
according to manufacturer's instructions. After 48 h of
transfection at 37°C, G418-sulfate (700 ng/µl for Eca-109 and 800
ng/µl for TE-1; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was
added to the cell culture medium throughout the experiment. Cells
were fed three times weekly and periodically assessed by western
blotting to ensure transgene expression. Thalidomide (25 mg/tablet;
ChangZhouZhiYao Jiangsu, China) was ground, dissolved, filtered and
adjusted to 2,000 mM with RPMI-1640 medium. Prior to treatment, the
thalidomide was diluted into 200 mM, and incubated for ≤72 h prior
to testing. SB203580 (S8307; Sigma-Aldrich; Merck KGaA) was diluted
using dimethyl sulfoxide into 1,000 µg/ml and further diluted into
0.05 µg/ml using RPMI-1640 medium prior to cell p38 blocking
incubation for 8 h.
Protein extraction and western blot
analysis
Cells were lysed with modified RIPA buffer (50 mM of
Tris, 150 mM of NaCl, 1% Triton X-100, 1% sodium deoxycholate, and
0.1% SDS) containing 25 µg/ml of leupeptin, 1 mM of sodium
orthovanadate, 2 mM of EDTA and 1 mM of phenylmethylsulfonyl
fluoride. The concentration of the protein samples was determined
using a BCA kit (Beyotime Institute of Biotechnology, Beijing,
China). A total of 20 µg of each protein sample was loaded onto an
8% SDS-PAGE gel, electrophoresed and blotted onto a polyvinylidene
difluoride membrane (Sigma-Aldrich; Merck KGaA). The membranes were
blotted with the first antibody (anti-p-p38) overnight at 4°C (cat.
no. AM063; Beyotime, Zhejiang, China), anti-IDO (cat. no.
H00003620-B01P; Abnova, Walnut, CA, USA), anti-Ku80 (cat. no.
BS2692; Bioworld Technology, Inc., St. Louis Park, MN, USA) and
anti-β-actin antibody (cat. no. MAB12983; Abnova) and with a
secondary antibody at room temperature for 1 h. Immunoreactivity
was detected using an enhanced chemiluminescence system (Xi'an
Jiaotong University) and normalized to β-actin.
Cell viability assay
Cell viability was assessed using an MTT assay.
Cells with stably transfected p-p38 and 48 h of transfection with
plasmid were seeded into 96-well plates at a density of
3×103 cells/well, and treated with or without
thalidomide at 0.2 mg/l for ≤3 days. At the end of the experiments,
20 µl of MTT (0.5 mg/ml in PBS) were added to the cells, incubated
for 4 h at 37°C, the growth medium was discarded, and 100 µl of
DMSO was added into each well. Following agitation for 10 min at
37°C on a shaker, the absorbance rate was read at 550 nm with a
scanning microtiter (PerkinElmer, Inc., Waltham, MA, USA). Data was
summarized as the percentage of control compared with untreated
cells.
Cell apoptosis analyses
Apoptosis in these cells treated with or without
thalidomide was evaluated using flow cytometric techniques using an
Annexin V-FLUOS Staining kit (Roche Applied Science), according to
manufacturer's instructions.
ELISA test
ELISA kits (Applygen Technologies, Inc., Beijing,
China) were used in order to test glucose consumption between
parental esophageal cancer cells and p38-transfected ones. Glucose
levels were determined in the supernatant of the medium at 0, 24,
48 and 72 h. Glucose levels were determined using the glucose
oxidase method (26), according to
manufacturer's instructions. Furthermore, 2-deoxy-D-glucose (2-DG;
10 mM) was used to block glucose metabolism (16,27) and
the dependence to glucose consumption was analyzed.
Statistical analysis
The χ2 test was performed to compare the
expression of p-p38 with clinicopathological data from patients.
