Introduction
Gliomas are the most common malignant tumors of the
central nervous system, accounting for ~50% of all intracranial
tumors. Gliomas are often located in functional areas exhibiting
invasive growth and leading to progressive deterioration; also
recurrences are common (1).
Treatments for glioma include surgery, radiotherapy, chemotherapy,
molecular biology approaches and gene therapy, with surgery being
the most common approach. However, complete removal is hard to
achieve, resulting in common unsatisfactory outcomes and a high
recurrence rate (2). The survival
time of patients with recurrent glioma is usually short. Without
timely treatment, survival time is usually shorter than 6 months.
In addition, the life quality of patients is usually poor.
Therefore, recurrent gliomas present an ongoing challenge for the
treatment of neurological diseases (3). Although in vitro radiotherapy can
effectively kill glioma tumor cells, it can also bring damage to
surrounding healthy brain tissue, skin and scalp, and the amount of
collateral damage increases with higher doses. In all, the
development of a novel treatment that can effectively kill tumor
cells and improve outcomes, while reducing the side effects of
radiation treatment is urgently needed (4,5).
125I seed implantation is a kind of in vivo
radiotherapy. 125I seed implantation achieves low dose
continuous exposure, high accuracy and killing of tumor cells in
short-range (6). In this study,
patients with recurrent glioma were treated with surgery combined
with 125I seed implantation, and their prognosis was
analyzed. Our study provides scientific evidence for the
effectiveness of this treatment.
Materials and methods
General information: Sixty-six patients with
recurrent gliomas were selected in Yidu Central Hospital of Weifang
from April, 2011 to March, 2014. Patients were randomly divided
into a surgery alone (control) group and a surgery combined with
125I seed implantation (observation) group, with 33
patients in each. The presence of a malignant glioma diagnosed by
pathological examination, with recurrent tumor diagnosed by head
MRI, measurable lesions and an expected survival longer than 8
weeks were all characteristics of the inclusion criteria for the
patients. Additionally, all signed informed consent forms. Patients
excluded from the study were those with severe uncontrollable
hypertension, transient ischemic attack, shock or cerebral
hemorrhage; patients with severe coagulation dysfunction; and
patients with a preoperative Karnofsky Performance Status (KPS)
score lower than 50 points. There were no significant differences
in the general information of patients between the two groups
(P>0.05) (Table I).
 | Table I.Comparison of general information
between two groups. |
Table I.
Comparison of general information
between two groups.
| Items | Control group
(n=33) | Observation group
(n=33) |
t/χ2-test | P-value |
|---|
| Sex
(male/female) | 16/17 | 18/15 | 0.061 | 0.806 |
| Age (years) | 40–70 | 40–75 |
|
|
| Average age
(years) | 54.78±6.49 | 54.32±6.58 | 0.286 | 0.776 |
| Preoperative KPS
score | 62.78±5.49 | 62.32±5.58 | 0.338 | 0.737 |
| Tumor site (n,
%) |
|
|
|
|
|
Superficial | 19 (57.58) | 21 (63.64) | 0.064 | 0.801 |
| Near
midline | 14 (42.42) | 12 (36.36) |
|
|
| Pathological grade
(n, %) |
|
|
|
|
| Grade
II | 8
(24.24) | 7
(21.21) | 0.011 | 0.995 |
| Grade
III | 15 (45.45) | 17 (51.52) |
|
|
| Grade
IV | 10 (30.30) | 9
(27.27) |
|
|
Methods
Surgical treatment
Patients in both groups were treated with surgery
under general anesthesia. A site on the original surgical incision
showed the shortest distance to the tumor surface. The bone flap
was lifted up and meninges were opened. Adhesions between the tumor
and brain tissues were dissected. Tumor and peripheral edema tissue
resection was maximized regardless of proximity to important brain
function area and its blood vessels. If the tumor capsule showed
serious hemorrhage necrosis, some of the necrotic tissue was
removed. If the tumor capsule showed cystic changes, fluid was
first removed by aspiration, and then the tumor was resected
piecemeal along the edges.
