Introduction
Multiple clinical studies have reported on the
factors involved in the development of oral tongue squamous cell
carcinoma (OTSCC), which include unsuitable tooth fillings or
prosthesis placement, smoking, alcohol consumption, inflammation,
precancerous lesions such as leukoplakia, infection, endocrine
disease, poor oral hygiene and heredity (1–4). Fan et
al (2) revealed that mechanical
trauma and galvanic phenomena, as a result of dental prosthesis
placement, may also have a major role in the etiology of OTSCC
(2), whereas Hougeir et al
(5) demonstrated that contact allergy
to metal dental restorations is a risk factor for OTSCC development
(5). However, in young and young
mature patients, the duration of such damage or exposure is short;
hence, these factors cannot be considered. The broad factors
involved in OTSCC development, including chemical and epigenetic
causes, appear to be less applicable to young mature patients than
to older patients (6). The present
study hypothesized that the lingual position of the mandibular
second molars, which are affected by features such as excessive
lingual inclination, may serve as a potential factor for the
development of OTSCC in young mature patients. Based on the
clinical experience of the authors, the excessive lingual
inclination across an extended period may be potentially associated
with the development of OTSCC. This chronic damage to the surface
of the tongue mucosa by a lingualized mandibular second molar may
induce malignant transformation (7).
OTSCC in young mature patients is associated with
particularly high rates of regional and distant metastases;
recurrence is more aggressive, with a higher fatality rate in such
cases (8–16). Thus, detailed examination of the
position of the mandibular molars may indirectly aid reduction of
the risk of developing OTSCC in young mature patients. In addition
to determination of the position of the mandibular second molars,
the tongue space is vital to ensuring a thorough and accurate
evaluation. The present study measured these two parameters using
computed tomography (CT), establishing an association between the
position of the mandibular second molar and the tongue space with
the development of OTSCC in young mature patients. A comparison of
the position of the mandibular second molar on the healthy side and
on the affected side in young mature patients with OTSCC was also
performed.
Materials and methods
Patients
A total of 21 patients with OTSCC with an intact
mandibular second molar on the affected side of the tongue were
included in the present study; the medical records of these 21
patients with OTSCC aged <50 years, who had undergone coronal
and axial CT prior to glossectomy (along with neck dissection in
certain cases) between April 2009 and December 2015 at the Section
of Maxillofacial Surgery in Tokyo Medical and Dental University,
were retrospectively examined. In the present study, patients aged
<50 years were considered to be ‘young mature patients’, as the
mean age at diagnosis of OTSCC is reportedly 60 years (2). With regard to the control group, 21
sex-matched patients with oral diseases, such as mandibular cysts,
ranulas, submandibular gland salivolithiasis, and maxillary
gingival carcinoma, with a similar age to that of the OTSCC group,
and with height and weight within 5% of the value of the OTSCC
group were included. None of the 42 patients had any prosthesis
placement, including metal inlays, metal onlays, full metal crowns,
bridges and prosthetics, periodontal or endodontic disease in the
mandibular second molars, or endocrine disease, such as diabetes
mellitus. The medical charts of the 21 patients with OTSCC and the
21 control patients, including the sex, age, and
tumor-node-metastasis classification of the tumor according to the
Union for International Cancer Control (17), were examined (Table I). The present study was retrospective
and followed the Declaration of Helsinki on ethics, and the
regional Ethical Review Board of Tokyo Medical and Dental
University (Tokyo, Japan) approved the study.
 | Table I.Clinical characteristics, including θ
and S values, of the 21 young mature patients with OTSCC and
21 control patients. |
Table I.