Then, Spearman and logistic regression analyses were utilized to
compare the expression of p-p38 between tumor and adjacent normal
tissues. Kaplan-Meier curve and logical rank test were used to
analyze survival data of the patients. All in vitro data are
presented as the mean of ≥3 individual experiments ± standard error
of the mean, which was statistically analyzed using the Student's
t-test. All statistical analyses were conducted using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Protein expression of p38 is
associated with poor clinical outcome of esophageal cancer
patients
In the present study, the protein expression of p38
was detected in a total of 228 patients with esophageal cancer, and
the corresponding normal tissues were analyzed using
immunohistochemistry. It was identified that the protein expression
of p38 was significantly upregulated in esophageal cancer tissues,
compared with paired non-cancerous tissues (Fig. 1); that is, p38 protein was expressed
in 157/228 (68.9%) esophageal cancer tissues, whereas 35/228
(15.4%) matched adjacent non-cancer tissues were p38-positive
(P<0.01). Furthermore, p38 expression was identified to be
significantly associated with tumor invasion (P=0.032), lymph nodes
metastasis (P=0.048), tumor recurrence (P=0.003), depression
(P=0.001) and overall survival (P<0.01) of patients with
esophageal cancer (Table I). In
addition, multivariate analysis revealed that p38 was associated
with overall survival and depression (Table I).
 | Table I.Associations between p38 expression
and clinicopathological features from patients with esophageal
cancer (n=228). |
Table I.
Associations between p38 expression
and clinicopathological features from patients with esophageal
cancer (n=228).
| Variables | No. of cases | p-p38-positive | P-value
(univariate) | P-value
(multivariate) | Risk ratio (95%
CI) |
|---|
| Age (years) |
|
| 0.116 |
|
|
|
≤59 | 134 | 86 (64.1) |
|
|
|
|
>59 | 94 | 71 (75.5) |
|
|
|
| Sex |
|
| 0.08 |
|
|
|
Male | 177 | 127 (71.8) |
|
|
|
|
Female | 51 | 30 (58.8) |
|
|
|
| Histological
types |
|
| 0.558 |
|
|
|
Squamous | 206 | 142 (68.9) |
|
|
|
|
Adenocarcinoma | 22 | 15 (68.2) |
|
|
|
| Invasion |
|
| 0.032 | 0.171 |
|
| T1 | 22 | 12 (54.5) |
|
|
|
| T2 | 31 | 18 (58.1) |
|
|
|
| T3 | 131 | 92 (70.2) |
|
|
|
| T4 | 38 | 33 (86.8) |
|
|
|
| Lymph node |
|
| 0.048 | 0.955 |
|
| N0 | 127 | 83 (65.4) |
|
|
|
| N1 | 83 | 59 (71.1) |
|
|
|
| N2 | 18 | 15 (83.3) |
|
|
|
| Metastasis |
|
| 0.024 | 0.086 |
|
|
Negative | 212 | 144 (67.9) |
|
|
|
|
Positive | 16 | 13 (81.2) |
|
|
|
| Depression
status |
|
| 0.001 | 0.040 | 2.382
(0.503–2.978) |
|
None | 38 | 16 (42.1) |
|
|
|
|
Mild | 31 | 15 (48.4) |
|
|
|
|
Modest | 76 | 59 (77.6) |
|
|
|
|
Severe | 60 | 47 (78.3) |
|
|
|
| Chemotherapy |
|
| 0.323 |
|
|
| CR | 16 | 12 (75.0) |
|
|
|
| PR | 64 | 43 (67.2) |
|
|
|
| SD | 40 | 27 (67.5) |
|
|
|
| PD | 70 | 57 (81.4) |
|
|
|
| Recurrence |
|
| 0.003 | 0.38 |
|
| OS |
|
| <0.001 | <0.001 | 0.903
(0.029–12.623) |
Kaplan-Meier curve analysis was performed, and the
data revealed that p38 expression was significantly associated with
poor overall (P=0.03) and disease-free (P=0.009) survival rates for
patients with esophageal cancer. In addition, survival was affected
by metastasis (P=0.001), depression (P=0.002) and chemotherapy
(P<0.01) based on log-rank survival analysis (Tables II and III). Multivariate stepwise logistic
regression analyses revealed that the protein expression of p38,
tumor invasion and depression were all independent predictors for
the survival time of patients.
 | Table II.Log-rank and Cox proportional hazards
regression model of prognostic variables for progress free survival
of patients with esophageal cancer. |
Table II.