Seed implantation
The National Research Centre of Isotope Technology
produced the 125I seed, in the China Institute of Atomic
Energy. The seed activity was reportedly 0.5–0.7 mCi. According to
the results of preoperative enhanced MRI results, the
125I seed was implanted within a 1 cm radius to the
center of the original tumor. Interval was 1.0 cm, depth was
0.5–1.0 cm and functional area was 80 Gy. After implantation,
gelatin sponge and hemostatic gauze were used to cover the
manipulation position to prevent shedding of seed and local
bleeding.
Postoperative treatment
After treatment, patients were subjected to
conventional anti-infection and antiepileptic prophylactic
treatments. Enhanced MRI examination was performed every 2 months
after surgery to observe any developing changes in the patients'
physical signs, and tumor volumes. Changes in KPS scores, ensuing
complications and survival time were recorded.
Evaluation criteria
The efficacy of treatment was evaluated 3 months
after surgery, according to the evaluation criteria for solid
tumors. Complete remission (CR) meant all visible lesions had
disappeared for >4 weeks after the surgical procedure. Partial
remission (PR) meant the tumor diameter was reduced >50% and
remained so for >4 weeks after surgery. A stable disease (SD)
meant the tumor was still present and had not improved to at least
to the level of the PR. Progression of disease (PD) was obvious if
the target lesion had increased in diameter by >20% or if a new
lesion appeared. The overall objective response rate (ORR) was
calculated by the equation ORR = (CR + PR)/total number, and the
disease control rate (DCR) by the equation DCR = (CR + PR +
SD)/total number.
The occurrence of any adverse reactions within 1
month after surgery was evaluated according to the Common
Terminology Criteria for Adverse Events (CTCAE) (7). Grade I patients had mild or no symptoms,
and no treatment was needed. Grade II patients had instrumental
activities of daily living limited by adverse reactions but only
local or non-invasive treatment was needed to improve their well
being. Grade III patients had their daily life activities limited,
they had severe responses or disability but no immediate threat to
life, they needed to be hospitalized or their hospital stay needed
to be extended. Grade IV patients experienced life-threatening
complications, needing emergency treatment. Finally, patients were
classified in grade V if death ensued due to complications.
The life qualities of patients at 6, 12 and 18
months after surgery were evaluated according to KPS scoring
criteria (8). The scores were
positively correlated with the life quality of the patients
(Table II).
 | Table II.KPS scoring criteria. |
Table II.
KPS scoring criteria.
| Status of
patients | Score | Status of
patients | Score |
|---|
| Normal, no symptoms
or abnormal physical signs | 100 | Dependant on others
for daily life and special care is needed | 40 |
| Normal activities
with minor illness | 90 | Seriously dependant
in daily life | 30 |
| Normal activities
with slight difficulties | 80 | Bed-ridden,
hospitalization is needed but no threat to life | 20 |
| Independent in daily
life but cannot work normally | 70 | Life-threatening
situation | 10 |
| Mostly independent in
daily life and help is needed occasionally | 60 | Death | 0 |
| Help is needed
frequently | 50 |
|
|
Statistical analysis
Data were processed using SPSS 19.0 (SPSS Inc.,
Chicago, IL, USA) software. Measurement data were expressed as mean
± standard deviation (mean ± SD), and t-tests were performed for
comparisons between groups. Count data were expressed as rate, and
comparisons between groups were performed using χ2
tests. Survival analysis was performed by Kaplan-Meier survival
analysis. Single factor analysis for survival was performed using
log-rank test. Factors with statistical significance in single
factor analysis were further subjected to multi-factor Cox
regression analysis. A P<0.05 was considered to be statistically
significant.
Results
The treatment efficacies in the two groups were
compared at 3 months after surgery. The results showed that ORR and
DCR in the observation group (69.69 and 93.94%, respectively) were
higher than those in the control group (42.42 and 69.69%,
respectively), and the differences were statistically significant
(P<0.05) (Table III).
 | Table III.Comparison of short-term efficacy in
the two groups (n, %). |
Table III.
