As one of the most intractable malignancies,
multiple myeloma (MM) has characteristics of infiltration and
growth of plasma cells, the most differentiated cells in the B-cell
lineage, in the bone marrow. MM typically affects elderly patients,
with ~33% patients are older than 75 years at diagnosis (1). MM is divided into two distinct genetic
subtypes based on chromosome content. The incidence of standard
risk is 60% and median overall survival (OS) is 8–10 years. The
incidence of intermediate is 20% and median OS is 4–5 years. The
incidence of high risk is 20% and median overall OS is 3 years
(2). Ramsenthaler et al
(3) reported that the most prevalent
symptoms were fatigue (98.8%, 95% CI 98.1–99.2%), pain (73%,
39.9–91.7), constipation (65.2%, 22.9–92.2) and tingling in the
hands/feet with 53.4% (0.4–99.7). The most common problems were
decreased physical functioning (98.9%, 98.2–99.3), decreased
cognitive functioning (80.2%, 40–96.1) and financial difficulties
(78.4%, 39.1–95.4). Renal impairment (RI) is a common feature of
symptomatic MM and may cause major management problems (4). RI affects up to 50% of patients with MM
(5). Renal failure (RF) is detected
in between 20 and 30% patients at the onset of MM, and in 50% of
patients during its progression (6).
The prognosis of patients with MM has significantly improved
following the introduction of novel concepts of immunomodulation
and proteasome inhibition in myeloma therapies (7). Bortezomib is the first-in-class
proteasome inhibitor that has been approved for the treatment of
patients with MM in the bone marrow. The present study reports the
case of a newly diagnosed MM patient, aged 83 years, in whom RI was
successfully treated with a bortezomib-based regimen.
An 83-year-old man was admitted to Lanzhou General
