Introduction
Breast cancer is one of the most common malignancies
in women. According to statistics, breast cancer accounts for 7–10%
of all malignancies and is next only to uterine cancer in women
(1). The pathogenesis of breast
cancer is often related to genetic factors. Pre- and postmenopausal
women between 40 and 60 years of age have the highest incidence and
tumors are commonly originated in mammary gland epithelial tissue
(1,2).
Statistical data published by WHO in 2008 indicated that new cases
of breast cancer reached 1.38 million/year, ranking second among
women. In addition, breast cancer accounted for 23% of new cases of
cancer and ranked fifth in the causes of cancer death. Thus, breast
cancer remains a serious threat to women's health (3,4).
The existing clinical treatments for breast cancer
usually have large adverse reactions, not only causing excruciating
pain but also seriously reducing the quality of life. Chinese herbs
have many advantages in traditional Chinese medicine. Its effective
ingredients are characterized by high efficiency and low toxicity,
and recently both domestic and foreign scientists have paid more
attention to Chinese herbs due to these important advantages. The
active ingredients from extracts of Chinese herbs play significant
roles in inhibiting cancer cell proliferation, inducing apoptosis,
and reducing the adverse effects of chemotherapy and radiotherapy
(5). In recent years, Chinese herbs
have become a hot spot due to minor side effects, anticancer and
immune regulatory effects (6).
Coumarin is a compound with the benzo-α-pyrone nucleus that has
many biological activities with broad medicinal value, including
anticancer, immune support, antivirus, anti-bacterial,
anti-oxidation, antiarrhythmic and anti-osteoporosis (7). Fraxetin is a simple coumarin compound
and is an active ingredient of traditional Chinese medicine Cortex
Fraxini. Fraxetin has received recent attention for its antitumor,
anti-oxidation effects, as well as other pharmacological effects
(8).
Bcl-2 can inhibit cell apoptosis and plays a
critical role in the regulation of apoptosis. In addition, Bcl-2
can protect cells from death, improve cell survival, and increase
the number of cells (9). Bax and
Bcl-2 belong to the same family, but Bax promotes apoptosis. Bax
antagonizes the apoptosis inhibitory effect of Bcl-2 gene
and directly promotes cell apoptosis by interaction with cells
(10). Factor-associated suicide
(Fas) is a cell death factor that can induce apoptosis, and when
Fas combines with the corresponding Fas ligand (FasL) on the cell
surface, it activates an intracellular-related apoptotic signaling
pathway (11,12).
The aim of the current study was to investigate the
effect of fraxetin on the proliferation and apoptosis of human
breast cancer cells MCF-7. We examined the mechanism by which
fraxetin induces apoptosis. This study lays the foundation for the
clinical treatment of breast cancer.
Materials and methods
Cell culture
MCF-7 breast cancer cells (Cell Bank, Chinese
Academy of Sciences, Shanghai, China) were cultured at 37°C in 5%
CO2 until 85% confluence was reached. Then, the cells
were digested with trypsin and cell suspension was diluted with
Dulbeccos modified Eagles medium (DMEM) (Gibco, Carlsbad, CA, USA)
containing 10% fetal bovine serum. After cell concentration was
adjusted to 2×108/liter, the cells were counted and
seeded in culture plates for the subsequent experiments.
Cell proliferation inhibition
rate
Cell proliferation was measured using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay after cells were treated with fraxetin (both from Sigma, St.
Louis, MO, USA). MCF-7 cells were seeded in 96-well plates at a
concentration of 1×105/ml and each well contained 100
µl. After 24 h, fraxetin was added to 10, 20, 40 and 60 µM final
concentrations. The control group was treated without fraxetin. The
cells were incubated for 24 and 48 h at 37°C in 5% CO2
and then culture medium was changed. MTT (10 µl) was added to each
well at a final concentration of 5 mg/ml. After incubating for 4 h,
the optical density (OD) at 570 nm was measured by a microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The
inhibition rate was calculated as: Inhibition rate (%) = (OD value
of control group - OD value of experimental group/OD value of
control group) × 100%.
