Introduction
Gastric cancer, one of the most common malignancies
of the digestive system, is the second leading cause of
cancer-associated mortality worldwide, with an increased frequency
in East Asia, particularly in China (1). Despite the decreased incidence and
mortality of gastric cancer in China over the past decades, this
malignancy remains a prominent burden to local health programs
(2). Currently, surgical resection is
the only curative treatment available for localized gastric cancer;
however, nearly half of patients diagnosed possess advanced to
late-stage cancer, with lymph node or distant metastasis, and are
not eligible for surgery (3,4). Among the patients presenting with
early-stage disease, even following a potentially curative
resection, 60% eventually develop distant metastasis or local
recurrence (5). The prognosis of
patients with advanced or recurrent gastric cancer remains poor,
with 5-year survival rates ranging between 5 and 17% in the Western
world, and have not changed significantly in the last 30 years
(6–8).
Thus, identification of novel biomarkers for early diagnosis or
prognosis is key to improving the prognosis of patients with
gastric cancer.
Forkhead box M1 (FOXM1), characterized by a
100-amino-acid winged-helix DNA-binding domain, is a member of the
forkhead transcription factor family. It serves an important role
in cell cycle regulation by promoting the transition from G1 to S
phase and progression to mitosis through cell division cycle 25
homolog B, cyclin-dependent kinase 1 and cyclin-dependent kinase
inhibitor 1B (1,9–11). Recent
studies have demonstrated that FOXM1 is overexpressed and serves an
essential role in the development and progression of distinct types
of human cancer, including hepatocellular carcinoma, non-small cell
lung cancer and breast cancer (12–14). In a
rhabdomyosarcoma cell line, FOXM1 silencing results in decreased
cell growth and survival (15).
Urokinase-type plasminogen activator (uPA), a member
of the uPA system, is a trypsin-like serine protease. The uPA
ligand binds to its receptor, a three-domain glycolipid-anchored
cell-surface protein, and mediates proteolysis by plasminogen or
growth factor activation (16–19).
Previous studies have demonstrated that increased expression of the
uPA system in tumor tissues is associated with pathological
characteristics and prognosis in numerous types of tumor due to its
roles in cell adhesion, migration and invasion, as well as
metastasis (17,20,21).
Plebani et al (22)
investigated uPA levels in gastric cancer and normal samples from
20 patients with gastric cancer using ELISA and identified
significantly increased uPA expression levels in cancer samples,
with decreased uPA receptor levels identified to be associated with
a prolonged survival time.
Although FOXM1 and uPA are considered to mediate
tumor cell differentiation, invasion and metastasis in human
cancer, the association of their expression levels with
clinicopathological characteristics in patients with gastric cancer
remains unknown. Similarly, the importance of FOXM1 expression and
its effect on uPA in gastric cancer biology as well as patient
prognosis are not well-understood. Therefore, the aim of the
present study was to investigate FOXM1 and uPA expression levels in
gastric tissue specimens and evaluate their association with the
clinicopathological features of gastric cancer as well as exploring
their individual or combined effects on the clinical outcome. The
results of the present study provide a basis for understanding the
clinical significance of FOXM1 and uPA in the prognosis of patients
with gastric cancer.
Materials and methods
Patients and tissue samples
A total of 436 patients (310 males and 126 females;
median age, 59.50 years; range, 17–99 years) with gastric cancer,
who underwent gastrectomy in Zhejiang Provincial People's Hospital
(Hangzhou, China) between March 1998 and December 2004, were
consecutively enrolled in the present study. Written informed
consent was obtained from each patient and the present study was
approved by the Ethics Committee of Zhejiang Provincial People's
Hospital. All patients met the following eligibility criteria: i)
Tumor histologically confirmed to be gastric cancer; ii) no
previous endoscopic mucosal resection, palliative resection,
preoperative chemotherapy or radiotherapy; and iii) 5 years of
follow-up data available, with follow-up ending in December 2008.
Clinicopathological data were obtained from operative and
pathological reports, including tumor location, size, Lauren type
(23), histological classification,
degree of differentiation, depth of tumor invasion,
tumor-node-metastasis (TNM) stage, lymph node status, vessel
invasion and distant metastasis status. All cases were classified
according to the World Health Organizations pathological
classification (2010) of gastric tumors (23). Survival time was
defined as between the date of surgery and the follow-up deadline
or date of mortality. In addition, 92 para-cancer tissues, >5 cm
away from the tumor lesion boundary, were randomly selected as
controls. All tissue samples were fixed in 10% neutral formalin
buffer (pH 7.4) at room temperature for 10 h, paraffin-embedded
(FFPE), and stored until used.
Tissue microarray (TMA)
TMAs were constructed using FFPE blocks from 436
tumor tissue samples and 92 normal mucosa specimens; all
experiments were performed on an automated TMA instrument. Briefly,
4-µm-thick sections were obtained from each tissue block and
stained with hematoxylin and eosin for 10 min at room temperature
to identify appropriate tumor areas. Core tumor tissue biopsies
(diameter, 2 mm) were obtained from individual FFPE blocks (donor
blocks) and arranged in recipient paraffin blocks (tissue array
blocks) using a trephine. Each TMA block contained >6 adjacent
non-cancerous mucosal specimens serving as internal controls.
Immunohistochemical staining and
evaluation
Immunohistochemical staining was performed to
analyze the expression of FOXM1 and uPA in 4-µm-thick TMA sections
including 436 gastric cancer and 92 adjacent non-cancerous tissue
samples. Briefly, TMA sections were deparaffinized twice for 15 min
in xylene, rehydrated in graded ethanol and incubated in 3%
H2O2 at room temperature for 15 min to block
endogenous peroxidase activity. The sections were microwaved in
0.01 M of citrate buffer (1.92 g anhydrous citric acid dissolved in
1 liter distilled water and adjusted to pH 7.4 with 1 N NaOH),
boiled for 15 min for antigen retrieval, and incubated with 10%
normal goat serum (cat. no. 50062Z; Thermo Fisher Scientific Inc.,
Waltham, MA, USA) at room temperature for 10 min to block
non-specific binding. Subsequently, the sections were incubated
with rabbit monoclonal anti-FOXM1 (1:500 dilution in PBS; ab207298;
Abcam, Cambridge, MA, USA) and anti-uPA (1:250 dilution in PBS;
ab169754; Abcam) antibodies overnight at 4°C, with normal goat
serum used as a negative control. Following incubation with
horseradish peroxidase-conjugated anti-rabbit secondary antibodies
(cat. no. ZDR-5306; OriGene Technologies, Inc., Rockville, MD, USA)
for 20 min at room temperature, 3-diaminobenzidine
tetrahydrochloride was used to visualize the signals.