Log-rank and Cox proportional hazards
regression model of prognostic variables for progress free survival
of patients with esophageal cancer.
| Prognostic
variable | P-value
(univariate) | P-value
(multivariate) | Risk ratio (95%
CI) |
|---|
| Age (years) | <0.001 | 0.376 |
|
| Sex | 0.243 |
|
|
| Stage (IA-IV) | <0.001 | 0.264 |
|
| p38 | <0.001 | 0.008 | 1.534
(0.256–6.814) |
| T | <0.001 | 0.001 | 1.186
(0.359–4.886) |
| N | <0.001 | 0.914 |
|
| M | <0.001 | 0.025 | 4.245
(0.158–9.617) |
| Depression
status | <0.001 | 0.056 |
|
|
Mild |
| 0.327 |
|
|
Modest |
| 0.045 | 0.433
(0.361–5.377) |
|
Severe |
| 0.001 | 0.455
(0.216–13.365) |
| Chemotherapy | 0.01 | 0.497 |
|
 | Table III.Log-Rank and Cox proportional hazards
regression model of prognostic variables for overall survival of
patients with esophageal cancer. |
Table III.
Log-Rank and Cox proportional hazards
regression model of prognostic variables for overall survival of
patients with esophageal cancer.
| Prognostic
variable | P-value
(univariate) | P-value
(multivariate) | Risk ratio (95%
CI) |
|---|
| Age (years) | 0.001 | 0.273 |
|
| Sex | 0.433 |
|
|
| Stage (IA-IV) | <0.01 | 0.129 |
|
| p38 | <0.01 | 0.03 | 1.677
(0.238–4.717) |
| T | <0.01 | 0.212 |
|
| N | <0.01 | 0.754 |
|
| M | <0.01 | 0.001 | 3.66
(0.406–10.192) |
| Depression
status | <0.01 | 0.002 |
|
|
Mild |
| 0.128 |
|
|
Modest |
| 0.02 | 0.433
(0.361–5.377) |
|
Severe |
| <0.01 | 0.455
(0.216–13.365) |
| Chemotherapy | <0.01 | <0.01 | 1.551
(0.106–17.219) |
Transfection of p38 increases cell
proliferation and glucose dependence in esophageal cancer cell
lines Eca-109, and TE-1