Hospital, Lanzhou Command, (Lanzhou, Gansu, China) on February 25,
2015, due to marked weakness, fatigue and a poor appetite. The
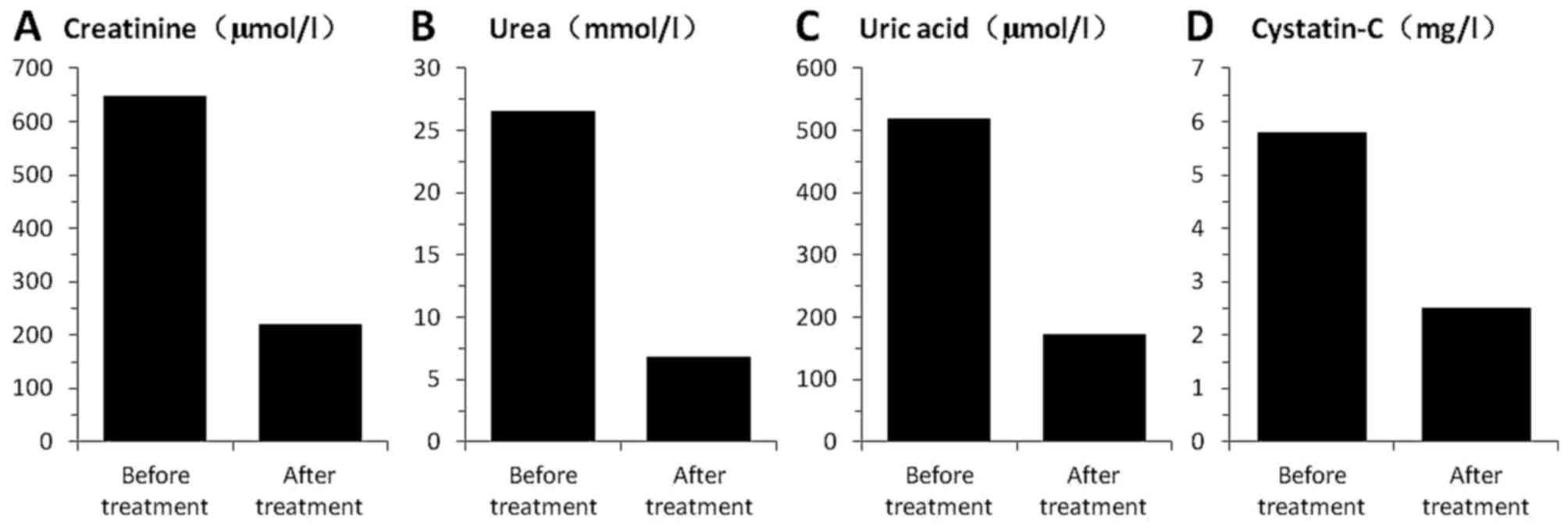
patient was admitted to the Department of Nephrology due to severe
RI (Fig. 1), with 647.0 µmol/l
creatinine (normal, (35–97 µmol/l), 26.60 mmol/l urea (normal,
2.40–8.20 mmol/l), 520.0 µmol/l uric acid (normal, 90.0–420.0
µmol/l) and 8.5 mg/l cystatin-C (normal, 0.00–1.16 mg/l). Blood
routine showed anemia with 61 g/l Hb, 512 mg/dl immunoglobulin
(Ig)G, 21.9 mg/dl IgA and 8.5 mg/dl IgM. There were local cystic
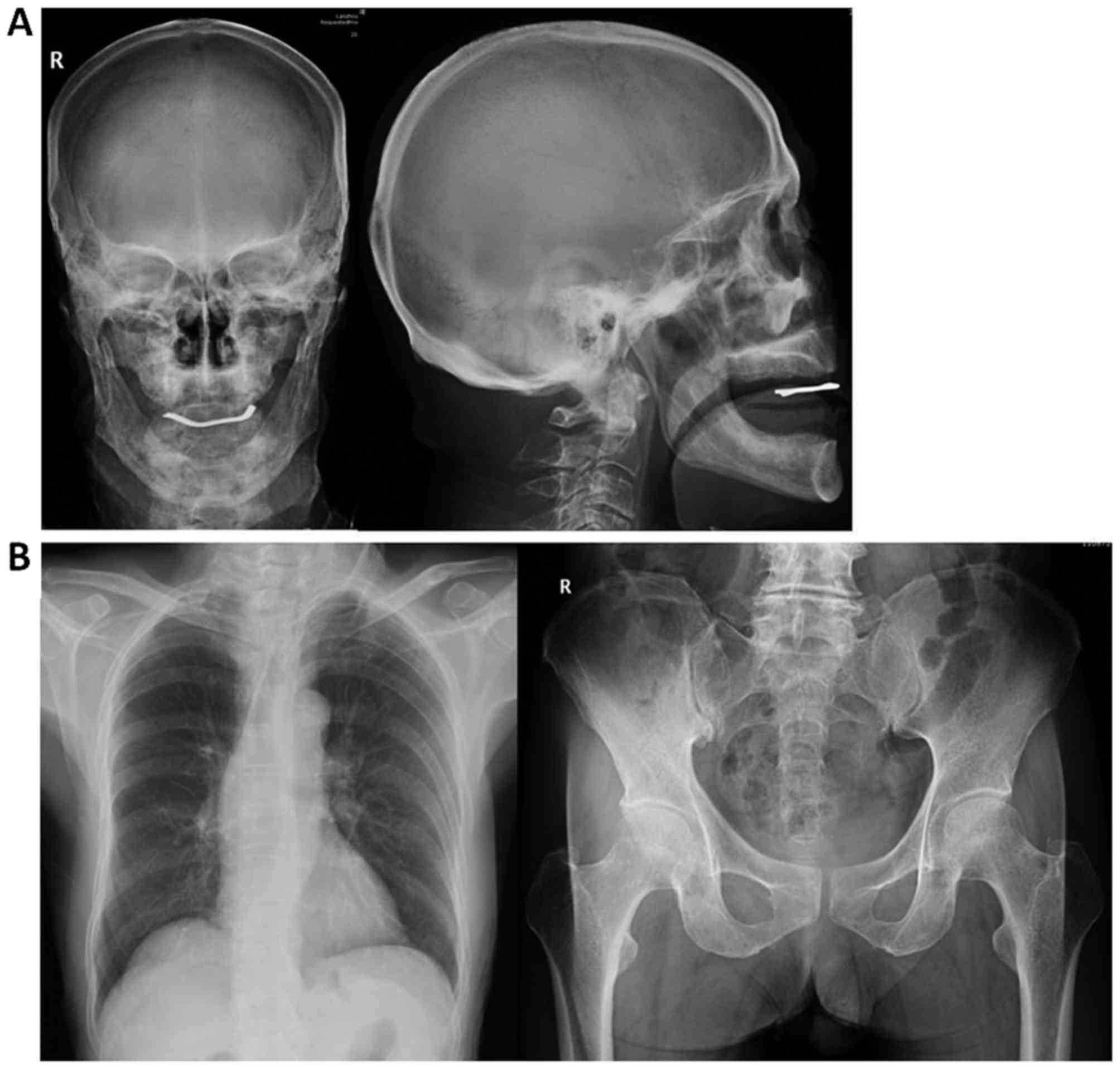
changes on the parietal bone, as determined by X-ray (Fig. 2), and an anomalous area of increased
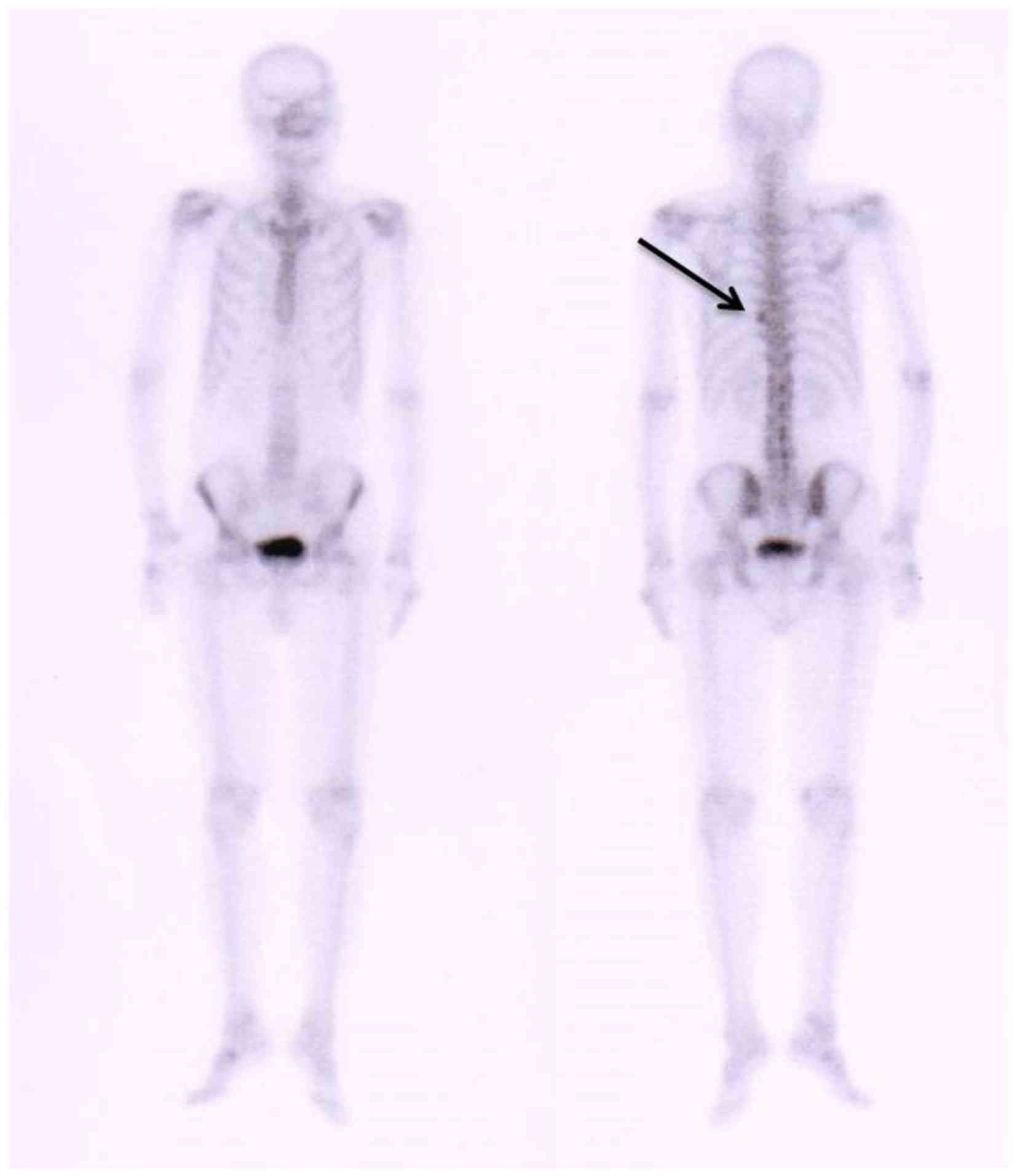
radioactivity on the left side of the 9th vertebral rib joints, as
determined by Technetium-99 m radionuclide bone imaging (injected,
25 mCi) (Fig. 3). Immunofixation
electrophoresis (8) indicated a λ
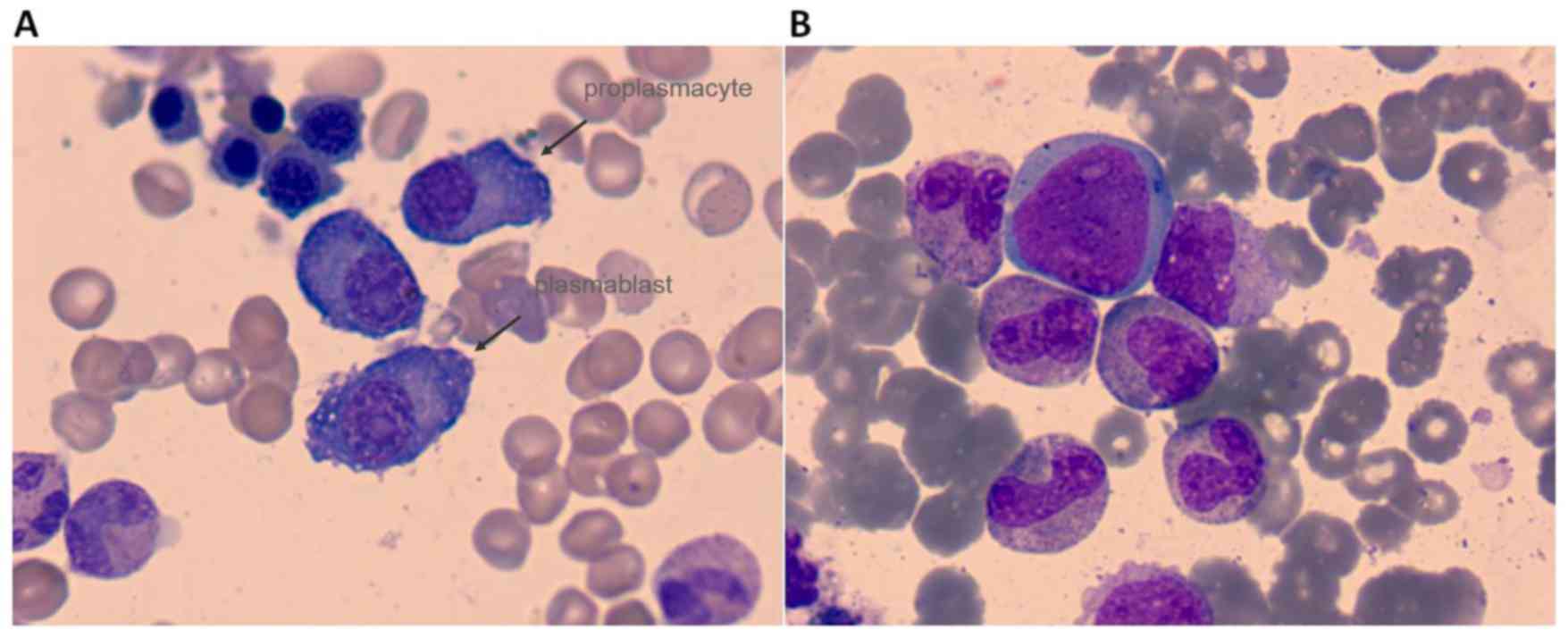
light chain-positive status. In total, 19.2% plasmablasts and
proplasmacytes were detected in the bone marrow (Fig. 4A), and the cell surface markers
cluster of differentiation (CD)13, CD33, CD38 and human leukocyte
antigen-antigen D related were positively detected by flow
cytometry, as described previously (9). Chromosome analysis showed that the
patient was 46, XY. Immunoglobulin heavy chain gene fracture
restructuring was positive (14%), as detected by fluorescence in
situ hybridization of the patient's bone marrow cells (10). These specific tests were performed as
MM may be accompanied by gene and chromosome mutations. For
example, the patients who presented with chromosomal abnormalities
del17p, t(14;16) or t(14;20) were genetically defined as high-risk
features (7). 1q21 amplification also