Counterstaining was performed with hematoxylin, staining for 10 min
at room temperature. Immunohistochemical evaluation was
independently performed by two pathologists blinded to the clinical
data. Average staining intensity in each case was classified into
one of the following four categories: 0, Negative; 1, weak
staining; 2, moderate staining; 3, intense staining. Meanwhile, the
proportion of cells with positive-staining were classified as
follows: 0, <5%; 1, 5–25%; 2, 26–50%; 3, 51–75%; 4, 76–100%. The
overall staining index was calculated as the sum of the staining
intensity score and proportion of positively stained cells. The
final composite scores were divided into four grades: 0–1 (−), 2–3
(+), 4–6 (++) and 6 (+++). Patients with a score of ‘−’ were
classified as the negative expression group, whereas those with +,
++ and +++ comprised the low, moderate and high expression groups,
respectively. Finally, low, moderate and high expression groups
were all considered to be FOXM1- and uPA-positive.
Statistical analysis
All statistical analyses were performed using SPSS
(version 13.0; SPSS Inc., Chicago, IL, USA) and GraphPad Prism
(version 5.01; GraphPad Software Inc., La Jolla, CA, USA).
Categorical data were assessed using χ2 or Fisher's
exact test. Survival curves were evaluated using the Kaplan-Meier
estimator method, with the log-rank test used to assess group
differences. Multivariate analysis using the Cox's proportional
hazards regression model was performed to assess FOXM1 and uPA
protein levels for their prognostic value. The associations of
FOXM1 and uPA expression with clinicopathological characteristics
were estimated using the Pearson's correlation method. Results are
presented as the mean ± standard deviaiton. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological
characteristics
A total of 436 patients with gastric cancer (310
males and 126 females) were included in the present study. On the
basis of the World Health Organization Classification Criteria
(2010) for gastric cancer (23), the
diagnoses included 28 mucinous adenocarcinomas, 16 papillary
adenocarcinomas, 65 signet ring cell carcinomas and 327 tubular
adenocarcinomas. A total of 3 cases were undifferentiated, 309 were
poorly differentiated, 109 were moderately differentiated and 15
cases were well-differentiated. According to the TNM staging
criteria, 90, 104, 173 and 69 cases were TNM stages I, II, III and
IV, respectively; 270 cases exhibited lymph node metastasis,
whereas 166 cases did not (P<0.05; Table I).
 | Table I.Association of FOXM1 and uPA
expression with various clinicopathological parameters in 436
patients with gastric cancer. |
Table I.
Association of FOXM1 and uPA
expression with various clinicopathological parameters in 436
patients with gastric cancer.
|
|
| FOXM1 |
| uPA |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | Patients (n,
436) | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| Age, years |
|
|
| 0.210 |
|
| 0.041 |
|
≤50 | 344 | 69 | 275 |
| 146 | 198 |
|
|