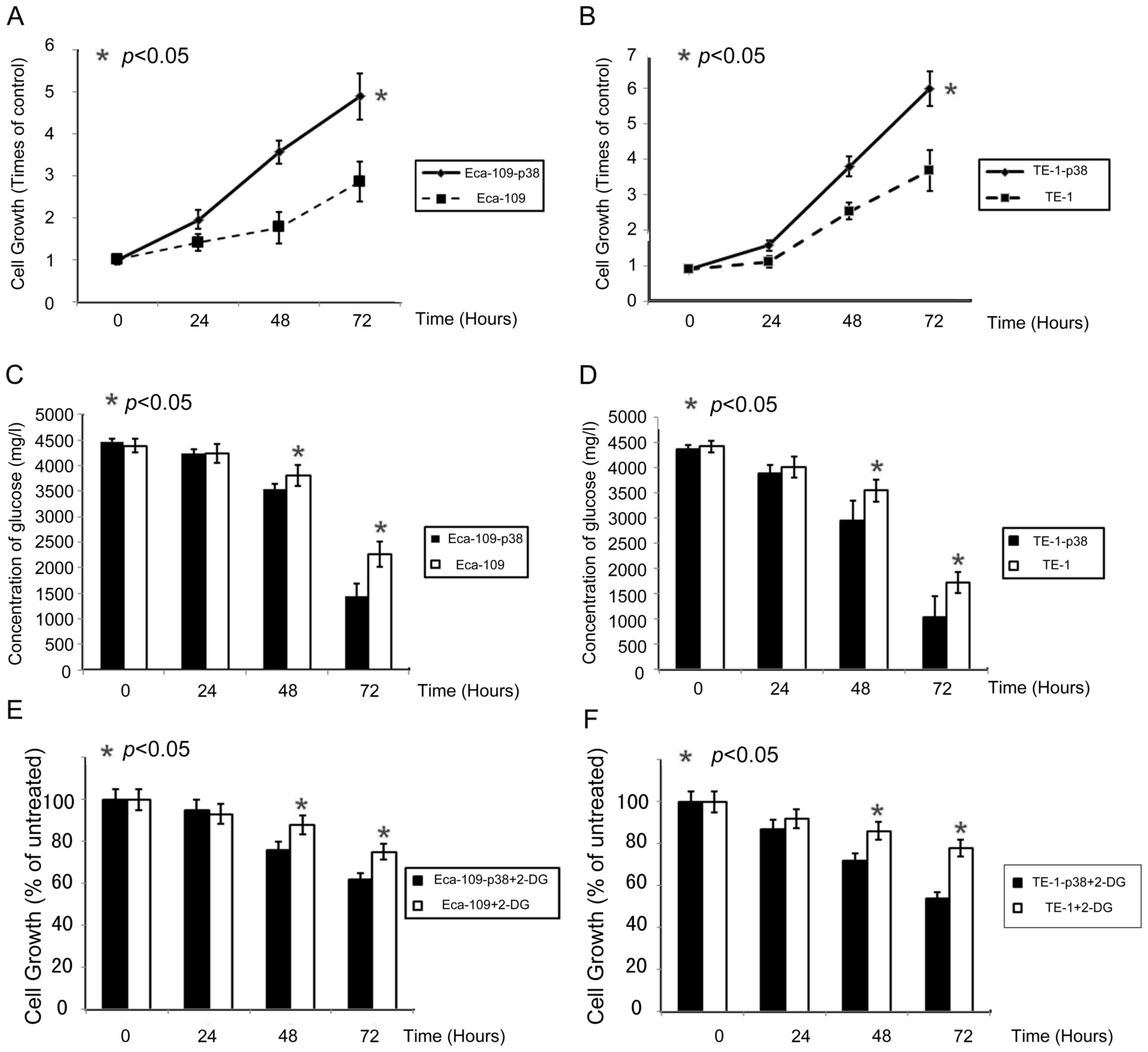
As previously reported (18), p38 can enhance cell proliferation in
esophageal cancer cell lines. In order to investigate the potential
of p38 to enhance cell growth in esophageal cancer cells (Eca-109
and TE-1), cell viability was measured using an MTT assay following
transfection of the p38 gene. Elevated p38 expression increased
Eca-109 and TE-1 proliferation in a time-dependent manner. p38 had
a definite effect on esophageal cancer cell growth. With the
upregulation of p38, cell growth was promoted at a statistically
significant level on days two and three (Fig. 2A and B).
Cells with elevated p38 exhibited a significant
increase in glucose consumption (Fig. 2C
and D). This indicates that p38 may influence cell glycolysis
in esophageal cancer cells directly. Growth of Eca-109 and TE-1 was
inhibited by incubation with 2-DG in a time-dependent manner
(Fig. 2E and F); the growth of cells
with elevated p38 expression was inhibited by 34 and 42% using 2-DG
at 72 h. Parental Eca-109 and TE-1 cells (in which p38 was normal)
were significantly less sensitive to 2-DG compared with cells with
enhanced p38 expression.
Increased p38 resist esophageal cancer
cells to thalidomide-induced cell death
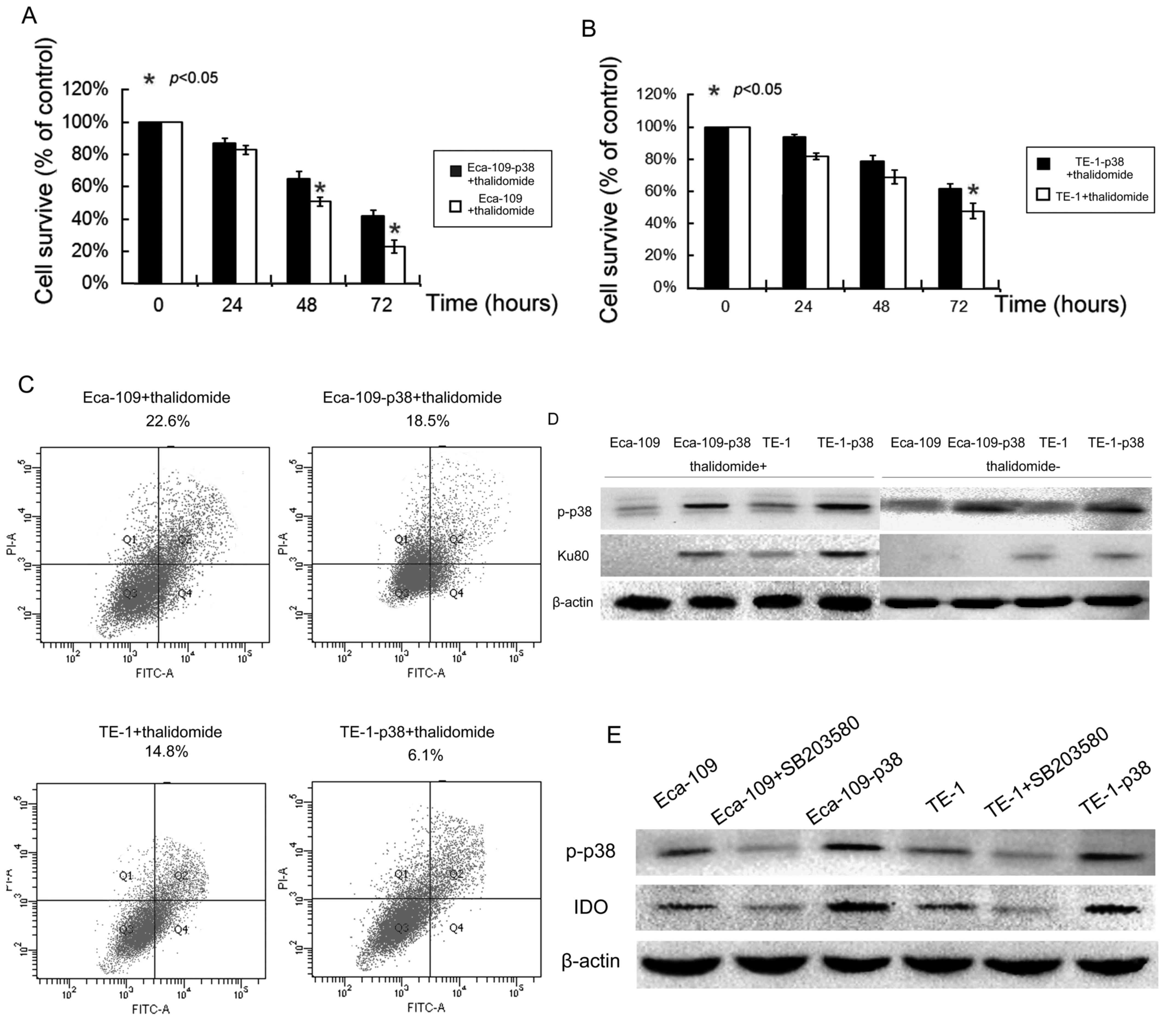
Since p38 protein is able to regulate chemotherapy
treatment (26), whether or not p38
expression can affect esophageal cancer cell response to
thalidomide treatment was determined. Following the transfection of
p38 protein in esophageal cancer cells, treatment 200 mM of
thalidomide for 72 h decreased the viability of parental esophageal
cancer cells. The upregulation of p38 in esophageal cancer cells