Clinical characteristics, including θ
and S values, of the 21 young mature patients with OTSCC and
21 control patients.
| Patient no. | Group | Age, years/sex | TNM
classification/Diagnosis on control patients | θ,° | S,
mm2 |
|---|
| 1 | OTSCC | 29/Male | T3N2bM0 | 62.7 | 10.9 |
|
| Control | 29/Male | Ranula | 70.8 | 10.9 |
| 2 | OTSCC | 38/Male | T1N0M0 | 59.3 | 10.3 |
|
| Control | 38/Male | Mandibular cyst | 75.4 | 12.3 |
| 3 | OTSCC | 35/Male | T1N0M0 | 54.5 | 10.8 |
|
| Control | 35/Male | Submandibular gland
salivolithiasis | 64.5 | 11.7 |
| 4 | OTSCC | 45/Male | T4N2bM0 | 65.8 | 9.8 |
|
| Control | 45/Male | Mandibular cyst | 70.3 | 11.1 |
| 5 | OTSCC | 43/Male | T1N0M0 | 62.2 | 9.4 |
|
| Control | 43/Male | Mandibular cyst | 66.2 | 12.4 |
| 6 | OTSCC | 43/Male | T1N0M0 | 64.1 | 10.2 |
|
| Control | 43/Male | Mandibular tumor | 57.2 | 11.5 |
| 7 | OTSCC | 33/Male | T2N0M0 | 58.7 | 10.9 |
|
| Control | 33/Male | Mandibular tumor | 74.9 | 11.6 |
| 8 | OTSCC | 34/Male | T2N0M0 | 75.5 | 10.6 |
|
| Control | 34/Male | Submandibular gland
salivolithiasis | 74.1 | 11.0 |
| 9 | OTSCC | 47/Male | T2N0M0 | 63.3 | 10.1 |
|
| Control | 47/Male | Mandibular
cyst | 67.8 | 12.6 |
| 10 | OTSCC | 46/Male | T1N0M0 | 59.3 | 10.9 |
|
| Control | 46/Male | Mandibular
cyst | 68.2 | 11.8 |
| 11 | OTSCC | 38/Male | T1N0M0 | 63.1 | 8.7 |
|
| Control | 38/Male | Mandibular
cyst | 69.5 | 12.1 |
| 12 | OTSCC | 36/Male | T2N0M0 | 79.5 | 10.7 |
|
| Control | 36/Male | Maxillary gingival
carcinoma | 76.8 | 10.9 |
| 13 | OTSCC | 37/Male | T3N0M0 | 59.6 | 10.0 |
|
| Control | 37/Male | Mandibular
tumor | 56.9 | 10.2 |
| 14 | OTSCC | 42/Female | T1N0M0 | 56.0 | 12.3 |
|
| Control | 42/Female | Mandibular