>50 | 92 | 24 | 68 |
| 50 | 42 |
|
| Sex |
|
|
| 0.420 |
|
| 0.727 |
|
Male | 310 | 63 | 247 |
| 141 | 169 |
|
|
Female | 126 | 30 | 96 |
| 55 | 71 |
|
| Location |
|
|
| 0.187 |
|
| 0.471 |
|
Cardia | 59 | 10 | 49 |
| 23 | 36 |
|
|
Body | 171 | 44 | 127 |
| 75 | 96 |
|
|
Antrum | 206 | 39 | 167 |
| 98 | 108 |
|
| Tumor size, cm |
|
|
| 0.142 |
|
| <0.001 |
|
<5 | 257 | 61 | 196 |
| 138 | 58 |
|
| ≥5 | 179 | 32 | 147 |
| 119 | 121 |
|
| Lauren
classification |
|
|
| <0.001 |
|
| 0.073 |
|
Intestinal | 251 | 46 | 205 |
| 121 | 130 |
|
|
Diffuse | 144 | 45 | 99 |
| 63 | 81 |
|
|
Mixed | 41 | 2 | 39 |
| 12 | 29 |
|
| Histology
classification |
|
|
| 0.727 |
|
| 0.471 |
|
Papillary | 16 | 3 | 13 |
| 6 | 10 |
|
|
Tubular | 327 | 70 | 257 |
| 150 | 177 |
|
|
Mucinous | 28 | 4 | 24 |
| 9 | 19 |
|
| Signet
ring cell | 65 | 16 | 49 |
| 31 | 34 |
|
|
Differentiation |
|
|
| 0.004 |
|
| 0.101 |
|
Weak | 15 | 8 | 7 |
| 11 | 4 |
|
|
Moderate | 109 | 22 | 87 |
| 51 | 58 |
|
|
Strong | 309 | 61 | 248 |
| 132 | 177 |
|
|
Undifferentiated | 3 | 2 | 1 |
| 2 | 1 |
|
| Invasion depth |
|
|
| <0.001 |
|
| <0.001 |
| T1 | 57 | 19 | 38 |
| 44 | 13 |
|
| T2 | 109 | 37 | 72 |
| 63 | 46 |
|
| T3 | 244 | 32 | 212 |
| 87 | 157 |
|
| T4 | 26 | 5 | 21 |
| 2 | 24 |
|
| TNM stages |
|
|
| <0.001 |
|
| <0.001 |
| I | 90 | 26 | 64 |
| 73 | 17 |
|
| II | 104 | 37 | 67 |
| 70 | 34 |
|
|
III | 173 | 24 | 149 |
| 47 | 126 |
|
| IV | 69 | 6 | 63 |
| 6 | 63 |
|
| Lymph node
metastasis |
|
|
| <0.001 |
|
| <0.001 |
| No | 166 | 62 | 104 |
| 127 | 39 |
|
|
Yes | 270 | 31 | 239 |
| 69 | 201 |
|
| Vessel
invasion |
|
|
| <0.001 |
|
| <0.001 |
| No | 183 | 60 | 123 |
| 125 | 58 |
|
|
Yes | 253 | 33 | 220 |
| 71 | 182 |
|
| Distant
metastasis |
|
|
| <0.001 |
|
| <0.001 |
| No | 375 | 87 | 288 |
| 191 | 184 |
|
|
Yes | 61 | 6 | 55 |
| 5 | 56 |
|
Clinical pathology of FOXM1 and uPA
expression in gastric cancer
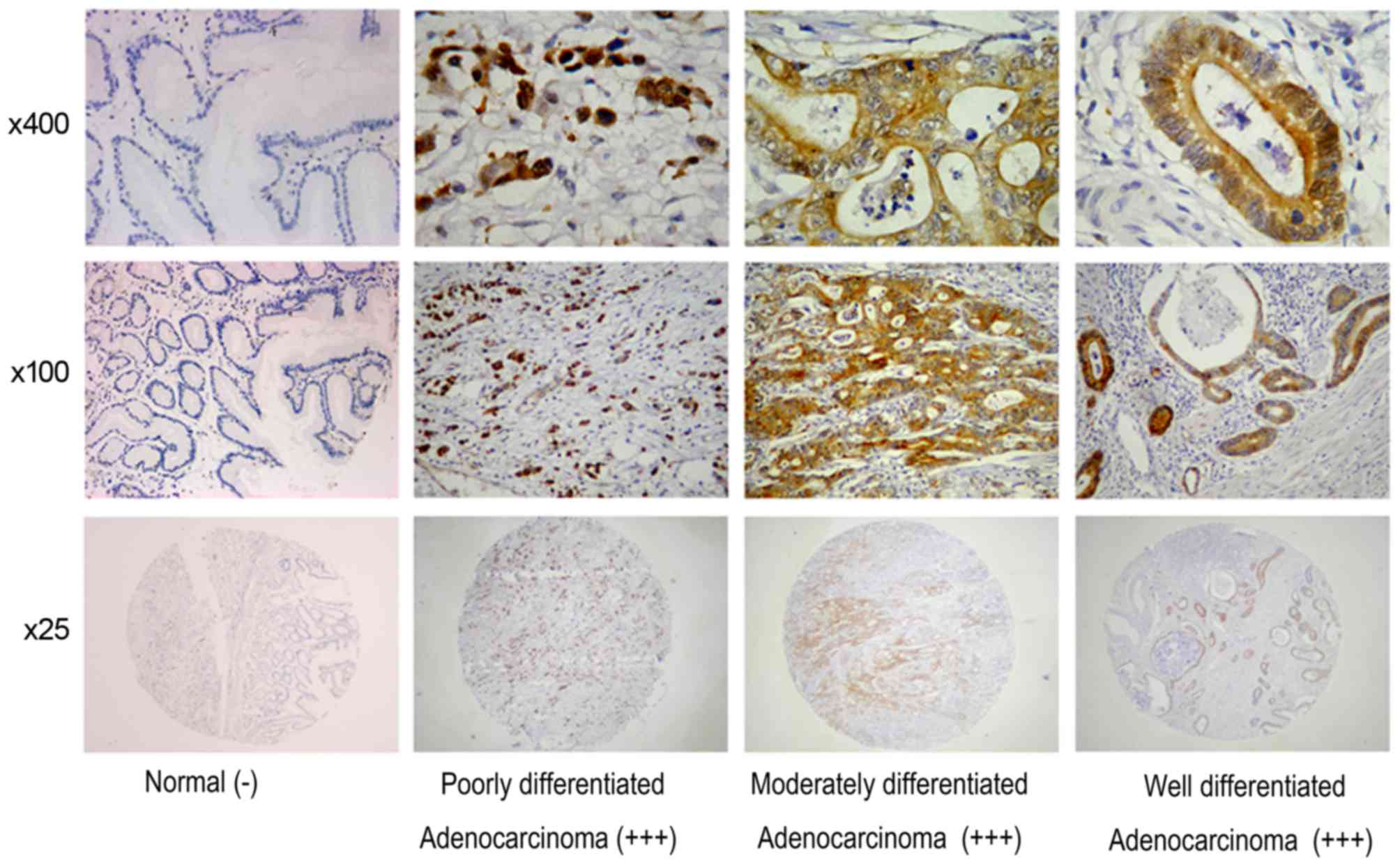
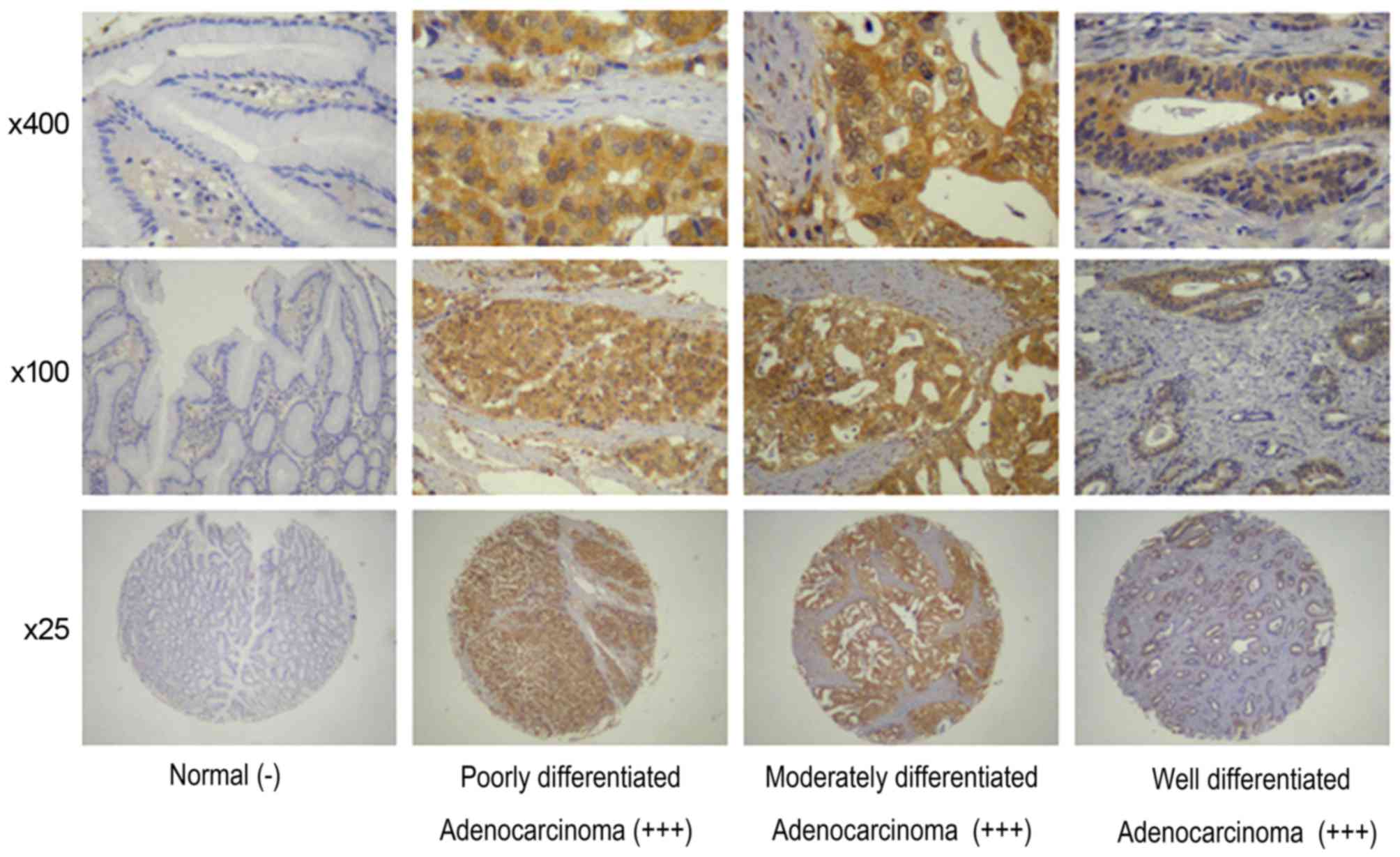
FOXM1 and uPA were identified in cancer cells; in
the 436 gastric cancer specimens, FOXM1 and uPA detection rates
were 78.67 (343/436) and 83.26% (363/436), respectively; however,
only a limited number of stained tissues were observed among the 92
para-cancer tissues (Table II;
Figs. 1 and 2). These results indicated that FOXM1 and
uPA expression levels were significantly increased in gastric
cancer compared with para-cancer tissues (P<0.05).
 | Table II.FOXM1 and uPA expression levels in
gastric cancer and paraneoplastic tissue specimens. |
Table II.
FOXM1 and uPA expression levels in
gastric cancer and paraneoplastic tissue specimens.
|
| FOXM1 | uPA |
|---|
|
|
|
|
|---|
| Variable | Gastric cancer
tissues | Para-cancer
tissues | χ2 | P-value | Gastric cancer
tissues | Para-cancer
tissues | χ2 | P-value |
|---|
| Negative | 93 | 75 | 127.026 | <0.001 | 73 | 78 | 172.806 | <0.001 |
| Positive |
|
|
|
|
|
|
|
|
|
Weak | 174 | 10 |
|
| 193 | 10 |
|
|
|
Moderate | 112 | 5 |
|
| 89 | 3 |
|
|
|
Strong | 57 | 2 |
|
| 81 | 1 |
|
|
| Total | 436 | 92 |
|
| 436 | 92 |