Morphological observation
MCF-7 cells were treated with 20, 40 and 60 µM of
fraxetin for 24 h, and the morphological changes were observed and
recorded with an inverted microscope (Nikon, Tokyo, Japan).
4,6-Diamidino-2-phenylindole (DAPI)
staining
MCF-7 cells were seeded in 6-well plates at a
density of 1×104 cells/well. After 24 h, the supernatant
was suctioned and the cells were cultured in medium containing 20,
40 or 60 µM of fraxetin for 24 h, then washed with pre-cooled
phosphate-buffered saline (PBS) 3 times. DAPI solution (1 µg/ml)
was added to each well, the cells were incubated at 37°C for 5 min
and washed with pre-cooled PBS again. The cells were observed and
images were captured using a fluorescence microscope (Nikon) in the
dark.
Fas/FasL mRNA expression by
RT-PCR
MCF-7 cells were seeded in 6-well plates at a
density of 1×104 cells/well. After 24 h, the supernatant
was suctioned and the cells were cultured in medium containing 20,
40 or 60 µM of fraxetin for 24 h, the cells were collected and
total RNA was extracted according to the instructions of the RNA
extraction kit (Invitrogen, Carlsbad, CA, USA). RNA concentration
and purity (A260/A280 >1.8, indicating pure RNA) were determined
by UV-Vis spectrophotometer (Hitachi, Tokyo, Japan). cDNA was
obtained via reverse transcription from mRNA according to the
instruction of the reverse transcription kit (Invitrogen). The
expression of target genes was then detected using cDNA as the
template by RT-PCR assay, according to the manufacturer's
instructions (Invitrogen), and glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) was used as an internal control. The
primer sequences for Fas and FasL (Takara, Dalian, China) are shown
in Table I. Amplification conditions
were: 95°C for 10 min, 95°C for 15 sec, 60°C for 1 min, with 40
cycles of amplification. The Ct value was automatically calculated
using the CFX Manager software (Bio-Rad Laboratories, Inc.), and
the relative quantification of gene expression was calculated using
the 2−ΔCt method, as per the formula: ΔCt (target gene)
= Ct (target gene) - Ct (control gene).
 | Table I.Primer sequences for
Fas/FasL. |
Table I.
Primer sequences for
Fas/FasL.
| Gene | Primer sequences |
|---|
| Fas | F:
5′-GGCATCTGGACCCTCCTACCTCTG-3′ |
|
| R:
5′-CCTTGGAGTTGATGTCAGTCACTTGG-3′ |
| FasL | F: 5′-
GGCCTGTGTCTCCTTGTGAT-3′ |
|
| R:
5′-TGCCAGCTCCTTCTGAAGTA-3′ |
| GAPDH | F:
5′-ATGGCACCGTCAAGGCTGAG-3′ |
|
| R:
5′-GCAGTGATGGCATGGACTGT-3′ |
Bax and Bcl-2 protein expression by
western blotting
MCF-7 cells were seeded in 6-well plates at a
density of 1×104 cells/well. After 24 h, the supernatant
was suctioned and the cells were cultured in medium containing 20,
40 or 60 µM of fraxetin for 24 h. The cells were collected and
lysed with cell lysis buffer (Biyuntian Biotechnology Research
Institute, Nantong, China), centrifuged for 15 min at high speed
and low temperature, and the supernatant was collected. The
extracted protein concentrations were determined using a BCA
protein quantification kit (Biyuntian Biotechnology Research
Institute, Nantong, China). Subsequently, 50 µg protein was
separated by sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE), and the proteins were transferred to a
PVDF membrane. The membrane was incubated in blocking buffer for 1
h at room temperature and primary rabbit anti-huamn polyclonal
antibodies against GAPDH, Bax and Bcl-2 (dilution, 1:1,000; cat.