cyst | 63.8 | 12.5 |
| 15 | OTSCC | 48/Female | T1N0M0 | 73.9 | 9.0 |
|
| Control | 48/Female | Submandibular gland
salivolithiasis | 70.1 | 11.1 |
| 16 | OTSCC | 43/Female | T1N0M0 | 71.5 | 10.1 |
|
| Control | 43/Female | Mandibular
tumor | 80.7 | 12.1 |
| 17 | OTSCC | 49/Female | TisN0M0 | 61.8 | 10.1 |
|
| Control | 49/Female | Mandibular
tumor | 57.9 | 11.2 |
| 18 | OTSCC | 43/Female | T1N0M0 | 73.2 | 11.2 |
|
| Control | 43/Female | Mandibular
cyst | 72.2 | 12.8 |
| 19 | OTSCC | 47/Female | T2N0M0 | 62.9 | 9.4 |
|
| Control | 47/Female | Radicular cyst | 72.3 | 10.3 |
| 20 | OTSCC | 38/Female | T1N0M0 | 79.3 | 10.8 |
|
| Control | 38/Female | Mandibular
tumor | 71.2 | 10.8 |
| 21 | OTSCC | 31/Female | T2N0M0 | 71.1 | 10.0 |
|
| Control | 31/Female | Mandibular
cyst | 78.6 | 11.2 |
Evaluation of OTSCC status
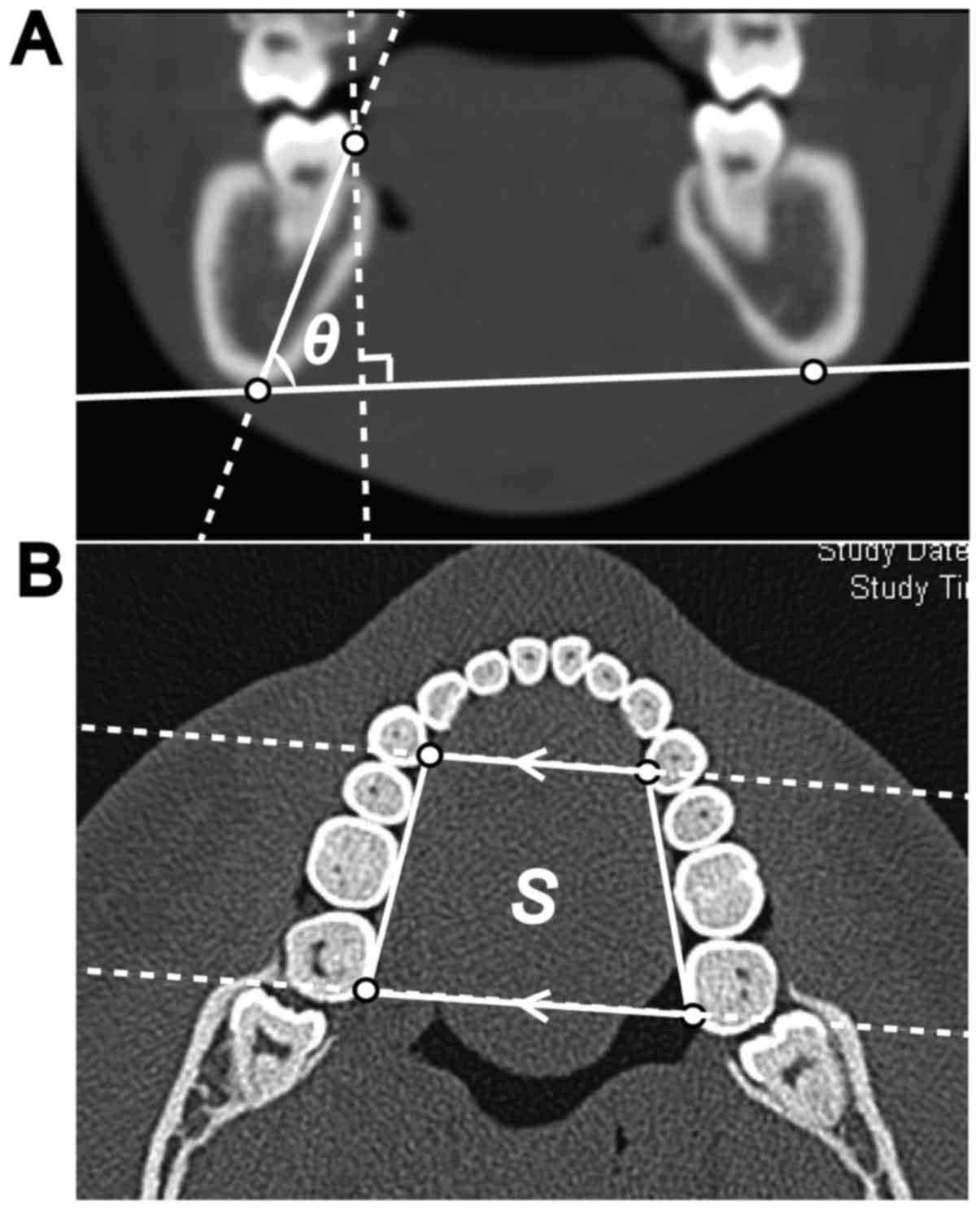
To evaluate the position of the mandibular second
molar, the angular inclination was measured. The angle of the
mandibular second molar on the affected side, against the
tangential line connecting the inferior borders of the bilateral
mandibular bone on coronal CT imaging (termed θ; Fig. 1), was also measured. The lingual point
of the mandibular second molar on the affected side was determined
as being the tangential point of the perpendicular line against the
tangential line that connected the inferior borders of the
bilateral mandibular bone. The angles of the mandibular second
molars in the healthy side and the affected side in each of the 21
young adult patients with OTSCC were also compared (Fig. 2). The measurement side of the control
group was the same side as that of the OTSCC group in each matched
patient.