significantly reduced the decrease in cell viability caused by
thalidomide treatment (P<0.05; Fig. 3A
and B).
Furthermore, the tumor cell apoptosis assay revealed
that the average apoptotic cell fraction markedly decreased in p38
overexpression cells compared with that in parental cells (Fig. 3C). Following treatment with
thalidomide for 24 h, cells with elevated p38 expression were
associated with a decrease in apoptosis in Eca-109 (81.9%) and TE-1
(41.2%) cells, respectively, compared with parental controls. This
finding suggests that p38 serves a role in tumor cell resistance to
thalidomide-induced apoptosis.
In order to discover the mechanism involved in
p38-induced resistance, Ku autoantigen 80 kDa (Ku80) was detected
and recruited following DNA fraction. After 48 h of incubation,
Ku80 in normal cells was markedly lower compared with in
p38-transfected Eca-109 and TE-1 cells (Fig. 3D), although there was no observable
difference prior to treatment with thalidomide.
P38 expression is associated with
higher IDO levels and depression
Coltella et al (15) reported that p38 was essential in
inducing the expression of IDO, a depression associated gene. In
order to investigate whether or not p38 alone could increase IDO
expression in esophageal cancer cells, western blotting in Eca-109
and TE-1 cells were performed without the addition of LPS. Results
revealed that IDO expression in Eca-109 and TE-1 was markedly
upregulated by p38 transfection compared with in parental cell
lines (Fig. 3E). Blocking p38 using
SB203580 markedly reduced IDO expression compared with parental
cells (Fig. 3E).