Comparison of short-term efficacy in
the two groups (n, %).
| Groups | CR | PR | SD | PD | ORR | DCR |
|---|
| Observation
group | 12 (36.36) | 11 (33.33) | 8 (24.24) | 2 (6.06) | 23 (69.69) | 31 (93.94) |
| Control group | 6
(18.18) | 8
(24.24) | 9 (27.27) | 10 (30.30) | 14 (42.42) | 23 (69.69) |
| χ2
test |
|
|
|
| 3.937 | 4.991 |
| P-value |
|
|
|
| 0.047 | 0.026 |
The occurrence of adverse reactions within 1 month
after surgery was compared between the two groups, no significant
differences in adverse reactions were found between the groups
(Table IV).
 | Table IV.Comparison of adverse reactions
between the groups (n, %). |
Table IV.
Comparison of adverse reactions
between the groups (n, %).
| Groups | Cases | Grade I | Grade II | Grade III | Grade IV | Grade V |
|---|
| Observation
group | 33 | 24 (72.73) | 7 (21.21) | 2 (6.06) | 0 (0.00) | 0 (0.00) |
| Control group | 33 | 26 (78.79) | 6 (18.18) | 1 (3.03) | 0 (0.00) | 0 (0.00) |
| χ2
test |
| 0.490 |
|
|
|
|
| P-value |
| 0.783 |
|
|
|
|
The postoperative KPS score was compared between the
two groups. The results showed that the KPS scores in the
observation group were significantly higher than those in the
control group at 6, 12 and 18 months after operation (P<0.05)
(Table V).
 | Table V.Comparison of KPS scores between the
two groups. |
Table V.
Comparison of KPS scores between the
two groups.
| Groups | 6 months after
operation | 12 months after
operation | 18 months after
operation |
|---|
| Observation