In addition, the tongue-space (S) was estimated as
being the area of the rectangle formed by the lingual edge of the
bilateral posterior corners of the mandibular second molars and
around the center of the lingual side of the first premolars on
axial CT images. The two vertices on the first premolars were
determined along a line parallel to the two lingual edges of the
bilateral posterior corners of the mandibular second molars, which
passed along the bilateral first premolars (Fig. 1). The slice height of the axial CT
image was determined as being half that of the crown height of the
mandibular second molar on the affected side. The corresponding
pairs of data points are presented in Table I.
Statistical analysis
All statistical analyses were conducted using SPSS
for Windows version 19.0 (IBM Corp., Armonk, NY, USA). In addition,
the distributions of θ and S values in patients with OTSCC
and in control patients were analyzed using the Mann-Whitney U test
(Fig. 3A and B). P<0.05 was
considered to indicate a statistically significant difference.
Results
The median age of the 21 young mature patients with
OTSCC (8 females and 13 males) was 43 years (range, 29–49 years).
The OTSCC lesion appeared to be in contact with the lingual side of
the mandibular second molar in all cases. With regard to the
clinical classification of the tumors, 52.4% of lesions were
classified as T1, 28.6% were classified as T2, 9.5% were classified
as T3, 4.8% were classified as T4 and 4.8% were classified as T-is
(Table I). The median angle on the
affected side was 71.3° in females (range, 56.0–79.3°) and 62.7° in
males (range, 54.5–79.5°) of the OTSCC group, and was 71.7° in
females (range, 57.9–80.7°) and 69.5° in males (range, 56.9–76.8°)
of the control group (Table I). If
the lingual position of the mandibular second molar were critical
for developing OTSCC, the θ value on the affected side would be
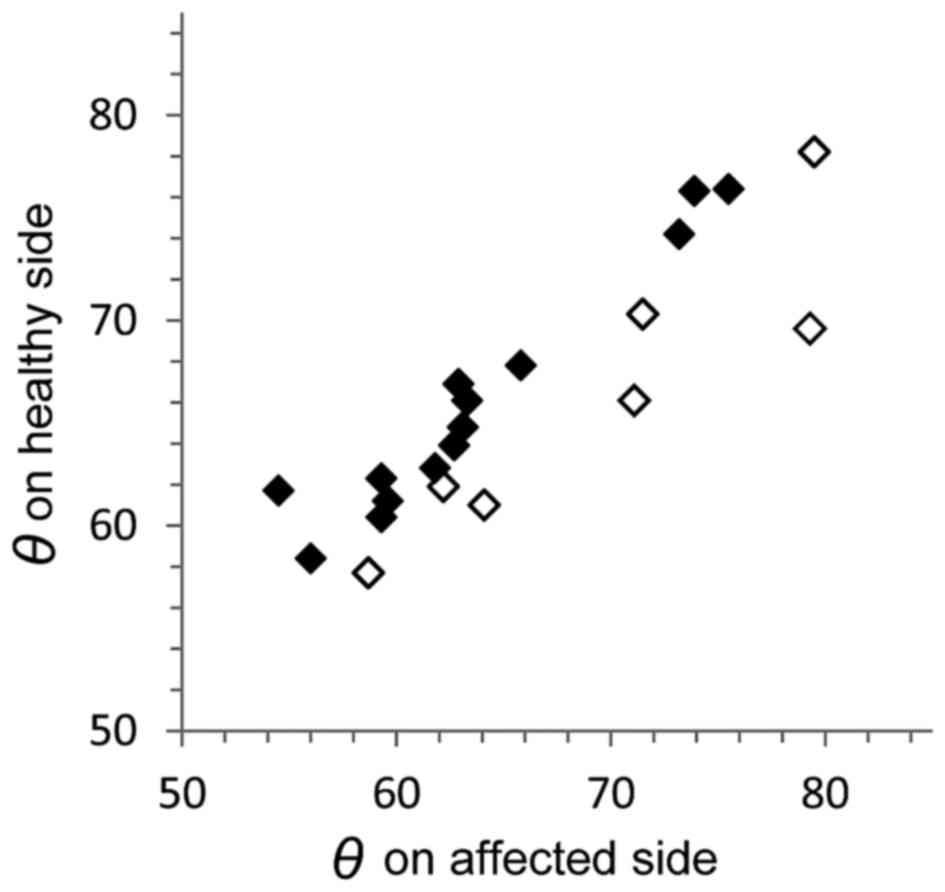
lower than that on the healthy side. Fig.