has very important prognostic value in multiple myeloma. Аmp1q21 is
one of the most common chromosomal abnormalities in patients with
new-onset MM and may appear in the course of disease progression.
The presence of аmp1q21 is an important prognostic factor and
should be included in the diagnostic study at disease onset and
progression (11). The patient was
transferred to Department of Hematology on February 28, 2015. The
patient's Karnofsky performance status (12) score was 70. A diagnosis of MM, λ light
chain type, stage IIIB (Durie-Salmon system) (13), was formed, and bortezomib and
dexamethasone regimen chemotherapy was administered from March 2,
2015 (bortezomib, 1.3 mg/m2, intravenous injection, days 1, 4, 8
and 11; dexamethasone, 40 mg, intravenous drip, days 1–4, 8–11). On
March 1 and March 3, 300 ml red blood cells were transfused into
the patient. RI was greatly improved following the bortezomib-based
chemotherapy (Fig. 1), and
plasmablasts and proplasmacytes were virtually eliminated, with
only 0.8% mature plasmacytes left in the bone marrow (Fig. 4B). The Hb level was maintained at ~90
g/l. The patient declined further bortezomib treatment due to
numbness and pain in the hands and fingertips. On April 23, 2015,
the patient was administered a melphalan and prednisone regimen
(melphalan, 4 mg/m2, oral administration, days 1–4; prednisone,
oral administration, 40 mg/m2, days 1–7). The patient was
discharged from hospital on 28 April 2015, with 100 mg thalidomide
prescribed every night for the long-term. The patient is currently
under follow-up every 4–6 weeks. Written informed consent was
obtained from the patient for the publication of the present
study.
MM is a clonal B-cell malignancy that causes bone
destruction and affects the immune system. Approximately 70% of