group | 84.67±3.23 | 71.35±3.28 | 60.68±3.24 |
| Control group | 76.27±3.64 | 62.47±3.36 | 45.83±3.78 |
| t-test | 9.215 | 8.812 | 11.084 |
| P-value | <0.001 | <0.001 | <0.001 |
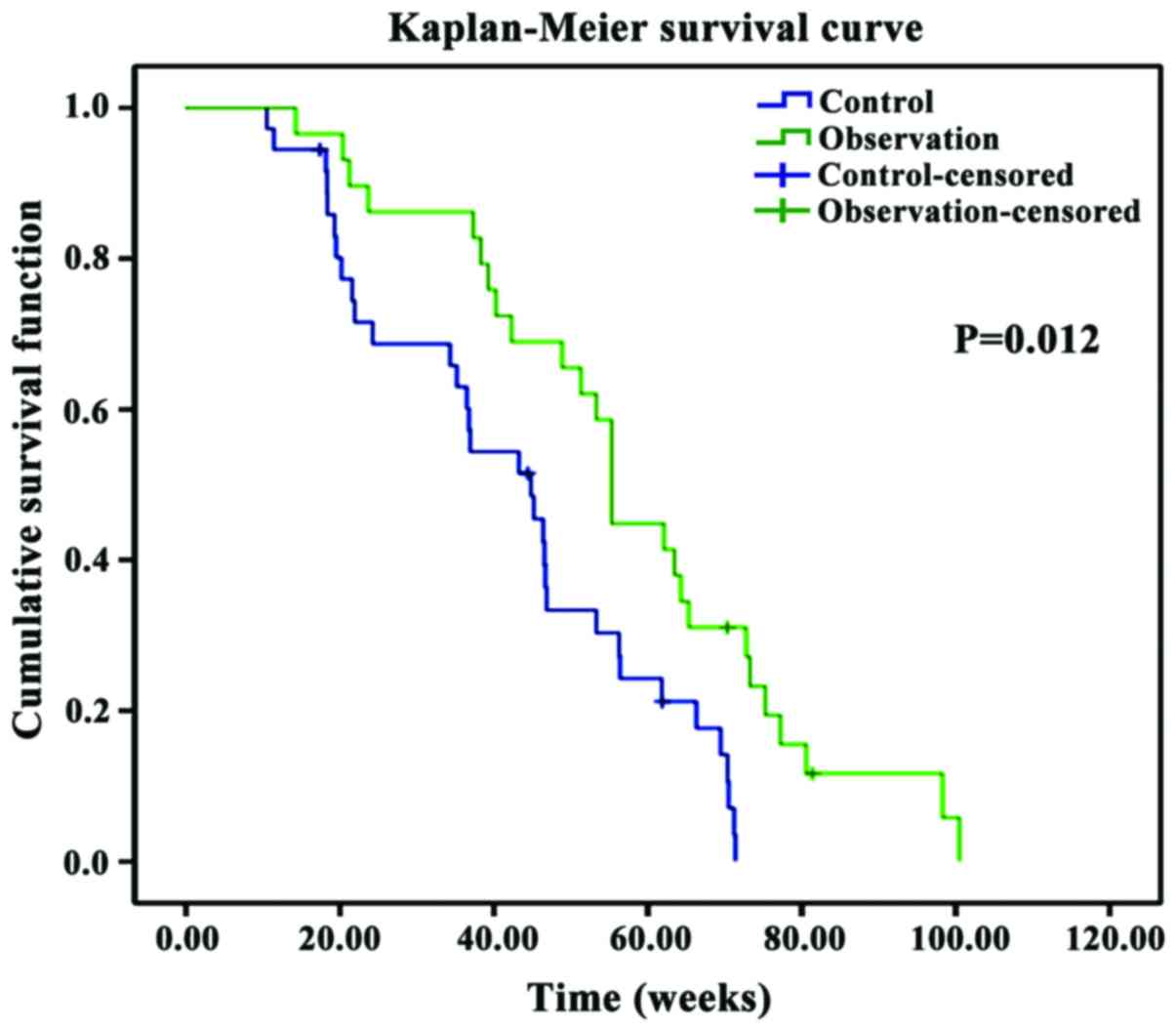
The survival of patients in the two groups was also
compared. Results showed that the mean survival time was longer and
the postoperative survival rate was higher in the observation group
when compared to the same parameters in the control group
(P<0.05) (Table VI and Fig. 1).
 | Table VI.Comparison of survival between two
groups. |
Table VI.
Comparison of survival between two
groups.
| Group | Cases | 6-month survival
rate (n, %) | 12-month survival
rate (n, %) | 18-month survival
rate (n, %) | Mean survival time
(week) |
|---|
| Observation
group | 33 | 32 (96.97) | 27 (81.82) | 19 (57.58) | 67.56±7.48 |
| Control group | 33 | 25 (75.76) | 18 (54.55) | 10 (30.30) | 52.64±7.53 |
|
χ2/t-test |
| 4.632 | 4.470 | 3.937 | 8.075 |
| P-value |
| 0.031 | 0.035 | 0.047 | <0.001 |
The Kaplan-Meier analysis showed that the survival
time was significantly longer in the observation group compared to
that in the control group (P<0.05).
The univariate analysis performed by a log-rank test
showed that preoperative KPS score, tumor site, tumor pathological
grade and degree of tumor resection were all adverse factors
influencing the prognosis of the patients (P<0.05) (Table VII).
 | Table VII.Univariate analysis for
prognosis. |
Table VII.
Univariate analysis for
prognosis.