|
|
FOXM1 and uPA levels were associated with invasion
depth, TNM stage, lymph node status, vessel invasion and distant
metastasis (P<0.05; Table I). In
addition, the FOXM1 expression level was associated with
differentiation and Lauren classification, and uPA expression was
associated with tumor size (P<0.05; Table I). However, no significant association
between other clinicopathological characteristics and FOXM1 and uPA
expression levels was identified.
The FOXM1 detection rate was increased in patients
with lymph node metastasis (88.5%, 239/270) compared with those
without lymph node metastasis (62.7%, 104/166; P<0.05). The
FOXM1 detection rate was also increased in patients with distant
metastasis (90.2%, 55/61) compared with those without (76.8%,
288/375; P<0.05). Patients with stage III and IV gastric cancer
exhibited increased FOXM1 detection rates (86.1 and 91.3%,
respectively) compared with those with stage I and II gastric
cancer (71.1 and 64.4%, respectively; P<0.05). Similarly, T3 and
T4 stage patients exhibited increased FOXM1 detection rates (86.9
and 80.8%, respectively) compared with T1 and T2 stage cases (66.7
and 66.1%, respectively). Furthermore, the FOXM1 detection rate was
86.9% (220/253) in gastric carcinoma specimens with vessel
invasion, which was increased compared with that obtained for
specimens without vessel invasion (67.2%, 123/183; P<0.05).
FOXM1 detection rates were increased in patients with intestinal
and mixed types (81.7 and 95.1%, respectively) compared with
diffuse-type cases (68.8%; P<0.05).
The uPA detection rate was increased in gastric
carcinoma specimens with tumor size ≥5 cm at 67.6% (121/179),
compared with tumor size <5 cm (22.6%, 58/257; P<0.05). The
uPA detection rate was increased in patients with lymph node
metastasis (74.4%, 201/270; P<0.05) compared with those without
(23.5%, 39/166; P<0.01). Meanwhile, uPA detection rates were
increased in specimens with vascular invasion and distant
metastasis at 71.9% (182/253) and 91.8% (56/61), respectively,
compared with cases without vascular invasion (31.6%, 58/183;
P<0.05) or distant metastasis (49.1%, 184/375; P<0.05). In
addition, uPA was detected in 18.8% (17/90) and 32.7% (34/104) of
TNM stage I and II samples, respectively, which was decreased
compared with TNM stage III and IV specimens (72.8% or 126/173 and
91.3% or 63/69, respectively; P<0.01). Notably, uPA expression
was associated with depth of invasion in gastric cancer; from T1 to
T4 stages, positive rates gradually increased (T1, 22.8%; T2,
42.2%; T3, 64.3%; T4, 92.3%; P<0.01). No significant association
between FOXM1 and uPA expression and the remaining
clinicopathological parameters was identified. Cox's multivariate
analysis revealed that age, TNM stage, distant metastasis and FOXM1
and uPA levels were independent prognostic factors (Table III). Thus, FOXM1 and uPA may be
independent predictors of prognosis in patients with gastric
cancer.
 | Table III.Multivariate Cox's proportional
hazard analysis of overall survival. |
Table III.