nos. 13937-1-AP, 23931-1-AP and 12789-1-AP; Sanying Biotechnology,
Wuhan, China) were added and the membrane was incubated overnight
at 4°C. After washing the membrane with TTBS, HRP-conjugated goat
anti-rabbit polyclonal secondary antibody (dilution, 1:2,000; cat.
no. SA00001-2; Sanying Biotechnology) was added and the membrane
was incubated at room temperature for 1 h. Enhanced
chemiluminescence (ECL) was added to the membrane and blots were
developed in the dark. Images were recorded with a gel imaging
system (Bio-Rad Laboratories, Richmond, CA, USA). GADPH was used as
the internal reference, and the gray-scale values were analyzed and
compared.
Statistical analysis
Data were presented as means ± standard deviations.
Data were analyzed by SPSS 17.0 (IBM Corp., Armonk, NY, USA) using
one-way ANOVA along with multiple comparison test. P<0.05 was
considered statistically significant.
Results
Effect of fraxetin on inhibition of
MCF-7 cell proliferation
We first examined the ability of fraxetin to inhibit
cell proliferation. MCF-7 cells were cultured in medium containing
0, 10, 20, 40 and 60 µM of fraxetin. After 24 h, the proliferation
of MCF-7 cells was significantly inhibited in all the groups
(Table II). The proliferation
inhibition rates were obtained in a dose- and time-dependent manner
(Table II). To examine the apoptotic
mechanism, 20, 40 and 60 µM of fraxetin were chosen as the drug
concentrations and incubation time was 24 h for the subsequent
experiments.
 | Table II.Inhibitory effects of fraxetin on
MCF-7 cell proliferation (mean ± SD). |
Table II.
Inhibitory effects of fraxetin on
MCF-7 cell proliferation (mean ± SD).
|
| Cell proliferation
inhibition rate (%) |
|---|
|
|
|
|---|
| Fraxetin (µM) | 24 h | 48 h |
|---|
| 0 | 0 | 0 |
| 10 |
9.25±0.12a |
12.32±1.13a |
| 20 |
18.13±0.31a |
42.32±2.31a |
| 40 |
35.67±1.32a |
58.22±2.28a |
| 60 |
46.23±1.56a |
62.17±2.02a |
Effect of fraxetin on MCF-7 cell
morphology
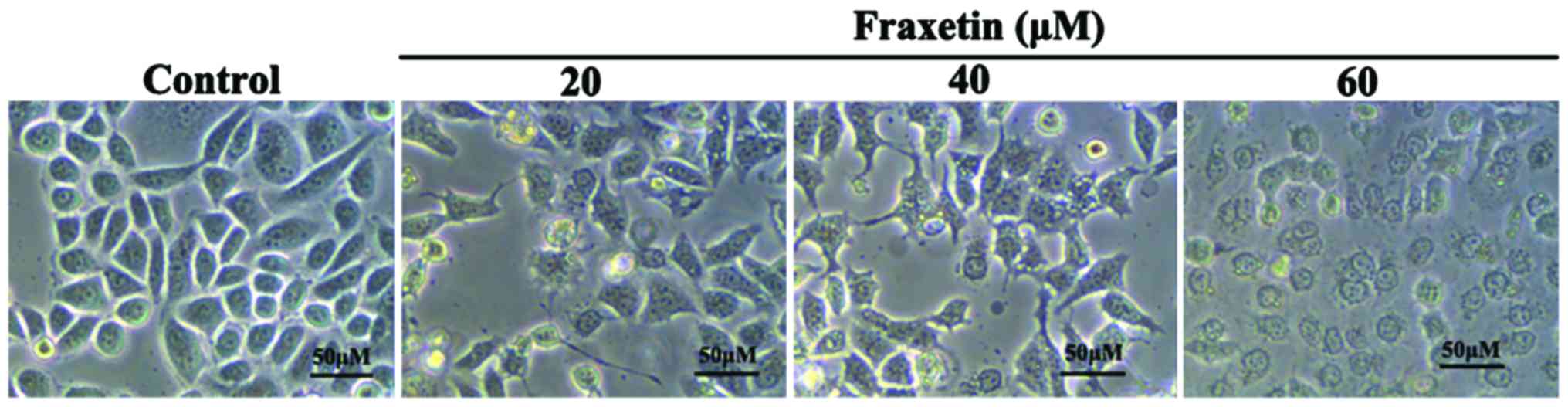
After MCF-7 cells were cultured for 24 h with 0, 20,
40 and 60 µM of fraxetin, the morphology of cells showed obvious
changes. We observed cell shrinkage, decreased cell adherence,
reduced cell number, and increased cell death number (Fig. 1). The changes in morphology exhibited
dose-dependence.