2 depicts scatter plots for the association between the θ value
on the healthy side and the affected side in the 21 patients with
OTSCC. The θ value on the affected side in 14 patients with OTSCC
(66.7%) was lower than that in the other 7 patients with OTSCC,
thus supporting the aforementioned reasoning.
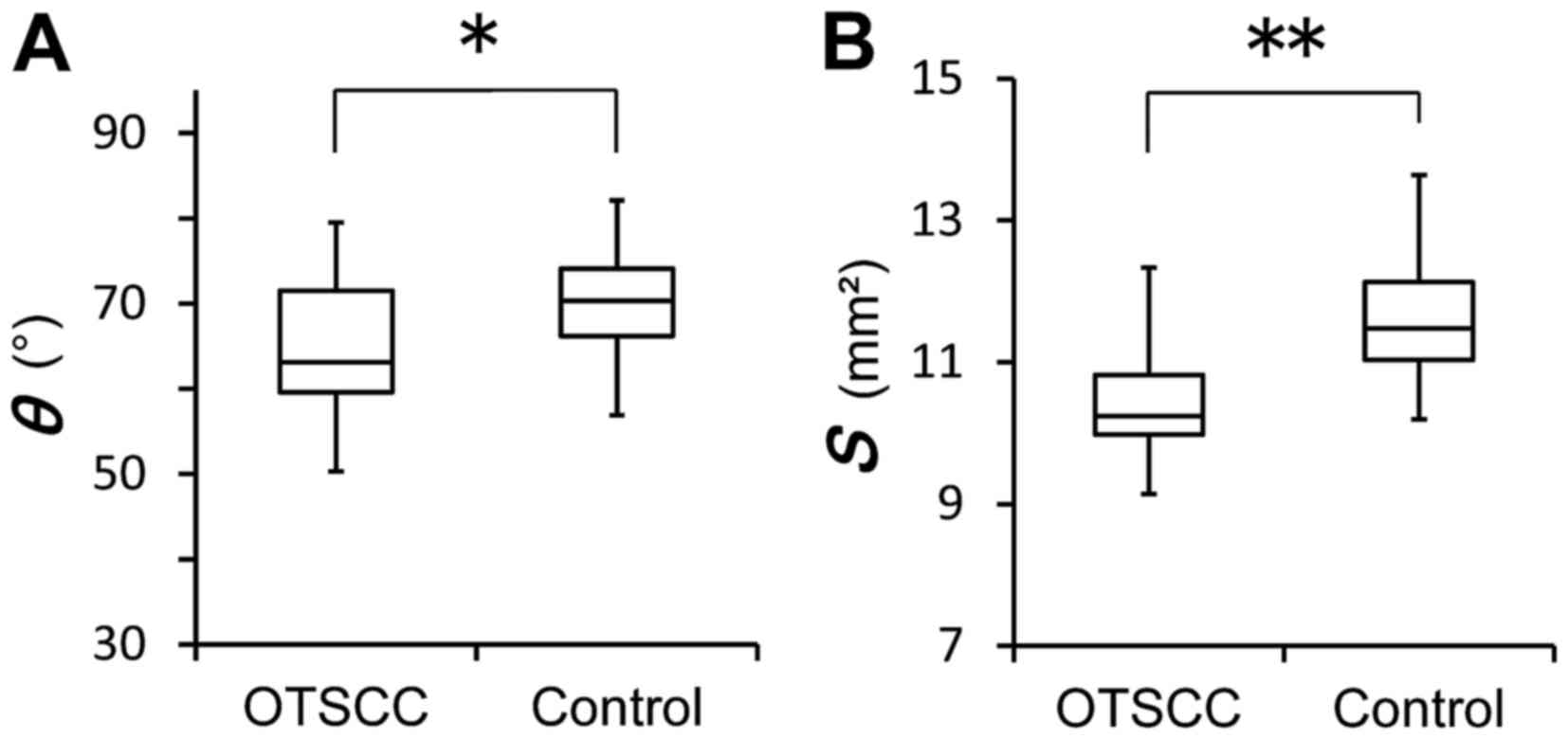
Furthermore, Mann-Whitney U test analysis confirmed
the presence of a significant difference in the distributions of θ
and S values between the OTSCC and control patients (P=0.016
and P<0.001, respectively; Fig. 3A and
B).
The number of young maturities with OTSCC who
habitually consumed alcohol was 3 (14.3%), the number who smoked
daily was 6 (28.6%) and the number who regularly consumed both
alcohol and smoked daily was 6 (28.6%). The number of control
patients who habitually consumed alcohol was 8 (38.1%), the number
who smoked daily was 8 (38.1%) and the number who regularly
consumed alcohol and smoked daily was 6 (28.6%).
Discussion
OTSCC is an uncommon malignancy among the young
mature generation (18–20). Thus far, no reports have examined the
association between the findings of physical examination of teeth
position and the development of OTSCC, to the best of our
knowledge. The present study aimed to evaluate the association
between the development of OTSCC in young maturities and the
mandibular second molar position and tongue space using coronal and
axial CT images. Previous studies have identified various risk
factors for OTSCC, including age, smoking status, alcohol
consumption and human papilloma virus infection (2,21–24). Furthermore, nutritional deficiency and
poor oral hygiene with misaligned dentition have also been