myeloma patients are >60 years old and 90% are >50 years old.
RI is a frequently occurring complication of symptomatic myeloma.
Moderate or severe RI occurs in 20–40% of newly diagnosed patients,
10% of who may require dialysis (14–18).
Antimyeloma therapy should be immediately administered for the
improvement of RI.
Bortezomib is the first-in-class proteasome
inhibitor that has been approved for the treatment of patients with
MM; it has a half-life independent of renal clearance (24) and data from initial phase II and III
trials, as well as data from patients undergoing dialysis,
indicating that bortezomib is safe to use in patients with renal
dysfunction (25,26), even in those undergoing dialysis
(27), without the requirement for
dose adjustments. In addition, certain studies have indicated that,
with regard to RI, the favorable activity of bortezomib may also be
as a result of a protective effect on renal cells, and due to
inflammatory and fibrotic cascade inhibition within the
microenvironment of the kidney (28,29).
However, with regard to RI, rapid and marked antimyeloma action,
and being non-renal metabolite, are the most significant advantages
of using bortezomib (30–32). The present data showed that kidney
function can rapidly be improved in MM patients, and even in older
patients, such as the 83-year-old treated in the present study. The
treatment appears to be safe and effective, and prospective trials
(33,34) showing high drug response rates of
myeloma and the kidneys in patients with moderate RI, in particular
to bortezomib, have further supported this stance. Another common
observation is that renal response correlates with tumor response
and is more common in newly diagnosed patients, compared with
late-state patients (35,36). The data from the present study are
consistent with this observation.