Effect of fraxetin on cell
apoptosis
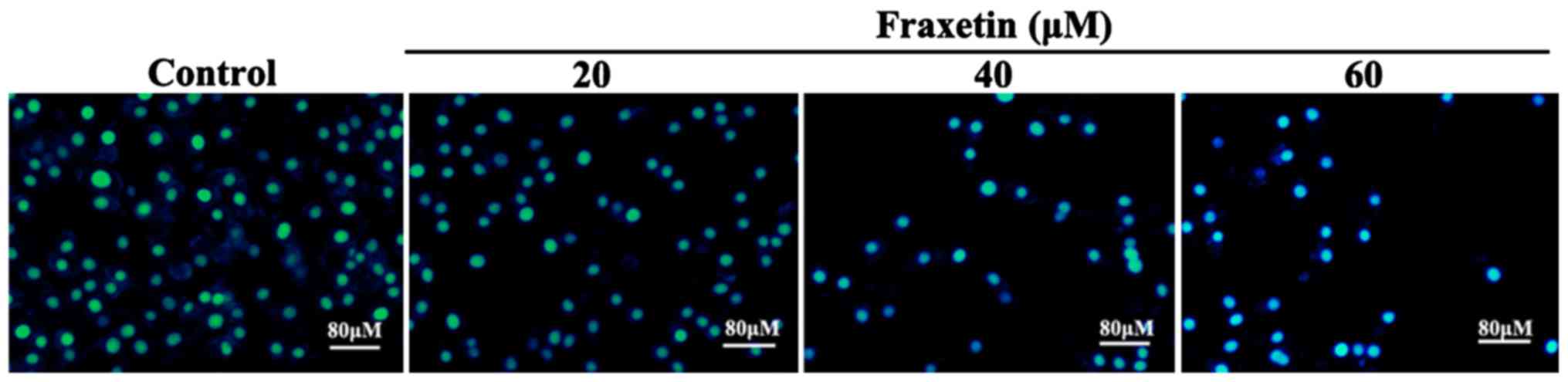
After MCF-7 cells were cultured for 24 h with 20, 40
and 60 µM of fraxetin, DAPI staining showed cell shrinkage and
nuclear chromatin condensation, suggesting the occurrence of
apoptosis (Fig. 2). Moreover, the
number of apoptotic cells increased with the increasing
concentrations of fraxetin.