In order to determine whether or not the IDO gene
was associated with depression, the medical record of 228
esophageal cancer patients were reviewed and the Zung Self-Rating
Depression Scale was used to assess the status of depression. The
depression status of 205 patients could be retrieved from patients
or their relatives. From the data obtained, it was identified that
167 patients (81.5%) had depression. Based on the analysis, p38
expression was identified to be associated with depression (P=0.04;
Table I). Logistic regression
analyses revealed that recurrence was associated with poor overall
(P=0.002) and disease-free (P=0.005) survival rates for patients
with esophageal cancer (Table II;
Fig. 1E and F).
Discussion
In the present study, it was demonstrated that p38
MAPK predicts poor prognosis in esophageal cancer and increases IDO
expression independent of LPS. It was also revealed that p38 MAPK
mediates cell proliferation, contributes to the development of
thalidomide resistance in vitro, enhances cellular
dependence on glucose, and increases p38-MAPK upregulated IDO gene
expression, which is associated with depressive symptoms, as
confirmed by multiple studies (28,29).
Recent studies of esophageal cancer on p38-MAPK
remain limited. Zheng et al (30) reported that in cancer cells in
vitro, p38 serves as an oncogene that promotes cell growth;
while at a stage prior to carcinogenesis, it suppress tumor growth.
Another study focusing on cell cycle revealed that p38 participates
in cancer G1/G0-phase arrest induced by obatoclax (18). In other cancer types, p38-MAPK also
served as a paradox; in which certain results demonstrated cancer
promoting, and others revealed suppressive functions of p38
(31). The results of the present
study in cancer tissue and cells confirmed the findings of the
aforementioned studies. In addition, the use of thalidomide was
assessed in order to assess the possibility of using this
anti-angiogenesis agent as an anticancer drug for the treatment of
esophageal cancer (32). The results
of the present study indicated that thalidomide was able to induce
apoptosis in esophageal cancer cell types Eca-109 and TE-1,
although its mechanism remains to be elucidated.
Signaling studies on depressive symptoms revealed
that the expression of IDO or decreased MAP kinase phosphatase 1 in
the hippocampus contributes to depression (33). In the present in vitro study,
it was demonstrated that p38-MAPK was not only an independent
predictor of poor outcome in esophageal cancer, but also aggravates
cancer-associated depression by enhancing IDO expression. In fact,
almost half of the patients enrolled in the study were not informed
that they suffered from esophageal cancer, as requested by their
relatives, who in these cases held power of attorney and therefore
medical rights over the patient. This may suggest that cancer
itself may trigger or aggravate depressive symptoms in these
patients, whether they have knowledge of the disease or not. In
addition, the findings of the present study suggest that patients
with increased p38 or IDO expression are prone to develop
depression and likely to require antidepressants. For those with
decreased IDO, a placebo effect may exist to a certain extent,
which requires further research (34).
However, it should be noted that the present study
was retrospective in nature. More detailed medical records and
follow-ups are required to understand the psychological status of
these patients. In addition, all factors that contribute to
cancer-associated depression were not included. In fact, a change
in eating habits following a surgical operation or forbidding a
patient to smoke is enough for a patient with a heavy addiction to
exhibit depressive symptoms, as well as surgical complications.
These problems could be resolved with detailed clinical history, in
which more cases would be enrolled in our next study.
Notwithstanding its limitations, the present study does suggest the
significance of esophageal-associated depressive symptoms, which
may be induced by p38 and IDO expression. Furthermore, this study
sheds new light on the role of p38 and its potential application in
the clinic. Further in vitro and in vivo studies are
required to determine the association between p38, IDO, and
esophageal cancer in depression.
Acknowledgements
The present study was supported by the National
Natural Scientific Foundation of China (grant no. 81501826).
References
|
1
|
Rubenstein JH and Shaheen NJ:
Epidemiology, diagnosis, and management of esophageal
adenocarcinoma. Gastroenterology. 149:302–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mortensen MB: Avoiding complications in
esophageal cancer surgery. Minerva Chir. 68:341–352.