There are several causes of RI in MM patients. The
circulating monoclonal light chains are filtered through the
glomeruli and reach the proximal tubule where they are catabolized.
The free light chains (FLCs) are endocytosed by the cells of the
proximal tubule through a receptor-mediated process, which results
in their degradation within lysosomes (37–40). Focal
loss of microvilli and inhibition of Na-K-ATPase pumps may lead to
reabsorption defects (41). Cast
nephropathy is another potential cause of RI. In a recent study,
24-h urine protein containing <25% albumin exhibited a
sensitivity of 0.98, a specificity of 0.94, a positive predictive
value of 0.75 and a negative predictive value of 0.99 for the
diagnosis of cast nephropathy (42).
For oliguria cases or when renal function is
deteriorating rapidly or during recovery, urine quantification of
light chains is unreliable. Serum FLCs have been used for the
assessment of patients with light chain amyloidosis and for
patients with oligo-secretory disease (43); however, in patients with RI and a
significant load of FLCs, the evaluation is not straightforward. In
patients with RI, κ and λ light chains increase and the normal κ/λ
ratio is different than in patients with normal renal function
(44,45). The current response criteria for
patients with measurable disease do not use the serum FLCs but the
urine 24-h light chain output (46).
Furthermore, the correlation of urine light chain excretion and
serum FLC levels is not linear, and large variations occur
(46). Thus, in patients with
significant RI, the evaluation of response to chemotherapy may be
challenging.
Serum cystatin-C is a sensitive marker of renal
dysfunction that has been applied by nephrologists for a number of
years. Terpos et al (47)
measured serum cystatin-C levels in newly diagnosed and pretreated
patients with MM and revealed that levels were increased in
patients with myeloma, even in individuals with normal serum
creatinine levels. Markers of renal damage, including neutrophil
gelatinase-associated lipocalin and kidney injury molecule-1 in the
serum and/or urine, are increased in patients with monoclonal
gammopathies, indicating that renal damage is present at an early
in the disease course (48). The
present results showed that cystatin-C decreased more than 1-fold
following bortezomib treatment. This is consistent with the results
described by Terpos et al (47). The suggestion that a decrease in
estimated glomerular filtration rate of >35% within 1 year,
without any other identifiable cause, could be used as an
indication for therapy is being considered. Reversal of renal
dysfunction and significant improvement of renal function have been
observed in several studies of patients with MM-related RI, and
even certain patients on dialysis became dialysis-independent
following treatment with bortezomib (27). However, it is also notable that
certain patients who do not achieve an objective myeloma response
may also show a significant improvement in renal function (33). Overall, significant improvement of
renal function was observed in 77–82% of patients treated with
bortezomib-based therapy (20).
Furthermore, this improvement appears to be independent of the dose
of steroids, although higher doses of dexamethasone may be
associated with a shorter time to renal recovery (20).
Although novel therapeutic agents, including the
proteasome inhibitor ixazomib and the immunomodulatory drug
pomalidomide have been introduced, the prognosis of MM patients
remains worse than that of the majority of other hematological
malignancies (49,50). In order to achieve any improvements in
treatment outcome for MM patients, it is necessary that the
molecular pathogenesis of the disease is understood to a greater
extent. A phase of asymptomatic expansion of clonal plasma cells,
also known as monoclonal gammopathy of undetermined significance
(MGUS), is progressed through by all MM cases (51,52). The
linear evolution of MM from MGUS to terminal phases, such as that
of extramedullary tumors and plasma cell leukemia via the
accumulation of novel mutations, is a long-held belief (51,52).
However, previous studies using next-generation sequencing revealed
the complex genomic architecture of MM. At each progression step,
novel mutations are acquired together with subclonal evolution from
reservoir clones with branching patterns. Individual subclones may
carry novel mutations and distinct phenotypes, including that of
drug sensitivity (53). Furthermore,
at the MGUS stage, minor clones are already present, which could
expand further along in the clinical course, causing relapse and/or
leukemic conversion (54). The final
aim of treatment is to eliminate all clones, including subclonal
populations with distinct biological characteristics. This may be
achievable by further the improvement of treatment strategies that
reflect the genomic landscape of the disease.
In summary, the present case indicates that
bortezomib is safe for MM patients >80 years old. The present
study also proved that bortezomib can improve the RI of myeloma
patients with a creatinine level of >600 µmol/l, and even of
those patients >80 years old. However, further cases are
required to confirm these findings.
This study was supported by a grant from the Natural
Science Foundation of Gansu Province (no. 145RJZA151).
|
1
|
Zweegman S, Engelhardt M and Larocca A:
EHA SWG on ‘Aging and Hematology’: Elderly patients with multiple
myeloma: Towards a frailty approach? Curr Opin Oncol. 29:315–321.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chesi M and Bergsagel PL: Molecular
pathogenesis of multiple myeloma: Basic and clinical updates. Int J
Hematol. 97:313–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ramsenthaler C, Kane P, Gao W, Siegert RJ,
Edmonds PM, Schey SA and Higginson IJ: Prevalence of symptoms in
patients with multiple myeloma: A systematic review and
meta-analysis. Eur J Haematol. 97:416–429. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dimopoulos MA and Terpos E: Renal
insufficiency and failure. Hematology Am Soc Hematol Educ Program.