Effect of fraxetin on expression of
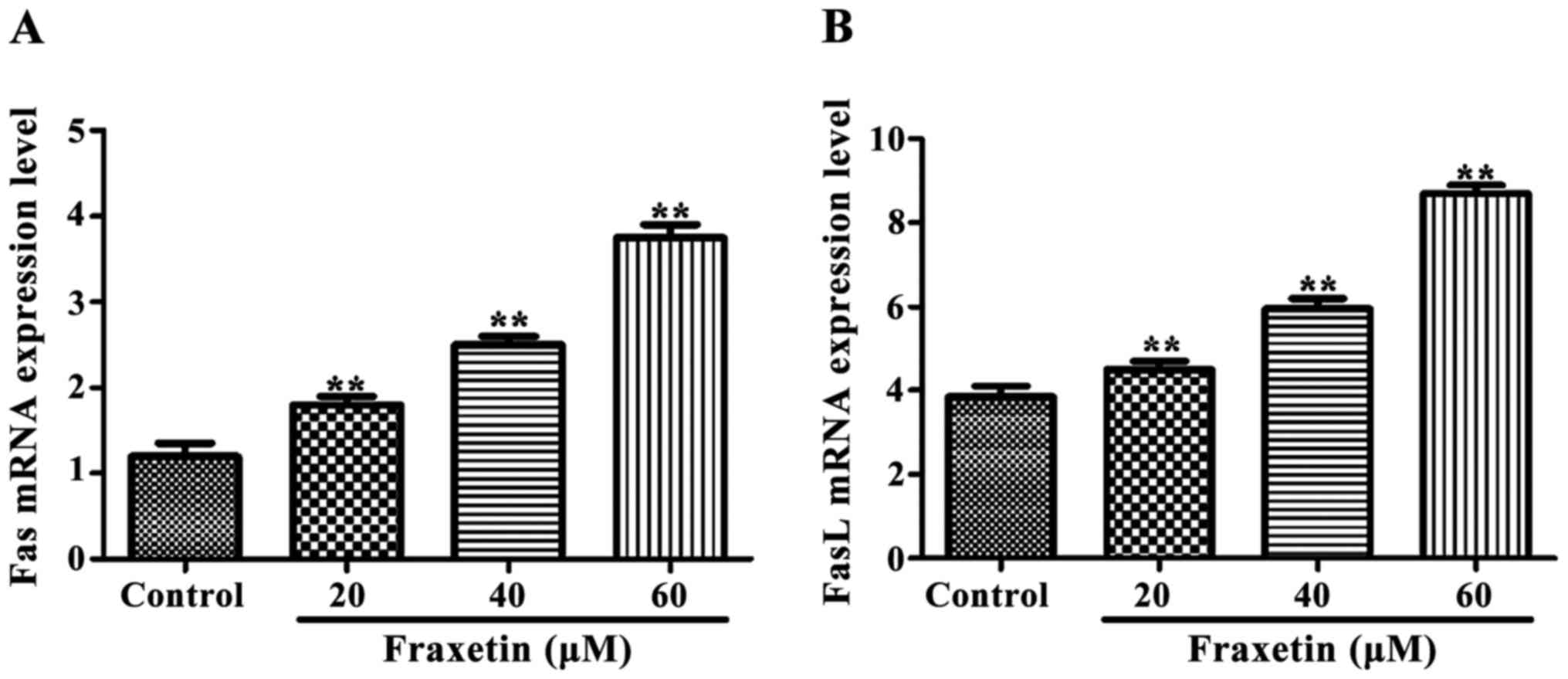
Fas and FasL mRNA
The expression of Fas and FasL mRNA
was significantly increased (P<0.01) after cells were incubated
for 24 h with 20, 40 and 60 µM of fraxetin compared to the control
cultured without fraxetin (Fig.
3).