identified as causative factors (25–27). A
number of prior studies have also examined associations between
dental prostheses, including metal crowns, bridges and prostheses,
and the development of OTSCC (2,28–30). Hougeir et al (5) proposed that an oral metal-contact
allergy was a factor that influenced the development of OTSCC. The
21 young mature patients with OTSCC in the present study had no
metal prostheses in or around the mandibular second molar; hence,
oral metal-contact allergy and poor prostheses were not considered
suitable risk factors for the patients in the present study.
However, the presence of oral galvanism, as proposed by Fan et
al (2), cannot be completely
ruled out. In addition, the present study could not exclude the
presence of pre-existing dysplasia or cancer.
As predicted, the majority of young mature patients
with OTSCC had a lower θ value on the affected side than on the
healthy side. A lower θ value was associated with a more lingual
position of the mandibular second molar, which would consequently
lead to a smaller S value. Thus, the development of OTSCC in
young mature patients was not only influenced by the lingual
position of the mandibular second molar, but also by the narrow
tongue space; the significant difference of the distributions of θ
and S values between young mature patients with OTSCC and
control patients supports this. No microglossia was observed in the
21 young mature patients with OTSCC; the 21 OTSCCs were in contact
with or compressed by the lingualized mandibular molars. The
significant difference in the tongue space noted in the present
study also supports this reasoning.