Multivariate Cox's proportional
hazard analysis of overall survival.
|
|
|
|
|
|
| 95% CI for HR |
|---|
|
|
|
|
|
|
|
|
|---|
| Covariates | B-value | SE | Wald | P-value | HR | Lower | Upper |
|---|
| Age | 0.012 | 0.005 | 4.872 | 0.027 | 1.012 | 1.001 | 1.023 |
| TNM stage | 0.565 | 0.143 | 15.711 | 0.000 | 1.760 | 1.331 | 2.327 |
| Distant
metastasis | 0.463 | 0.206 | 5.064 | 0.024 | 1.589 | 1.062 | 2.379 |
| FOXM1
expression | 3.220 | 0.837 | 14.789 | 0.000 | 25.023 | 4.89 | 129.125 |
| uPA expression | 0.969 | 0.455 | 4.533 | 0.033 | 2.635 | 1.080 | 6.428 |
|
Differentiation | 0.270 | 0.136 | 3.919 | 0.048 | 1.309 | 1.003 | 1.710 |
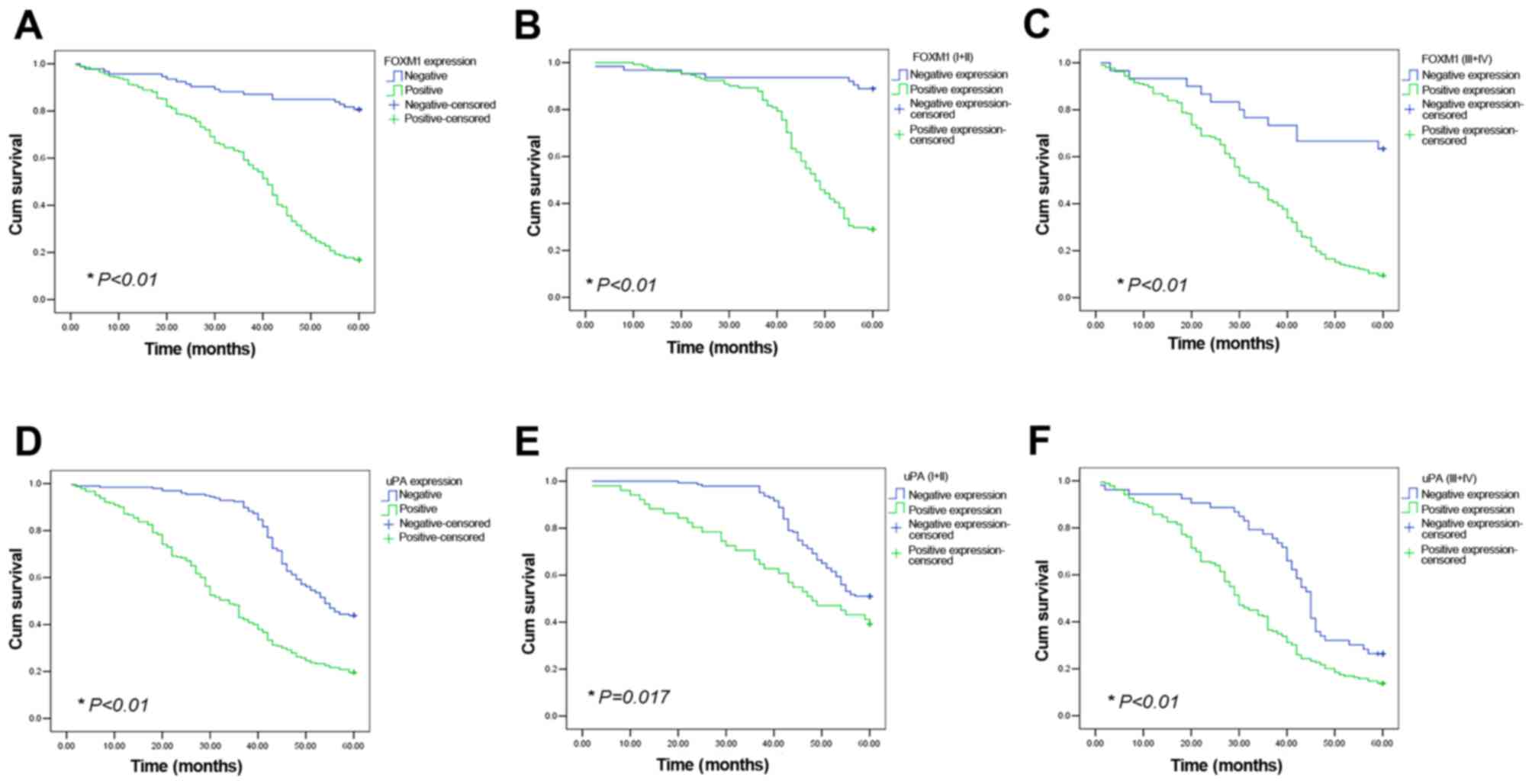
Increased FOXM1 and uPA expression
levels are associated with poor prognosis
In the patient cohort of the present study (n=436),
the mean survival time in FOXM1-positive patients (38.37±0.878
months) was significantly decreased compared with FOXM1-negative
patients (54.17±1.49 months, P<0.01); the 5-year survival rate
was also significantly decreased in FOXM1-positive patients (16.9%,
58/343) compared with FOXM1-negative cases (80.6%, 75/93;
P<0.01; Fig. 3A). FOXM1-positive
patients exhibited decreased survival rates in the TNM grade I and
II, and TNM grade III and IV groups, respectively, compared with
patients not expressing these proteins (P<0.01; Fig. 3B and C). Similarly, mean survival time
in uPA-positive patients was significantly decreased compared with
uPA-negative cases (34.72±1.14 vs. 50.37±0.83 months; P<0.01);
the 5-year survival rate was significantly decreased in
uPA-positive patients (19.6%, 47/240) compared with uPA-negative
cases (43.9%, 86/196; P<0.01; Fig.
3D). In the TNM grade I and II, and TNM grade III and IV
groups, uPA-positive patients also exhibited decreased survival
rates compared with patients not expressing these proteins
(P<0.01; Fig. 3E and F).