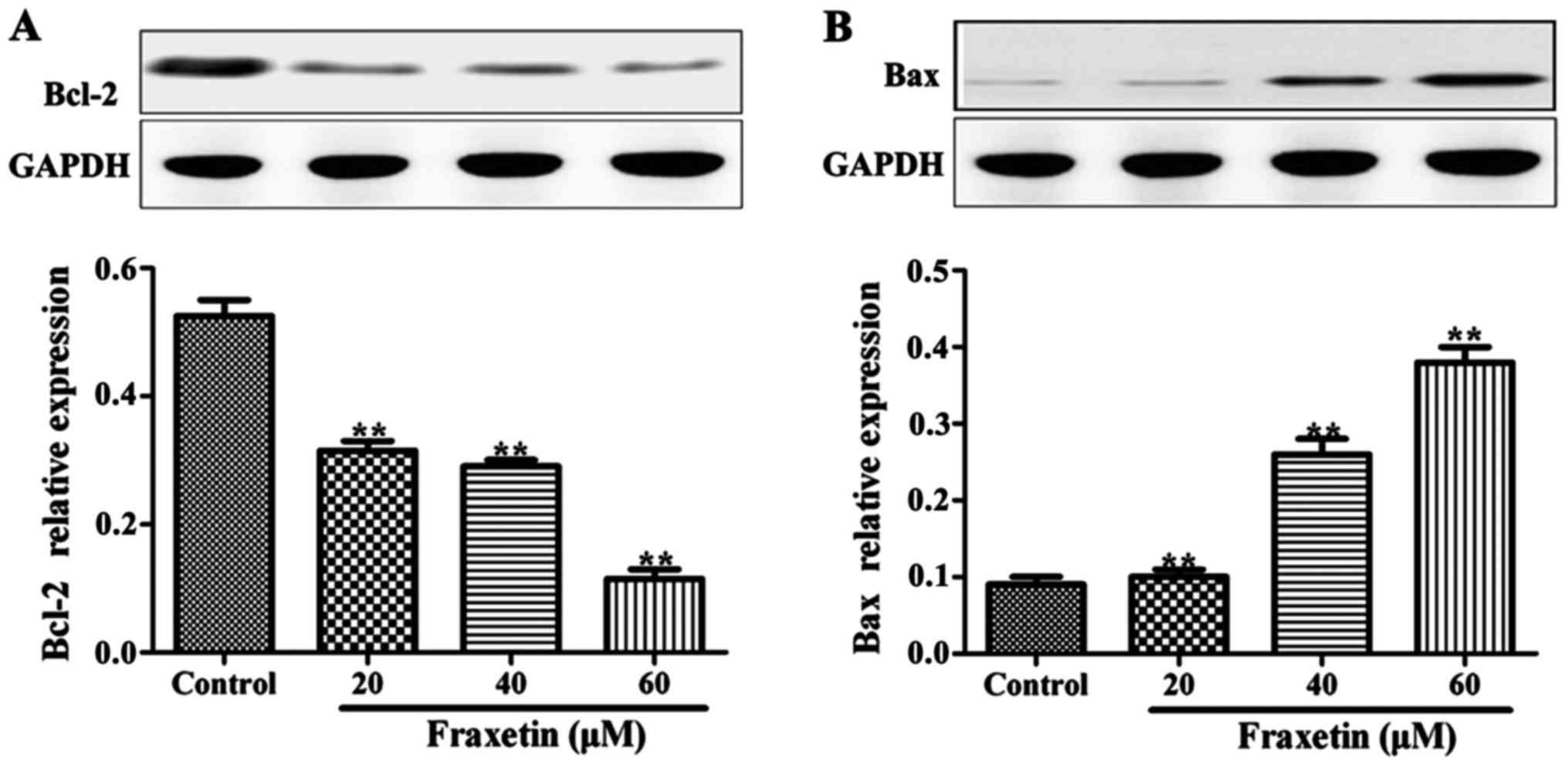
Effect of fraxetin on Bax and Bcl-2
protein expression levels
After the cells were cultured for 24 h with 20, 40
and 60 µM of fraxetin, the expression levels of Bax protein were
significantly increased compared to the cells cultured without
fraxetin (P<0.01). By contrast, the expression levels of Bcl-2
were significantly decreased in the wells treated with fraxetin
(Fig. 4). The expression levels of
Bax and Bcl-2 changed in a dose-dependent manner.
Discussion
The increasing incidence of breast cancer makes it a
serious threat to the lives of women and to their quality of life.