2010:431–436. 2010.PubMed/NCBI
|
|
5
|
Knudsen LM, Hippe E, Hjorth M, Holmberg E
and Westin J: Renal function in newly diagnosed multiple myeloma-a
demographic study of 1353 patients. The Nordic Myeloma Study Group.
Eur J Haematol. 53:207–212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rekhtina IG and Mendeleeva LP: Current
approaches to treating of patients with multiple myeloma with renal
failure: Questions and proofs. Ter Arkh. 89:112–117. 2017.(In
Russian; Abstract available in Russian from the publisher).
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Joseph NS, Gentili S, Kaufman JL, Lonial S
and Nooka AK: High-risk mutiple myiloma: Definition and management.
Clin Lymphoma Myeloma Leuk. 17S:S80–S87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caillon H, Irimia A, Simon JS, Axel A,
Sasser K, Scullion MJ, Ligneel T, Nouadje G, Moreau P and Dejoie T:
Overcoming the interference of daratumumab with immunofixation
electrophoresis (IFE) using an industry-developed dira test:
Hydrashift 2/4 daratumumab. Blood. 128:2063. 2016.
|
|
9
|
Puig N, Sarasquete ME, Balanzategui A,
Martínez J, Paiva B, García H, Fumero S, Jiménez C, Alcoceba M,
Chillón MC, et al: Critical evaluation of ASO RQ-PCR for minimal
residual disease evaluation in multiple myeloma. A comparative
analysis with flow cytometry. Leukemia. 28:391–397. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ross FM, Avet-Loiseau H, Ameye G,
Gutiérrez NC, Liebisch P, O'Connor S, Dalva K, Fabris S, Testi AM,
Jarosova M, et al: Report from the european myeloma network on
interphase FISH in multiple myeloma and related disorders.
Haematologica. 97:1272–1277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abramova TV, Obukhova TN, Mendeleeva LP,
Pokrovskaya OS, Gribanova EO, Ryzhko VV, Grebenyuk LA, Nareyko MV,
Solovyev MV, Votyakova OM, et al: Prognostic value of 1q21
amplification in multiple myeloma. Ter Arkh. 89:32–38. 2017.(In
Russian). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Modesto AP, Usvyat L, Calice-Silva V,
Spigolon DN, Figueiredo AE, de Moraes TP, Olandoski M, Shimakura
SE, Barretti P, Kotanko P and Pecoits-Filho R: Impact of the
karnofsky performance status on survival and its dynamics during
the terminal year of peritoneal dialysis patients. Perit Dial Int:
pii. pdi.2015.00241. 2017. View Article : Google Scholar
|
|
13
|
Qian J, Jin J, Luo H, Jin C, Wang L, Qian
W and Meng H: Analysis of clinical characteristics and prognostic
factors of multiple myeloma: A retrospective single-center study of
787 cases. Hematology. 22:472–476. 2017.PubMed/NCBI
|
|
14
|
Alexanian R, Barlogie B and Dixon D: Renal
failure in multiple myeloma. Pathogenesis and prognostic
implications. Arch Intern Med. 150:1693–1695. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blade´ J, Fernández-Llama P, Bosch F,
Montolíu J, Lens XM, Montoto S, Cases A, Darnell A, Rozman C and
Montserrat E: Renal failure in multiple myeloma: Presenting
features and predictors of outcome in 94 patients from a single
institution. Arch Intern Med. 158:1889–1893. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kyle RA, Gertz MA, Witzig TE, Lust JA,
Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson
DR, et al: Review of 1027 patients with newlydiagnosed multiple
myeloma. Mayo Clin Proc. 78:21–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eleutherakis-Papaiakovou V, Bamias A, Gika
D, Simeonidis A, Pouli A, Anagnostopoulos A, Michali E,
Economopoulos T, Zervas K and Dimopoulos MA: Greek Myeloma Study
Group: Renal failure in multiple myeloma: Incidence, correlations,
and prognostic significance. Leuk Lymphoma. 48:337–341. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Torra R, Bladé J, Cases A, López-Pedret J,
Montserrat E, Rozman C and Revert L: Patients with multiple myeloma
requiring long-term dialysis: Presenting features, response to
therapy, and outcome in a series of 20 cases. Br J Haematol.
91:854–859. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eleftherakis-Papapiakovou E, Kastritis E,
Roussou M, Gkotzamanidou M, Grapsa I, Psimenou E, Nikitas N, Terpos
E and Dimopoulos MA: Renal impairment is not an independent adverse
prognostic factor in patients with multiple myeloma treated upfront
with novel agent-based regimens. Leuk Lymphoma. 52:2299–2303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dimopoulos MA, Roussou M, Gkotzamanidou M,
Nikitas N, Psimenou E, Mparmparoussi D, Matsouka C,
Spyropoulou-Vlachou M, Terpos E and Kastritis E: The role of novel
agents on the reversibility of renal impairment in newly diagnosed
symptomatic patients with multiple myeloma. Leukemia. 27:423–429.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chanan-Khan AA, San Miguel JF, Jagannath
S, Ludwig H and Dimopoulos MA: Novel therapeutic agents for the
management of patients with multiple myeloma and renal impairment.