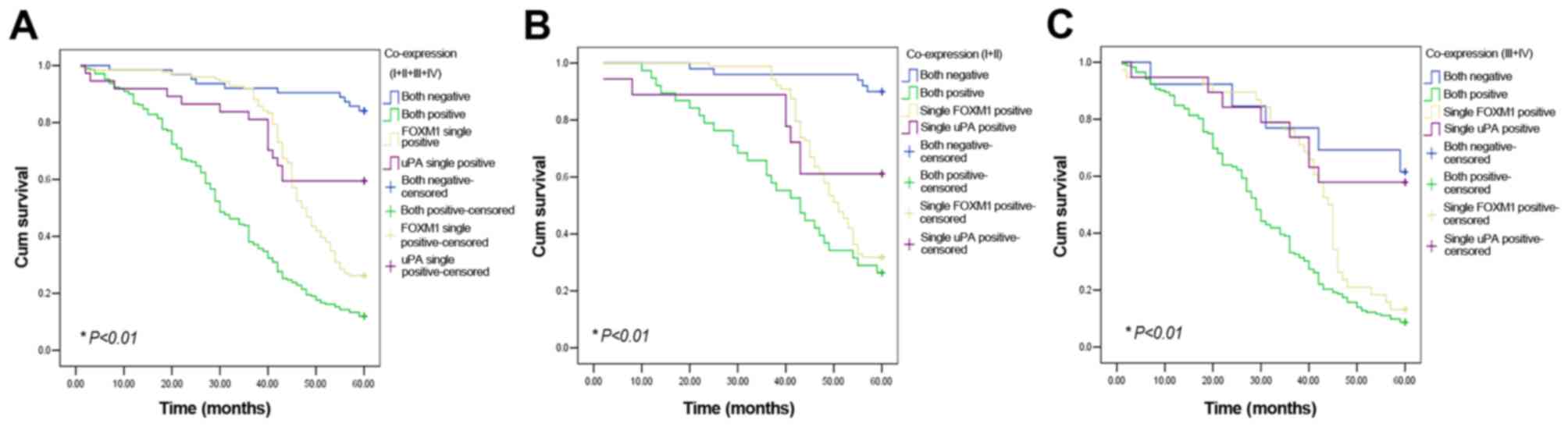
Association of FOXM1, uPA and
co-expression with patient prognosis
There were 63 gastric cancer cases not expressing
either FOXM1 or uPA, and 210 patients with gastric cancer
expressing both proteins simultaneously. A total of 126 gastric
cancer cases were positive for only FOXM1; furthermore, 37 gastric
cancer cases only exhibited uPA expression. Mean 5-year survival in
patients with uPA and FOXM1 double-negative cancer (84.1%, 53/63)
was markedly increased compared with cases expressing only uPA
(59.5%, 22/37) or FOXM1 (26.2%, 33/126), and markedly increased
compared with patients with uPA and FOXM1 double-positive cancer
(11.9%, 25/210). The mean survival of uPA and FOXM1 double-positive
patients was significantly decreased compared with uPA and FOXM1
double-negative patients (32.62±1.13 vs. 56.44±1.36 months;
P<0.01; Fig. 4A). In the TNM grade
I and II, and TNM grade III and IV groups, uPA and FOXM1
double-positive patients also exhibited a poorer prognosis compared
with double-negative or single-positive patients (P<0.05;
Fig. 4B and C).
Discussion
The results of the present study indicated that
FOXM1 and uPA expression levels were significantly increased in
gastric cancer compared with para-cancerous tissues. Notably,
increased FOXM1 and uPA protein levels were associated with
clinicopathological factors such as tumor size, depth of invasion,
TNM stage, lymph node, vessel invasion and distant metastasis. In
addition, patients with gastric cancer with increased FOXM1 and uPA
levels exhibited poorer prognosis compared with decreased FOXM1 and
uPA expression. Multivariate Cox's proportional hazards analysis
revealed that age, invasion depth, FOXM1 level and uPA content were
all independent indicators for overall survival time in gastric
cancer. These results indicated that FOXM1 and uPA protein
overexpression in gastric cancer is associated with malignant
behavior and poor overall survival.
The FOXM1 gene (~25 kb), located at the 12p13-3
chromosome region and composed of 10 exons, exhibits the common
characteristics of the family of FOX transcription factors: A
conserved DNA sequence with a winged-helix domain (24). Important functions of FOXM1 include
regulation of the cell cycle, promotion of cell proliferation, and
inhibition of cell aging and apoptosis (9). Pathological overexpression of FOXM1 may
induce the malignant proliferation of tumor cells (25). In contrast with the results of the
present study, Okada et al (26) and Li et al (27) identified no significant association
between FOXM1 overexpression and clinicopathological factors
including pathological T factor, nodal involvement and histological
differentiation. A possible reason for this discrepancy is that
these studies comprised small sample sizes, and may not have
allowed adequate exploration of the association between FOXM1
expression and clinicopathological factors. In the present study,
FOXM1 expression was identified to be significantly associated with
the prognosis of patients with gastric cancer; patients with
increased FOXM1 expression exhibited a decreased survival time
compared with those not expressing FOXM1. Hui et al
(28) identified that patients with
esophageal squamous cell carcinoma with nuclear FOXM1 expression
are younger compared with those without nuclear expression.
Therefore, there is a requirement for more detailed age-specific
analyses to clarify the association between age and FOXM1
expression in human cancer.
FOXM1 upregulation has been detected in a broad
range of human cancer cell lines and cancer types, indicating that
FOXM1 is associated with aggressive behavior of tumor cells in
vitro (24,29,30). The
results of the present study provided further support to this
hypothesis, revealing FOXM1 to be an independent prognostic factor
in gastric cancer. Previous studies indicated that other factors
may be more significant than FOXM1 in predicting prognosis in early
disease stage, including tumor location and lymph node metastasis;
whereas in advanced stages, angiogenesis, which is promoted by
FOXM1, may influence tumor growth rates more significantly
(30,31). The results of the present study
suggested that FOXM1 expression in gastric cancer serves an
unfavorable prognostic role in stage I to IV patients, supporting
the hypothesis formulated by the aforementioned studies.