|
|
|
|
| cLog-rank test |
|---|
|
|
|
|
|
|
|---|
| Items | Proportion (n,
%) | Median survival
time (weeks) | 95% confidence
interval (95% CI) | χ2
test | P-value |
|---|
| Age |
|
|
|
|
|
| <60
years | 30 (45.45) | 54.93 | 23.62–62.75 | 0.122 | 0.531 |
| ≥60
years | 36 (54.55) | 52.43 | 20.74–61.62 |
|
|
| Sex |
|
|
|
|
|
|
Male | 34 (51.52) | 55.82 | 21.53–63.84 | 0.006 | 0.943 |
|
Female | 32 (48.48) | 56.63 | 22.72–68.47 |
|
|
| Tumor pathological
grade |
|
|
|
|
|
| Grade
II | 15 (22.73) | 74.86 | 29.82–81.39 | 23.759 | <0.001 |
| Grade
III | 32 (48.48) | 56.75 | 28.71–66.32 |
|
|
| Grade
IV | 19 (28.79) | 41.32 | 14.36–56.73 |
|
|
| Tumor site |
|
|
|
|
|
|
Superficial | 39 (59.09) | 63.48 | 25.76–78.32 | 7.994 | 0.019 |
| Near
midline | 27 (40.91) | 51.56 | 23.48–59.65 |
|
|
| Tumor resection
degree |
|
|
|
|
|
| Total
resection | 45 (68.18) | 66.57 | 28.31–79.46 | 9.264 | 0.017 |
|
Subtotal resection | 21 (32.82) | 50.72 | 24.46–58.23 |
|
|
| Preoperative KPS
score |
|
|
|
|
|
|
≥70 | 44 (66.67) | 63.62 | 28.33–70.36 | 11.543 | 0.001 |
|
<70 | 22 (33.33) | 54.58 | 23.48–59.53 |
|
|
| Tumor diameter
(cm) |
|
|
|
|
|
|
>3 | 30 (45.45) | 56.39 | 27.73–74.92 | 0.469 | 0.541 |
| ≤3 | 36 (54.55) | 58.47 | 24.86–76.53 |
|
|
The multivariate Cox regression analysis for
prognosis showed that preoperative KPS score, tumor pathological
grade and degree of tumor resection were all independent risk
factors of prognosis (P<0.05) (Table VIII).
 | Table VIII.Multivariate Cox regression analysis
for prognosis. |
Table VIII.
Multivariate Cox regression analysis
for prognosis.
| Factors | B | SE | Wald | HR | 95% CI | P-value |
|---|
| Preoperative KPS
score | 0.789 | 0.030 | 9.021 | 3.215 | 1.731–6.158 | 0.008 |
| Degree of tumor
resection | 0.331 | 0.512 | 3.783 | 1.231 | 0.975–2.957 | 0.014 |
| Tumor pathological
grade | 0.467 | 0.673 | 5.327 | 9.013 | 3.456–14.854 | 0.026 |
Discussion
Gliomas usually develop deep within the brain. These
tumors usually cause poor life quality and show invasive growth and
characteristics leading to high mortality rates like rapid
progression and a low curing rate (9). Clinical manifestations of glioma include
malignant vomiting, headaches, optic disc edema, neurological
deficits, psychological changes, hemiplegia and ataxia, and
invasion and distant metastasis can easily occur (10). Surgery is the preferred treatment for
gliomas. However, gliomas can frequently recur within 6–10 months
after surgery. Once recurrence has happened, neurological damage in
patients increases, the area of the nerve structures damaged is
enlarged, and short-term decline in the level of awareness ensues,
eventually leading to brain failure and death (11). The main reason for recurrence is the
existence of glioma cell infiltration around the primary tumor, and
most of the recurrent lesions are found in an area within 2 cm
around the tumor, so control of local recurrence is essential for
treatment of malignant gliomas (12).
Treatment of recurrent gliomas requires secondary
surgery or chemotherapy and radiotherapy. However, the blood-brain
barrier increases difficulties in delivering drugs to brain, so
chemotherapy drugs cannot be used to effectively treat gliomas. In
addition, chemotherapy is usually accompanied by severe adverse
side effects, seriously affecting life quality of the patients
(13). Radiation therapy can be used
to reduce the size of the tumor through ionization, which in turn
alleviates the symptoms and extends the survival time of patient
(14). However, before reaching tumor
tissues, radiation will first bring damage to surrounding brain
tissue and subcutaneous structures, and this damage can be
increased with the increase in radiation dose (15).