Breast cancer cells are prone to drug resistance and adverse
reaction to first-line chemotherapy drugs are obstacles to breast
cancer treatment. Therefore, the choice of targeted therapy, the
rationalization of individual treatment regimen, and the harm
reduction in therapeutic drugs have been primary aims for breast
cancer treatment (13). At present,
drugs used for targeted therapy are lacking; therefore, effective
drugs with low toxicity that are cost-effective have become a
societal priority.
In some tumors, the upregulation of Bcl-2 protects
cells from death or prolonged lifespan (14), indicating that Bcl-2 is critical for
tumor survival. When Bcl-2 forms homodimers, it inhibits apoptosis.
By contrast, Bax promotes apoptosis when its expression increases
and binds Bcl-2 forming heterodimers or when Bax forms homodimers
(15). Additionally, Bcl-2
downregulation induces apoptosis in multiple myeloma (16,17). The
abnormal expression of Fas/FasL is associated with a variety of
diseases, including immune system diseases, tumor immunity, and
transplantation immunity (18). The
Fas/FasL signaling pathway also plays an important role in tumor
inhibition: Fas expression is downregulated or lost in malignant
tumor cells and when tumor cells are transformed, Fas is almost
completely absent (19).
In the present study, we have shown that fraxetin
significantly inhibited the proliferation of MCF-7 cells and
induced morphological changes, including cell shrinkage and nuclear
chromatin condensation. Additionally, fraxetin upregulated the
expression of FasL and Fas mRNA. A positive
expression of Fas found in non-small cell lung cancer tissues and
normal tissues was 46.7 and 100%, respectively (20,21). Tumor
cells avoid Fas/Fas-mediated apoptosis by downregulating the
expression of Fas and preventing binding to its ligand FasL.
Findings of that study are similar to those of the present study,
further confirming that fraxetin can activate the apoptotic pathway
and induce apoptosis of MCF-7 cells by upregulating the expression
of Fas and FasL mRNA. Furthermore, fraxetin induced
the expression of Bax while the expression of Bcl-2 was decreased,
further inducing tumor cell apoptosis. The study by Xi et al
(22) showed that carnosol induced
Bax upregulation in leukemic cells and downregulation of Bcl-2
protein by 34–53%.
In conclusion, the present study has demonstrated
that fraxetin inhibits the proliferation of the MCF-7 breast cancer
cell line and induces apoptosis of MCF-7 cells. The induction of
apoptosis may be achieved by upregulating FasL, Fas and Bax and
downregulating Bcl-2. In conclution, our results provide
experimental support for the therapeutic effect of fraxetin on
breast cancer.
References
|
1
|
Amatya B, Khan F and Galea MP: Optimizing
post-acute care in breast cancer survivors: a rehabilitation
perspective. J Multidiscip Healthc. 10:347–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McGurie WL: Breast cancer prognostic
factors: Evaluation guidelines. Natl Cancer Inst. 83:154–155. 1991.
View Article : Google Scholar
|
|
3
|
Abrahamsen JF, Bakken AM, Bruserud Ø and
Gjertsen BT: Flow cytometric measurement of apoptosis and necrosis
in cryopreserved PBPC concentrates from patients with malignant
diseases. Bone Marrow Transplant. 29:165–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zwick E, Bange J and Ullrich A: Receptor
tyrosine kinase as targets for anticancer drugs. Trends Mol Med.