Cancer cell invasion is accomplished by the
concerted action of a number of extracellular proteolytic enzyme
systems, including the uPA system, which is one of the most
important. Although referred to as a kinase, uPA possesses no
kinase activity and in turn, by limited proteolysis, may be
converted into active uPA (32).
Previous studies using a variety of models have demonstrated that
uPA is causally involved in promoting cancer invasion and
metastasis. For example, Bekes et al (33) revealed that uPA participates in an
early phase of prostate cancer dissemination (i.e. the initial
escape of tumor cells from the primary site). In addition, Tang and
Han (34) reported that the uPA
system serves an important role in breast cancer growth, invasion
and metastasis, possibly through the
Ras/extracellular-signal-regulated kinase or p38 mitogen-activated
protein kinase signaling pathway. Similarly, Heiss et al
(35) demonstrated that uPA receptor
expression on the tumor cell surface may be considered an
independent biomarker for predicting bone marrow micro-metastasis
in gastric cancer. As demonstrated by the results of the present
study, uPA expression was significantly increased in gastric cancer
compared with para-cancer tissues, and was associated with depth of
invasion, TNM stage, lymph node, vessel invasion and distant
metastasis. Meanwhile, Cox's regression analysis revealed that uPA
expression was an independent prognostic factor in patients with
gastric cancer. In addition, previous studies demonstrated that uPA
overexpression is associated with poor outcome in various types of
cancer. For example, Horvatic et al (36) assessed 105 patients with
differentiated thyroid carcinoma and normal matched tissues using
ELISA, and identified that increased uPA levels represent an
independent unfavorable prognostic factor in patients with
differentiated thyroid carcinoma. Wu et al (37) reported that combination analysis of
uPA and epithelial cadherin expression levels in laryngeal cancer
may be useful for predicting tumor metastatic risk and patient
prognosis. In the present study, patients with stage III and IV
gastric cancer expressing increased uPA levels exhibited
significantly poorer survival compared with uPA-negative patients,
indicating that uPA may serve an important role in tumor
progression and metastasis in gastric cancer.
A previous study demonstrated that RNA
interference-mediated FOXM1 knockdown inhibits HeLa cell growth,
with significantly decreased uPA levels in vitro (38). In addition, Ahmad et al
(39) demonstrated a similar pattern
in breast cancer cell lines. However, the association between FOXM1
levels and uPA expression levels in patients with gastric cancer
remains incompletely understood. The results of the present study
demonstrated that FOXM1 upregulation leads to uPA overexpression in
gastric cancer. Furthermore, Kaplan-Meier estimator survival
analysis revealed that patients with tumors exhibiting increased
expression levels of both FOXM1 and uPA exhibited significantly
poorer prognosis compared with those coexpressing these proteins at
decreased levels. These results suggested that FOXM1 and uPA may
interact to promote tumorigenesis and their combined expression may
be used as an independent prognostic marker in gastric cancer.
In conclusion, the results of the present study
suggested that increased FOXM1 and uPA expression levels in gastric
cancer are significantly associated with aggressive progression and
poor prognosis. Thus, overexpression of FOXM1 and uPA may possess
potential diagnostic value and indicate poor prognosis in patients
with gastric cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81502090 and
81470109), the Zhejiang Provincial Natural Science Foundation of
China (grant no. LY14H160039) and the Medicine and Health Research
Foundation of Zhejiang (grant no. 2013KYB022).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang J, Dang P, Raut CP, Pandalai PK,
Maduekwe UN, Rattner DW, Lauwers GY and Yoon SS: Comparison of a
lymph node ratio-based staging system with the 7th AJCC system for
gastric cancer: Analysis of 18,043 patients from the SEER database.
Ann Surg. 255:478–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nashimoto A: Current status of treatment
strategy for elderly patients with gastric cancer. Int J Clin
Oncol. 18:969–970. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nadauld LD, Garcia S, Natsoulis G, Bell
JM, Miotke L, Hopmans ES, Xu H, Pai RK, Palm C, Regan JF, et al:
Metastatic tumor evolution and organoid modeling implicate TGFBR2
as a cancer driver in diffuse gastric cancer. Genome Biol.
15:4282014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keighley MR: Gastrointestinal cancers in
Europe. Aliment Pharmacol Ther. 18 Suppl 3:S7–S30. 2003. View Article : Google Scholar
|
|
7
|
Fang N, Zhang HQ, He B, Xie M, Lu S, Wan
YY and Wang NR: Clinicopathological characteristics and prognosis
of gastric cancer with malignant ascites. Tumour Biol.
35:3261–3268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wierstra I and Alves J: FOXM1, a typical
proliferation-associated transcription factor. Biol Chem.
388:1257–1274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang IC, Chen YJ, Hughes D, Petrovic V,
Major ML, Park HJ, Tan Y, Ackerson T and Costa RH: Forkhead box M1
regulates the transcriptional network of genes essential for
mitotic progression and genes encoding the SCF (Skp2-Cks1)
ubiquitin ligase. Mol Cell Biol. 25:10875–10894. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Costa RH: FoxM1 dances with mitosis. Nat
Cell Biol. 7:108–110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kopanja D, Pandey A, Kiefer M, Wang Z,
Chandan N, Carr JR, Franks R, Yu DY, Guzman G, Maker A and
Raychaudhuri P: Essential roles of FoxM1 in Ras-induced liver
cancer progression and in cancer cells with stem cell features. J
Hepatol. 63:429–436. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Zhang J, Cui X, Yang Y, Li M, Qu
J, Li J and Wang J: FoxM1: A novel tumor biomarker of lung cancer.
Int J Clin Exp Med. 8:3136–3140. 2015.PubMed/NCBI
|
|
14
|
Bergamaschi A, Madak-Erdogan Z, Kim YJ,
Choi YL, Lu H and Katzenellenbogen BS: The forkhead transcription
factor FOXM1 promotes endocrine resistance and invasiveness in
estrogen receptor-positive breast cancer by expansion of stem-like
cancer cells. Breast Cancer Res. 16:4362014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wan X, Yeung C, Kim SY, Dolan JG, Ngo VN,
Burkett S, Khan J, Staudt LM and Helman LJ: Identification of
FoxM1/Bub1b signaling pathway as a required component for growth
and survival of rhabdomyosarcoma. Cancer Res. 72:5889–5899. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferraris GM and Sidenius N: Urokinase
plasminogen activator receptor: A functional integrator of
extracellular proteolysis, cell adhesion, and signal transduction.