Findings from the present study also revealed that
alcohol consumption and smoking status may not be directly
associated with OTSCC development in young maturities. The data
suggest that these factors may not influence OTSCC development in
young mature patients. However, the role of alcohol consumption and
smoking in OTSCC development cannot be excluded, due to the limited
cohort size of the present study.
Based on the findings of the present study,
orthodontic treatment in young mature patients, particularly that
involving bilateral molar-distance extension and tongue-space
widening, would be suitable for excluding one of the factors of
OTSCC development.
To conclude, the present study assessed the
association between the position of the intact mandibular second
molar and the development of OTSCC in young mature patients. The
angle of the mandibular second molar and the area of the tongue
space were measured using coronal and axial CT images. Mann-Whitney
U test analysis indicated that the angle of the mandibular second
molar and the tongue-space area differed significantly between
young mature patients with OTSCC and matched controls. The angle of
the mandibular second molar on the affected side in 66.7% of young
mature patients with OTSCC was lower than that on the healthy side.
Although there are several factors that may have a role in OTSCC
development in young mature patients, the position of the
mandibular second molar and tongue space may be a substantial
contributor.
Glossary
Abbreviations
Abbreviations:
|
OTSCC
|
oral tongue squamous cell
carcinoma
|
|
CT
|
computed tomography
|
References
|
1
|
Lissowska J, Pilarska A, Pilarski P,
Samolczyk-Wanyura D, Piekarczyk J, Bardin-Mikolłajczak A, Zatonski
W, Herrero R, Munoz N and Franceschi S: Smoking, alcohol, diet,
dentition and sexual practices in the epidemiology of oral cancer
in Poland. Eur J Cancer Prev. 12:25–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan H, Yoon KY, Kim SM, Myoung H, Lee JH
and Kim MJ: Relationship between squamous cell carcinoma of the
tongue and the position of dental prosthesis. J Adv Prosthodont.
7:129–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zafereo ME, Xu L, Dahlstrom KR, Viamonte
CA, El-Naggar AK, Wei Q, Li G and Sturgis EM: Squamous cell
carcinoma of the oral cavity often overexpresses p16 but is rarely
driven by human papillomavirus. Oral Oncol. 56:47–53. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amagasa T, Yamashiro M and Uzawa N: Oral
premalignant lesions: From a clinical perspective. Int J Clin
Oncol. 16:5–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hougeir FG, Yiannias JA, Hinni ML, Hentz
JG and el-Azhary RA: Oral metal contact allergy: A pilot study on
the cause of oral squamous cell carcinoma. Int J Dermatol.
45:265–271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegelmann-Danieli N, Hanlon A, Ridge JA,
Padmore R, Fein DA and Langer CJ: Oral tongue cancer in patients
less than 45 years old: Institutional experience and comparison
with older patients. J Clin Oncol. 16:745–753. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato T: A study on effect of mechanical
irritation in development and progression of tongue cancer. Kokubyo
Gakkai Zasshi. 62:532–550. 1995.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hilly O, Shkedy Y, Hod R, Soudry E,
Mizrachi A, Hamzany Y, Bachar G and Shpitzer T: Carcinoma of the
oral tongue in patients younger than 30 years: Comparison with
patients older than 60 years. Oral Oncol. 49:987–990. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jones JB, Lampe HB and Cheung HW:
Carcinoma of the tongue in young patients. J Otolaryngol.
18:105–108. 1989.PubMed/NCBI
|
|
10
|
Garavello W, Spreafico R and Gaini RM:
Oral tongue cancer in young patients: A matched analysis. Oral
Oncol. 43:894–897. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JO, Sun DI, Cho KJ, Joo YH, Yoo HJ
and Kim MS: Clinical outcome of squamous cell carcinoma of the
tongue in young patients: A stage-matched comparative analysis.