Seed implantation therapy is a new type of
brachytherapy, and is also called interstitial brachytherapy. Based
on the inverse square law, the radiation dose is reduced
substantially with distance from the source. Energy release can
usually reach 80% of its total within the area 1 cm round the seed
implant, and tumor cells within this range can be effectively
killed, while the damage to normal cells within this area is
reduced due to the rapid decline in dose (16,17). The
results of this study showed the effective rate of treatment in the
observation group was significantly higher than that in the control
group at 6 months after operation (P<0.05), but no significant
differences in adverse reactions were found between the groups
(P>0.05). The KPS scores in the observation group were
significantly higher than those in the control group at 6, 12 and
18 months after operation (P<0.05), the reason probably being
that the half-life of the 125I seed is 59.4 day,
providing effective radiation for four half-lives, and releasing
low-energy radiation to kill tumor cells, which improves outcomes
(17). Moreover, the dose
distribution is more uniform in seed implantation treatment
compared with other treatments, so infections caused by surgery can
be reduced without inducing significant increase in normal brain
tissue damage (17). The
125I seed can provide 240 days of continuous
irradiation, so that the treatment effect can last longer.
Therefore, symptoms of patients can be effectively alleviated and
life quality can be significantly improved (16).
Surgical treatment of recurrent gliomas requires
maximum resection of tumoral tissues sparing important cortical
functional areas (18). Seed
implantation should be combined with MRI, to ensure optimal
placement, the implanted particles should show triangle or square
shape (19). In order to avoid the
shedding of 125I seed and hemorrhages, the seed should
be fixed in position with a gelatin sponge. The puncture point
should be treated with hemostatic treatment, and there should be
constant monitoring to detect internal bleeding.
This study included analyses of prognostic factors.
Multivariate Cox regression showed that preoperative KPS scores,
tumor pathological grade and degree of tumor resection were
independent risk factors of prognosis (P<0.05). All those three
factors can affect the patient's neurological status. Life quality
of patients with recurrent glioma is clinically reflected in their
KPS score, and KPS scoring is an important part of prognosis
prediction providing importance guidance. The median survival time
of patients with KPS scores ≥70 was significantly higher than that
of patients with KPS scores <70. This is because tumor
metastasis and invasion can be indirectly evaluated by the KPS
score. The degree of tumor resection can affect the depth and
location of 125I seed implantation, resulting in better
efficacies when resection is highly successful so that the
inhibitory effect of 125I seed implantation is optimal.
Also, the survival rate of recurrent gliomas is decreased with the
increase of pathological grade, which is consistent with the
results of case studies world-wide, meaning that pathological grade
is a risk factor of prognosis (20).
We are aware of the limitations of our study due to
the small sample size, so larger studies are needed. Nevertheless,
based on our findings, surgery combined with 125I seed
implantation can effectively inhibit the growth of recurrent
gliomas, improve the patient's condition, delay the recurrence of
the tumors, and extend the survival time.
References
|
1
|
Lu HC, Ma J, Zhuang Z, Qiu F, Cheng HL and
Shi JX: Exploring the regulatory role of isocitrate dehydrogenase
mutant protein on glioma stem cell proliferation. Eur Rev Med
Pharmacol Sci. 20:3378–3384. 2016.PubMed/NCBI
|
|
2
|
Eckel-Passow JE, Lachance DH, Molinaro AM,
Walsh KM, Decker PA, Sicotte H, Pekmezci M, Rice T, Kosel ML,
Smirnov IV, et al: Glioma Groups based on 1p/19q, IDH, and TERT
promoter mutations in tumors. N Engl J Med. 372:2499–2508. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sakai K, Shimodaira S, Maejima S, Udagawa
N, Sano K, Higuchi Y, Koya T, Ochiai T, Koide M, Uehara S, et al:
Dendritic cell-based immunotherapy targeting Wilms' tumor 1 in
patients with recurrent malignant glioma. J Neurosurg. 123:989–997.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munck AF, Rosenschold P, Costa J,
Engelholm SA, Lundemann MJ, Law I, Ohlhues L and Engelholm S:
Impact of [18F]-fluoro-ethyl-tyrosine PET imaging on target
definition for radiation therapy of high-grade glioma. Neuro Oncol.