Semin Thromb Hemost. 39:347–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sidenius N and Blasi F: The urokinase
plasminogen activator system in cancer: Recent advances and
implication for prognosis and therapy. Cancer Metastasis Rev.
22:205–222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duffy MJ: The urokinase plasminogen
activator system: Role in malignancy. Curr Pharm Des. 10:39–49.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rabbani SA and Mazar AP: The role of the
plasminogen activation system in angiogenesis and metastasis. Surg
Oncol Clin N Am. 10(393–415): x2001.
|
|
20
|
Duffy MJ, Maguire TM, McDermott EW and
O'Higgins N: Urokinase plasminogen activator: A prognostic marker
in multiple types of cancer. J Surg Oncol. 71:130–135. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choong PF and Nadesapillai AP: Urokinase
plasminogen activator system: A multifunctional role in tumor
progression and metastasis. Clin Orthop Relat Res. 415
Suppl:S46–S58. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Plebani M, Herszènyi L, Carraro P, De
Paoli M, Roveroni G, Cardin R, Tulassay Z, Naccarato R and Farinati
F: Urokinase-type plasminogen activator receptor in gastric cancer:
Tissue expression and prognostic role. Clin Exp Metastasis.
15:418–425. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu B, El Hajj N, Sittler S, Lammert N,
Barnes R and Meloni-Ehrig A: Gastric cancer: Classification,
histology and application of molecular pathology. J Gastrointest
Oncol. 3:251–261. 2012.PubMed/NCBI
|
|
24
|
Halasi M and Gartel AL: FOX(M1) news-it is
cancer. Mol Cancer Ther. 12:245–254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zeng J, Wang L, Li Q, Li W, Björkholm M,
Jia J and Xu D: FoxM1 is up-regulated in gastric cancer and its
inhibition leads to cellular senescence, partially dependent on p27
kip1. J Pathol. 218:419–427. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Okada K, Fujiwara Y, Takahashi T, Nakamura
Y, Takiguchi S, Nakajima K, Miyata H, Yamasaki M, Kurokawa Y, Mori
M and Doki Y: Overexpression of forkhead box M1 transcription
factor (FOXM1) is a potential prognostic marker and enhances
chemoresistance for docetaxel in gastric cancer. Ann Surg Oncol.
20:1035–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li X, Qi W, Yao R, Tang D and Liang J:
Overexpressed transcription factor FOXM1 is a potential diagnostic
and adverse prognostic factor in postoperational gastric cancer
patients. Clin Transl Oncol. 16:307–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hui MK, Chan KW, Luk JM, Lee NP, Chung Y,
Cheung LC, Srivastava G, Tsao SW, Tang JC and Law S: Cytoplasmic
Forkhead box M1 (FoxM1) in esophageal squamous cell carcinoma
significantly correlates with pathological disease stage. World J
Surg. 36:90–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen CH, Chien CY, Huang CC, Hwang CF,
Chuang HC, Fang FM, Huang HY, Chen CM, Liu HL and Huang CY:
Expression of FLJ10540 is correlated with aggressiveness of oral
cavity squamous cell carcinoma by stimulating cell migration and
invasion through increased FOXM1 and MMP-2 activity. Oncogene.
28:2723–2737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Banerjee S, Kong D, Li Y and
Sarkar FH: Down-regulation of Forkhead Box M1 transcription factor
leads to the inhibition of invasion and angiogenesis of pancreatic
cancer cells. Cancer Res. 67:8293–8300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Q, Zhang N, Jia Z, Le X, Dai B, Wei D,
Huang S, Tan D and Xie K: Critical role and regulation of
transcription factor FoxM1 in human gastric cancer angiogenesis and
progression. Cancer Res. 69:3501–3509. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Petersen LC, Lund LR, Nielsen LS, Danø K
and Skriver L: One-chain urokinase-type plasminogen activator from
human sarcoma cells is a proenzyme with little or no intrinsic
activity. J Biol Chem. 263:11189–11195. 1988.PubMed/NCBI
|
|
33
|
Bekes EM, Deryugina EI, Kupriyanova TA,
Zajac E, Botkjaer KA, Andreasen PA and Quigley JP: Activation of
pro-uPA is critical for initial escape from the primary tumor and
hematogenous dissemination of human carcinoma cells. Neoplasia.
13:806–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang L and Han X: The urokinase
plasminogen activator system in breast cancer invasion and
metastasis. Biomed Pharmacother. 67:179–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Heiss MM, Simon EH, Beyer BC, Gruetzner
KU, Tarabichi A, Babic R, Schildberg FW and Allgayer H: Minimal
residual disease in gastric cancer: Evidence of an independent
prognostic relevance of urokinase receptor expression by
disseminated tumor cells in the bone marrow. J Clin Oncol.
20:2005–2016. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Herceg Horvatic G, Herceg D, Kralik M,
Kulic A, Bence-Zigman Z, Tomic-Brzac H, Bracic I, Kusacic-Kuna S
and Prgomet D: Urokinase plasminogen activator and its inhibitor
type-1 as prognostic factors in differentiated thyroid carcinoma
patients. Otolaryngol Head Neck Surg. 149:533–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu HY, Shen XH, Ni RS, Qian XY and Gao X:
Expression of E-cadherin and uPA and their prognostic value in
carcinoma of human larynx. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke
Za Zhi. 44:1024–1028. 2009.(In Chinese). PubMed/NCBI
|
|
38
|
Chen H, Zou Y, Yang H, Wang J and Pan H:
Downregulation of FoxM1 inhibits proliferation, invasion and
angiogenesis of HeLa cells in vitro and in vivo. Int J Oncol.
45:2355–2364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ahmad A, Wang Z, Kong D, Ali S, Li Y,
Banerjee S, Ali R and Sarkar FH: FoxM1 down-regulation leads to
inhibition of proliferation, migration and invasion of breast
cancer cells through the modulation of extra-cellular matrix
degrading factors. Breast Cancer Res Treat. 122:337–346. 2010.
View Article : Google Scholar : PubMed/NCBI
|