8:17–23. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Olaku O and White JD: Herbal therapy use
by cancer patients: A literature review on case reports. Eur J
Cancer. 47:508–514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiménez-Orozco FA, López-González JS,
Nieto-Rodriguez A, Velasco-Velázquez MA, Molina-Guarneros JA,
Mendoza-Patiño N, García-Mondragón MJ, Elizalde-Galvan P,
León-Cedeño F and Mandoki JJ: Decrease of cyclin D1 in the human
lung adenocarcinoma cell line A-427 by 7-hydroxycoumarin. Lung
Cancer. 34:185–194. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Finn GJ, Creaven BS and Egan DA: Daphnetin
induced differentiation of human renal carcinoma cells and its
mediation by p38 mitogen-activated protein kinase. Biochem
Pharmacol. 67:1779–1788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kaneko T, Tahara S and Takabayashi F:
Inhibitory effect of natural coumarin compounds, esculetin and
esculin, on oxidative DNA damage and formation of aberrant crypt
foci and tumors induced by 1,2-dimethylhydrazine in rat colons.
Biol Pharm Bull. 30:2052–2057. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cory S, Huang DCS and Adams JM: The Bcl-2
family: Roles in cell survival and oncogenesis. Oncogene.
22:8590–8607. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brady HJ and Gil-Gomez G: Bax. The
pro-apoptotic Bcl-2 family member, Bax. Int J Biochem Cell Biol.
30:647–650. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Myong NH: Tissue microarray analysis of
Fas and FasL expressions in human non-small cell lung carcinomas
with reference to the p53 and bcl-2 overexpression. J Korean Med
Sci. 20:770–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chang JS, Hsu YL, Kuo PL, Chiang LC and
Lin CC: Upregulation of Fas/Fas ligand mediated apoptosis by
gossypol in an immortalized human alveolar lung cancer cell line.
Clin Exp Pharmacol Physiol. 31:716–722. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Venditto VJ and Simanek EE: Cancer
therapies utilizing the camptothecins: A review of the in vivo
literature. Mol Pharm. 7:307–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Packham G and Cleveland JL: c-Myc and
apoptosis. Biochim Biophys Acta. 1242:11–28. 1995.PubMed/NCBI
|
|
15
|
Lu QL, Abel P, Foster CS and Lalani EN:
bcl-2: Role in epithelial differentiation and oncogenesis. Hum
Pathol. 27:102–110. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SH, Ahn KS, Jeong SJ, Kwon TR, Jung
JH, Yun SM, Han I, Lee SG, Kim DK, Kang M, et al: Janus activated
kinase 2/signal transducer and activator of transcription 3 pathway
mediates icariside II-induced apoptosis in U266 multiple myeloma
cells. Eur J Pharmacol. 654:10–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thoennissen NH, Iwanski GB, Doan NB,
Okamoto R, Lin P, Abbassi S, Song JH, Yin D, Toh M, Xie WD, et al:
Cucurbitacin B induces apoptosis by inhibition of the JAK/STAT
pathway and potentiates antiproliferative effects of gemcitabine on
pancreatic cancer cells. Cancer Res. 69:5876–5884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ryan AE, Shanahan F, O'Connell J and
Houston AM: Fas ligand promotes tumor immune invasion of colon
cancer in vivo. Cell Cycle. 5:246–249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Korkolopoulou P, Saetta AA, Levidou G,
Gigelou F, Lazaris A, Thymara I, Scliri M, Bousboukea K,
Michalopoulos NV, Apostolikas N, et al: c-FLIP expression in
colorectal carcinomas: Association with Fas/FasL expression and
prognostic implications. Histopathology. 51:150–156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimada H, Takeda A, Arima M, Okazumi S,
Matsubara H, Nabeya Y, Funami Y, Hayashi H, Gunji Y, Suzuki T, et
al: Serum p53 antibody is a useful tumor marker in superficial
esophageal squamous cell carcinoma. Cancer. 89:1677–1683. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Law PT and Wong N: Emerging roles of
microRNA in the intracellular signaling networks of hepatocellular
carcinoma. J Gastroenterol Hepatol. 26:437–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xi S, Kevin FD and Kimak M: Decreased
SIRT1, expression by promoter methylation in squamous cell
carcinogenesis. J Natl Cancer Inst. 98:181–189. 2006. View Article : Google Scholar : PubMed/NCBI
|