2013.PubMed/NCBI
|
|
3
|
Brescia AA, Broderick SR, Crabtree TD,
Puri V, Musick JF, Bell JM, Kreisel D, Krupnick AS, Patterson GA
and Meyers BF: Adjuvant therapy for positive nodes after induction
therapy and resection of esophageal cancer. Ann Thorac Surg.
101:200–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pozza A, Erroi FR, Scarpa M, Polese L,
Rampazzo L and Norberto L: Palliative therapy for esophageal
cancer: Laser therapy alone is associated with a better functional
outcome. Updates Surg. 67:61–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson KG, Chochinov HM, Skirko MG, Allard
P, Chary S, Gagnon PR, Macmillan K, De Luca M, O'Shea F, Kuhl D, et
al: Depression and anxiety disorders in palliative cancer care. J
Pain Symptom Manage. 33:118–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grabsch B, Clarke DM, Love A, McKenzie DP,
Snyder RD, Bloch S, Smith G and Kissane DW: Psychological morbidity
and quality of life in women with advanced breast cancer: A
cross-sectional survey. Palliat Support Care. 4:47–56. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pelletier G, Verhoef MJ, Khatri N and
Hagen N: Quality of life in brain tumor patients: The relative
contributions of depression, fatigue, emotional distress, and
existential issues. J Neurooncol. 57:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Breitbart W: Identifying patients at risk
for, and treatment of major psychiatric complications of cancer.
Support Care Cancer. 3:45–60. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Irving G and Lloyd-Williams M: Depression
in advanced cancer. Eur J Oncol Nurs. 14:395–399. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kadan-Lottick NS, Vanderwerker LC, Block
SD, Zhang B and Prigerson HG: Psychiatric disorders and mental
health service use in patients with advanced cancer: A report from
the coping with cancer study. Cancer. 104:2872–2881. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar B, Koul S, Petersen J, Khandrika L,
Hwa JS, Meacham RB, Wilson S and Koul HK: p38 mitogen-activated
protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer
by modulation of MMP-2 and MMP-9 activity. Cancer Res. 70:832–841.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiong S, Grijalva R, Zhang L, Nguyen NT,
Pisters PW, Pollock RE and Yu D: Up-regulation of vascular
endothelial growth factor in breast cancer cells by the
heregulin-beta1-activated p38 signaling pathway enhances
endothelial cell migration. Cancer Res. 61:1727–1732.
2001.PubMed/NCBI
|
|
13
|
Kumar V, Behera R, Lohite K, Karnik S and
Kundu GC: p38 kinase is crucial for osteopontin-induced furin
expression that supports cervical cancer progression. Cancer Res.
70:10381–10391. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo X, Ma N, Wang J, Song J, Bu X, Cheng
Y, Sun K, Xiong H, Jiang G, Zhang B, et al: Increased p38-MAPK is
responsible for chemotherapy resistance in human gastric cancer
cells. BMC Cancer. 8:3752008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coltella N, Rasola A, Nano E, Bardella C,
Fassetta M, Filigheddu N, Graziani A, Comoglio PM and Di Renzo MF:
p38 MAPK turns hepatocyte growth factor to a death signal that
commits ovarian cancer cells to chemotherapy-induced apoptosis. Int
J Cancer. 118:2981–2990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Looby E, Abdel-Latif MM, Athié-Morales V,
Duggan S, Long A and Kelleher D: Deoxycholate induces COX-2
expression via Erk1/2-, p38-MAPK and AP-1-dependent mechanisms in
esophageal cancer cells. BMC Cancer. 9:1902009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yousif NG, Al-Amran FG, Hadi N, Lee J and
Adrienne J: Expression of IL-32 modulates NF-κB and p38 MAP kinase
pathways in human esophageal cancer. Cytokine. 61:223–227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong D, Gu C, Shi L, Xun T, Li X, Liu S
and Yu L: Obatoclax induces G1/G0-phase arrest via
p38/p21(waf1/Cip1) signaling pathway in human esophageal cancer
cells. J Cell Biochem. 115:1624–1635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zoga M, Oulis P, Chatzipanagiotou S,
Masdrakis VG, Pliatsika P, Boufidou F, Foteli S, Soldatos CR,