Clin Cancer Res. 18:2145–2163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaballa MR, Laubach JP, Schlossman RL,
Redman K, Noonan K, Mitsiades CS, Ghobrial IM, Munshi N, Anderson
KC and Richardson PG: Management of myeloma-associated renal
dysfunction in the era of novel therapies. Expert Rev Hematol.
5:51–66; quiz 67–68. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Costa LJ, Abbas J, Ortiz-Cruz KL, Kang Y
and Stuart RK: Outcomes of patients with multiple myeloma and renal
impairment treated with bortezomib, cyclophosphamide, and
dexamethasone without plasma exchange. Eur J Haematol. 89:432–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mulkerin D, Remick S and Takimoto C:
Safety, tolerability and pharmacology of bortezomib in cancer
patients with renal failure requiring dialysis: Results from a
prospective phase 1 study. ASH Annu Meet Abstr. 110:34772007.
|
|
25
|
Jagannath S, Barlogie B, Berenson JR,
Singhal S, Alexanian R, Srkalovic G, Orlowski RZ, Richardson PG,
Anderson J, Nix D, et al: Bortezomib in recurrent and/or refractory
multiple myeloma. Initial clinical experience in patients with
impaired renal function. Cancer. 103:1195–1200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
San-Miguel JF, Richardson PG, Sonneveld P,
Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL,
Ben-Yehuda D, Lonial S, et al: Efficacy and safety of bortezomib in
patients with renal impairment: Results from the APEX phase 3
study. Leukemia. 22:842–849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chanan-Khan AA, Kaufman JL, Mehta J,
Richardson PG, Miller KC, Lonial S, Munshi NC, Schlossman R,
Tariman J and Singhal S: Activity and safety of bortezomib in
multiple myeloma patients with advanced renal failure: A
multicenter retrospective study. Blood. 109:2604–2606. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ying WZ, Wang PX, Aaron KJ, Basnayake K
and Sanders PW: Immunoglobulin light chains activate nuclear
factor-κB in renal epithelial cells through a Src-dependent
mechanism. Blood. 117:1301–1307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sarközi R, Perco P, Hochegger K, Enrich J,
Wiesinger M, Pirklbauer M, Eder S, Rudnicki M, Rosenkranz AR, Mayer
B, et al: Bortezomib-induced survival signals and genes in human
proximal tubular cells. J Pharmacol Exp Ther. 327:645–656. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Uttamsingh V, Lu C, Miwa G and Gan LS:
Relative contributions of the five major human cytochromes P450,
1A2, 2C9, 2C19, 2D6, and 3A4, to the hepatic metabolism of the
proteasome inhibitor bortezomib. Drug Metab Dispos. 33:1723–1728.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pekol T, Daniels JS, Labutti J, Parsons I,
Nix D, Baronas E, Hsieh F, Gan LS and Miwa G: Human metabolism of
the proteasome inhibitor bortezomib: Identification of circulating
metabolites. Drug Metab Dispos. 33:771–777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Labutti J, Parsons I, Huang R, Miwa G, Gan
LS and Daniels JS: Oxidative deboronation of the peptide boronic
acid proteasome inhibitor bortezomib: Contributions from reactive
oxygen species in this novel cytochrome P450 reaction. Chem Res
Toxicol. 19:539–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dimopoulos MA, Richardson PG, Schlag R,
Khuageva NK, Shpilberg O, Kastritis E, Kropff M, Petrucci MT,
Delforge M, Alexeeva J, et al: VMP (Bortezomib, Melphalan, and
Prednisone) is active and well tolerated in newly diagnosed
patients with multiple myeloma with moderately impaired renal
function, and results in reversal of renal impairment: Cohort
analysis of the phase III VISTA study. J Clin Oncol. 27:6086–6093.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Morabito F, Gentile M, Mazzone C, Rossi D,
Di Raimondo F, Bringhen S, Ria R, Offidani M, Patriarca F, Nozzoli
C, et al: Safety and efficacy of
bortezomib-melphalan-prednisone-thalidomide followed by
bortezomib-thalidomide maintenance (VMPT-VT) versus
bortezomib-melphalan-prednisone (VMP) in untreated multiple myeloma
patients with renal impairment. Blood. 118:5759–5766. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ludwig H, Adam Z, Hajek R, Greil R,
Tóthová E, Keil F, Autzinger EM, Thaler J, Gisslinger H, Lang A, et
al: Light chain-induced acute renal failure can be reversed by
bortezomib-doxorubicin-dexamethasone in multiple myeloma: Results
of a phase II study. J Clin Oncol. 28:4635–4641. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dimopoulos MA, Roussou M, Gavriatopoulou
M, Zagouri F, Migkou M, Matsouka C, Barbarousi D, Christoulas D,
Primenou E, Grapsa I, et al: Reversibility of renal impairment in
patients with multiple myeloma treated with bortezomib-based
regimens: Identification of predictive factors. Clin Lymphoma
Myeloma. 9:302–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Batuman V, Dreisbach AW and Cyran J:
Light-chain binding sites on renal brush-border membranes. Am J
Physiol. 258:F1259–F1265. 1990.PubMed/NCBI
|
|
38
|
Batuman V and Guan S: Receptor-mediated
endocytosis of immunoglobulin light chains by renal proximal tubule
cells. Am J Physiol. 272:F521–F530. 1997.PubMed/NCBI
|
|
39
|
Batuman V, Verroust PJ, Navar GL, Kaysen
JH, Goda FO, Campbell WC, Simon E, Pontillon F, Lyles M, Bruno J
and Hammond TG: Myeloma light chains are ligands for cubilin
(gp280). Am J Physiol. 275:F246–F254. 1998.PubMed/NCBI
|
|
40
|
Santostefano M, Zanchelli F, Zaccaria A,
Poletti G and Fusaroli M: The ultrastructural basis of renal
pathology in monoclonal gammopathies. J Nephrol. 18:659–675.