Clin Exp Otorhinolaryngol. 3:161–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sarkaria JN and Harari PM: Oral tongue
cancer in young adults less than 40 years of age: Rationale for
aggressive therapy. Head Neck. 16:107–111. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soudry E, Preis M, Hod R, Hamzany Y, Hadar
T, Bahar G, Strenov Y and Shpitzer T: Squamous cell carcinoma of
the oral tongue in patients younger than 30 years:
Clinicopathologic features and outcome. Clin Otolaryngol.
35:307–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Manuel S, Raghavan SK, Pandey M and
Sebastian P: Survival in patients under 45 years with squamous cell
carcinoma of the oral tongue. Int J Oral Maxillofac Surg.
32:167–173. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mallet Y, Avalos N, Le Ridant AM, Gangloff
P, Moriniere S, Rame JP, Poissonnet G, Makeieff M, Cosmidis A,
Babin E, et al: Head and neck cancer in young people: A series of
52 SCCs of the oral tongue in patients aged 35 years or less. Acta
Otolaryngol. 129:1503–1508. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Popovtzer A, Shpitzer T, Bahar G, Marshak
G, Ulanovski D and Feinmesser R: Squamous cell carcinoma of the
oral tongue in young patients. Laryngoscope. 114:915–917. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pindborg JJ, Reichart PA and Smith CJ:
Histological typing of cancer and precancer of the oral mucosa. 2nd
edition. Springer-Verlag; Berlin: 1997, View Article : Google Scholar
|
|
18
|
McGregor GI, Davis N and Robins RE:
Squamous cell carcinoma of the tongue and lower oral cavity in
patients under 40 years of age. Am J Surg. 146:88–92. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Venables CW and Craft IL: Carcinoma of the
tongue in early adult life. Br J Cancer. 21:645–650. 1967.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Newman AN, Rice DH, Ossoff RH and Sisson
GA: Carcinoma of the tongue in persons younger than 30 years of
age. Arch Otolaryngol. 109:302–304. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mashberg A, Boffetta P, Winkelman R and
Garfinkel L: Tobacco smoking, alcohol drinking, and cancer of the
oral cavity and oropharynx among U.S. veterans. Cancer.
72:1369–1375. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boffetta P, Mashberg A, Winkelmann R and
Garfinkel L: Carcinogenic effect of tobacco smoking and alcohol
drinking on anatomic sites of the oral cavity and oropharynx. Int J
Cancer. 52:530–533. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwartz SM, Daling JR, Doody DR, Wipf GC,
Carter JJ, Madeleine MM, Mao EJ, Fitzgibbons ED, Huang S, Beckmann
AM, et al: Oral cancer risk in relation to sexual history and
evidence of human papilloma virus infection. J Natl Cancer Inst.
90:1626–1636. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Laronde DM, Hislop TG, Elwood JM and Rosin
MP: Oral cancer: Just the facts. J Can Dent Assoc. 74:269–272.
2008.PubMed/NCBI
|
|
25
|
Preston-Martin S, Henderson BE and Pike
MC: Descriptive epidemiology of cancers of the upper respiratory
tract in Los Angeles. Cancer. 49:2201–2207. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cox B, Taylor K and Treasure E: Trends in
oral cancer by subsite in New Zealand. Eur J Cancer B Oral Oncol.
31B:113–117. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Macfarlane GJ, Sharp L, Porter S and
Franceschi S: Trends in survival from cancers of the oral cavity
and pharynx in Scotland: A clue as to why the disease is becoming
more common? Br J Cancer. 73:805–808. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jainkittivong A, Aneksuk V and Langlais
RP: Oral mucosal conditions in elderly dental patients. Oral Dis.
8:218–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lockhart PB, Norris CM Jr and Pulliam C:
Dental factors in the genesis of squamous cell carcinoma of the
oral cavity. Oral Oncol. 34:133–139. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kinnebrew M, Gettleman L, Carr RF and
Beazley R: Squamous cell carcinoma of the tongue in a young woman.
Report of a case with etiologic considerations. Oral Surg Oral Med
Oral Pathol. 58:696–698. 1984. View Article : Google Scholar : PubMed/NCBI
|