Nikolaou C and Papageorgiou C: Indoleamine 2,3-dioxygenase and
immune changes under antidepressive treatment in major depression
in females. In Vivo. 28:633–638. 2014.PubMed/NCBI
|
|
20
|
Zhou W, Dantzer R, Budac DP, Walker AK,
Mao-Ying QL, Lee AW, Heijnen CJ and Kavelaars A: Peripheral
indoleamine 2,3-dioxygenase 1 is required for comorbid
depression-like behavior but does not contribute to neuropathic
pain in mice. Brain Behav Immun. 46:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu WL, Lin YH, Xiao H, Xing S, Chen H,
Chi PD and Zhang G: Epstein-Barr virus infection induces
indoleamine 2,3-dioxygenase expression in human monocyte-derived
macrophages through p38/mitogen-activated protein kinase and NF-κB
pathways: Impairment in T cell functions. J Virol. 88:6660–6671.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujigaki H, Saito K, Fujigaki S, Takemura
M, Sudo K, Ishiguro H and Seishima M: The signal transducer and
activator of transcription 1alpha and interferon regulatory factor
1 are not essential for the induction of indoleamine
2,3-dioxygenase by lipopolysaccharide: Involvement of p38
mitogen-activated protein kinase and nuclear factor-kappaB
pathways, and synergistic effect of several proinflammatory
cytokines. J Biochem. 139:655–662. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fu X, Lawson MA, Kelley KW and Dantzer R:
HIV-1 Tat activates indoleamine 2,3 dioxygenase in murine
organotypic hippocampal slice cultures in a p38 mitogen-activated
protein kinase-dependent manner. J Neuroinflammation. 8:882011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nomura M, Shitara K, Kodaira T, Hatooka S,
Mizota A, Kondoh C, Yokota T, Takahari D, Ura T and Muro K:
Prognostic impact of the 6th and 7th American Joint Committee on
Cancer TNM staging systems on esophageal cancer patients treated
with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 82:946–952.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng Y, Li K, Diao D, Zhu K, Shi L, Zhang
H, Yuan D, Guo Q, Wu X, Liu D and Dang C: Expression of KIAA0101
protein is associated with poor survival of esophageal cancer
patients and resistance to cisplatin treatment in vitro. Lab
Invest. 93:1276–1287. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burrin JM and Price CP: Performance of
three enzymic methods for filter paper glucose determination. Ann
Clin Biochem. 21:411–416. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng Y, Diao D, Zhang H, Guo Q, Wu X,
Song Y and Dang C: High glucose-induced resistance to
5-fluorouracil in pancreatic cancer cells alleviated by
2-deoxy-D-glucose. Biomed Rep. 2:188–192. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wichers MC and Maes M: The role of
indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of
interferon-alpha-induced depression. J Psychiatry Neurosci.
29:11–17. 2004.PubMed/NCBI
|
|
29
|
Kohl C and Sperner-Unterweger B: IDO and
clinical conditions associated with depressive symptoms. Curr Drug
Metab. 8:283–287. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng ST, Zhang CS, Qin X, Gen YH, Liu T,
Sheyhidin I and Lu XM: The status of phosphorylated p38 in
esophageal squamous cell carcinoma. Mol Biol Rep. 39:5315–5321.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pal A, Huang W, Li X, Toy KA,
Nikolovska-Coleska Z and Kleer CG: CCN6 modulates BMP signaling via
the Smad-independent TAK1/p38 pathway, acting to suppress
metastasis of breast cancer. Cancer Res. 72:4818–4828. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu JP, Sun SP, Sun ZQ, Ni XC, Wang J, Li
Y, Hu LJ and Li DQ: Clinical trial of thalidomide combined with
radiotherapy in patients with esophageal cancer. World J
Gastroenterol. 20:5098–5103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun YR, Wang XY, Li SS, Dong HY and Zhang
XJ: β-asarone from Acorus gramineus alleviates depression by
modulating MKP-1. Genet Mol Res. 14:4495–4504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cuijpers P and Cristea IA: What if a
placebo effect explained all the activity of depression treatments?
World Psychiatry. 14:310–311. 2015. View Article : Google Scholar : PubMed/NCBI
|