17:757–763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laack NN, Sarkaria JN and Buckner JC:
Radiation therapy oncology group (RTOG) 98–02: Controversy or
consensus in the treatment of newly diagnosed low grade glioma
(LGG). Semin Radiat Oncol. 25:197–202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Guo F, Zhang WL, Huang DS, Hong L
and Han T: Clinical application of 125I particle
implantation in children with rhabdomysarcoma of the head and neck.
Zhongguo Dang Dai Er Ke Za Zhi. 14:437–440. 2012.(In Chinese).
PubMed/NCBI
|
|
7
|
van Zweeden AA, van der Vliet HJ, Wilmink
JW, Meijerink MR, Meijer OW, Bruynzeel AM, van Tienhoven G,
Giovannetti E, Kazemier G, Jacobs MA, et al: Phase I clinical trial
to determine the feasibility and maximum tolerated dose of
panitumumab to standard gemcitabine-based chemoradiation in locally
advanced pancreatic cancer. Clin Cancer Res. 21:4569–4575. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chambless LB, Kistka HM, Parker SL,
Hassam-Malani L, McGirt MJ and Thompson RC: The relative value of
postoperative versus preoperative Karnofsky Performance Scale
scores as a predictor of survival after surgical resection of
glioblastoma multiforme. J Neurooncol. 121:359–364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cohen AL and Colman H: Glioma biology and
molecular markers. Cancer Treat Res. 163:15–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Paw I, Carpenter RC, Watabe K, Debinski W
and Lo HW: Mechanisms regulating glioma invasion. Cancer Lett.
362:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pang C, Guan Y, Zhao K, Chen L, Bao Y, Cui
R, Li G and Wang Y: Up-regulation of microRNA-15b correlates with
unfavorable prognosis and malignant progression of human glioma.
Int J Clin Exp Pathol. 8:4943–4952. 2015.PubMed/NCBI
|
|
12
|
Ceccarelli M, Barthel FP, Malta TM,
Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh
A, Pagnotta SM, et al: TCGA Research Network: Molecular profiling
reveals biologically discrete subsets and pathways of progression
in diffuse glioma. Cell. 164:550–563. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma KX, Wang HJ, Li XR, Li T, Su G, Yang P
and Wu JW: Long noncoding RNA MALAT1 associates with the malignant
status and poor prognosis in glioma. Tumour Biol. 36:3355–3359.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rizzo AE and Yu JS: Radiation therapy for
glioma stem cells. Adv Exp Med Biol. 853:85–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McTyre E, Lucas JT, Helis C, Farris M,
Soike M, Mott R, Laxton AW, Tatter SB, Lesser GJ, Strowd RE, et al:
Outcomes for anaplastic glioma treated with radiation therapy with
or without concurrent temozolomide. Am J Clin Oncol. Mar
15–2017.(Epub ahead of print). doi: 10.1097/COC.0000000000000380.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dyk PT, Richardson S, Badiyan SN, Schwarz
JK, Esthappan J, Garcia-Ramirez JL and Grigsby PW: Outpatient-based
high-dose-rate interstitial brachytherapy for gynecologic
malignancies. Brachytherapy. 14:231–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Denecke T, Stelter L, Schnapauff D,
Steffen I, Sinn B, Schott E, Seidensticker R, Puhl G, Gebauer B,
Hänninen EL, et al: CT-guided interstitial brachytherapy of
hepatocellular carcinoma before liver transplantation: An
equivalent alternative to transarterial chemoembolization? Eur
Radiol. 25:2608–2616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao X, Bai HX, Zou Y and Yang L: Letter:
Reoperation for recurrent high-grade glioma: Does tumor genetics
play a role? Neurosurgery. 76:E496–E497. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi S, Yang J and Sun D: CT-guided
125I brachytherapy on pulmonary metastases after
resection of colorectal cancer: A report of six cases. Oncol Lett.
9:375–380. 2015.PubMed/NCBI
|
|
20
|
Li G, Zhang Z, Tu Y, Jin T, Liang H, Cui
G, He S and Gao G: Correlation of microRNA-372 upregulation with
poor prognosis in human glioma. Diagn Pathol. 8:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|