2005.PubMed/NCBI
|
|
41
|
Guan S, el-Dahr S, Dipp S and Batuman V:
Inhibition of Na-K-ATPase activity and gene expression by a myeloma
light chain in proximal tubule cells. J Investig Med. 47:496–501.
1999.PubMed/NCBI
|
|
42
|
Leung N, Gertz M, Kyle RA, Fervenza FC,
Irazabal MV, Eirin A, Kumar S, Cha SS, Rajkumar SV, Lacy MQ, et al:
Urinary albumin excretion patterns of patients with cast
nephropathy and other monoclonal gammopathy-related kidney
diseases. Clin J Am Soc Nephrol. 7:1964–1968. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mayo MM and Johns GS: Serum free light
chains in the diagnosis and monitoring of patients with plasma cell
dyscrasias. Contrib Nephrol. 153:44–65. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hutchison CA, Cockwell P, Harding S, Mead
GP, Bradwell AR and Barnett AH: Quantitative assessment of serum
and urinary polyclonal free light chains in patients with type II
diabetes: An early marker of diabetic kidney disease? Expert Opin
Ther Targets. 12:667–676. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hutchison CA, Plant T, Drayson M, Cockwell
P, Kountouri M, Basnayake K, Harding S, Bradwell AR and Mead G:
Serum free light chain measurement aids the diagnosis of myeloma in
patients with severe renal failure. BMC Nephrol. 9:112008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Durie BG, Harousseau JL, Miguel JS, Bladé
J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J,
Sonneveld P, et al: International uniform response criteria for
multiple myeloma. Leukemia. 20:1467–1473. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Terpos E, Katodritou E, Tsiftsakis E, et
al: Cystatin-C is a sensitive marker of renal impairment with an
independent predictive value for survival in multiple myeloma;
reduction post bortezomib monotherapy. ASH Annu Meet Abstr.
110:14842007.
|
|
48
|
Dimopoulos MA, Christoulas D, Kastritis E,
et al: Tubular damage is ubiquitous in newly-diagnosed patients
with multiple myeloma: Comparison of three urinary and two serum
markers of kidney injury. ASH Annu Meet Abstr. 120:29192012.
|
|
49
|
Gupta N, Goh YT, Min CK, Lee JH, Kim K,
Wong RS, Chim CS, Hanley MJ, Yang H, Venkatakrishnan K, et al:
Pharmacokinetics and safety of ixazomib plus
lenalidomide-dexamethasone in Asian patients with
relapsed/refractory myeloma: A phase 1 study. J Hematol Oncol.
8:1032015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rychak E, Mendy D, Shi T, Ning Y, Leisten
J, Lu L, Miller K, Narla RK, Orlowski RZ, Raymon HK, et al:
Pomalidomide in combination with dexamethasone results in
synergistic anti-tumour responses in pre-clinical models of
lenalidomide-resistant multiple myeloma. Br J Haematol.
172:889–901. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Weiss BM, Abadie J, Verma P, Howard RS and
Kuehl WM: A monoclonal precedes multiple myeloma in most patients.
Blood. 113:5418–5422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Agarwal A and Ghobrial IM: Monoclonal
gammopathy of undetermined significance and smoldering multiple
myeloma: A review of the current understanding of epidemiology,
biology, risk stratification, and management of myeloma precursor
disease. Clin Cancer Res. 19:985–994. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bahlis NJ: Darwinian evolution and tiding
clones in multiple myeloma. Blood. 120:927–928. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Magrangeas F, Avet-Loiseau H, Gouraud W,
Lodé L, Decaux O, Godmer P, Garderet L, Voillat L, Facon T, Stoppa
AM, et al: Minor clone provides a reservoir for relapse in multiple
myeloma. Leukemia. 27:473–481. 2013. View Article : Google Scholar : PubMed/NCBI
|