Introduction
Prostate cancer is the most common type of
malignancy and the second leading cause of cancer-associated
mortalities in the USA (1). Prostate
cancer cells first respond to androgen ablation therapy, but
long-term anti-androgen treatment results in loss of
responsiveness. Prostate cancer types may eventually develop to be
androgen-independent with markedly metastatic behavior. Prostate
cancer cells metastasize to other parts of the human body;
therefore, prostate cancer has deleterious effects on the survival
time and quality of life of patients. Until 2007, ~80% of patients
who were diagnosed with prostate cancer developed bone metastasis
(2,3).
Thus, understanding the molecular mechanisms underlying the
metastasis of prostate cancer is critical for the prevention and
therapy of prostate cancer metastasis.
Pharmaceutical compounds extracted from mushrooms
have demonstrated benefits for the treatment of a variety of
diseases, including various types of cancer, immunological
disorders and neurodegenerative diseases (4–6).
Ganoderma lucidum, a basidiomycetous fungus, is a type of
mushroom that has widely been used as a traditional medicine for
thousands of years, particularly in Asia (7). A number of bioactive chemical
substances, including polysaccharides, triterpenoids and proteins
are extracted from the fruiting bodies, cultured mycelia and spores
of G. lucidum (8). Previous
studies have indicated that the active compounds isolated from its
fruiting body ‘Lingzhi’ participate in a variety of biological
processes, including anti-inflammatory, antioxidant, antitumor and
immunomodulatory activities (9–13).
Triterpenes isolated from G. lucidum (GLT) also exhibit
cytotoxic activity against mouse sarcoma and mouse lung carcinoma
cells (14).
G. lucidum has been reported to inhibit the
metastasis of human prostate cancer cells (15,16);
however, because extracts of G. lucidum contain numerous
bioactive compounds, the exact functional compound remains to be
clarified. The present study was performed to elucidate the role of
GLT on prostate cancer cells. The present study demonstrated that
GLT significantly inhibited cell viability and induced apoptosis in
highly invasive DU-145 prostate cancer cells. Additionally, GLT
suppressed the migration and invasion via inhibition of matrix
metalloproteinase (MMP) expression.
Materials and methods
Cell culture and reagents
DU-145 human prostate cancer cells were obtained
from the American Type Culture Collection (Manassas, VA, USA).
DU-145 cells were maintained in F-12 medium containing penicillin
(50 U/ml), streptomycin (50 U/ml) and 10% fetal bovine serum (FBS;
all from Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
at 37°C in a humidified incubator containing 5% CO2. GLT
was purchased from Nanjing Zhongke Pharmaceutical Co. Ltd.
(Nanjing, China). The principal component of GLT is ganoderic acid
H, with a purity of ~99%. GLT was dissolved in dimethyl sulfoxide
(DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at a
concentration of 40 mg/ml and stored at 4°C (the final
concentration of DMSO in the controls and GLT was <0.1% to
exclude its toxicity). DMSO was used as the control.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.). RNA was reverse-transcribed using a SuperScript First Strand
cDNA system (Invitrogen; Thermo Fisher Scientific, Inc.), according
to the manufacturer's protocol. The following primers were used for
amplification of MMP-2: Sense primer 5′-GTCCACTGTTGGTGGGAACT-3′
(sense) and 5′-CTCCTGAATGCCCTTGATGT-3′ (antisense); Thermocycling
conditions were: 94°C for 2 min, followed by 30 cycles of 94°C for
30 sec, 55°C for 30 sec and 68°C for 1 min, then 68°C for 10
min.
MMP-9, 5′-GACAAGAAGTGGGGCTTCTG-3′ (sense) and
5′-TCAAAGACCGAGTCCAGCTT-3′ (antisense); Thermocycling conditions
were: 94°C for 2 min followed by 30 cycles of 94°C for 30 sec, 60°C
for 30 sec and 72°C for 1 min, then 72°C for 10 min.
β-actin (internal control)
5′-CGAAACTACCTTCAACTCCATCA-3′ (sense) and
5′-CGGACTCGTCATACTCCTGCT-3′ (antisense). Thermocycling conditions
were 94°C for 2 min followed by 30 cycles of 94°C for 30 sec, 58°C
for 30 sec and 68°C for 1 min, then 68°C for 10 min. The PCR
products were analyzed by 2% agarose gel electrophoresis and
confirmed by their appropriate size. The image was taken with the
gel imaging analysis system and the gray scale values were analyzed
using ImageJ software (V2.1.4.7; National Institutes of Health,
Bethesda, MD, USA). Experiments were repeated three times.
Cell viability assay
Cell viability was determined using an MTT assay,
according to the manufacturer's protocol (Promega Corporation,
Madison, WI, USA). Briefly, DU-145 cells cultured in a 96-well
plate at a density of 1×104/cm2 were treated
with 0.1, 0.5, 1 and 5 mg/ml GLT for 24 h and with 2 mg/ml GLT for
1, 2 and 3 days. At the end of the incubation period, absorption
was determined using a microplate reader at 570 nm.
Wound-healing assay
DU-145 cells were cultured in 6-cm plates until
confluent and subsequently the monolayer was scratched using a fine
sterile pipette tip to produce a narrow wound. The medium and
debris were aspirated away and replaced with fresh medium in the
presence of 0.1 and 2 mg/ml GLT. Images were captured prior to and
6, 12 and 24 h after wounding using a Nikon TMS-F phase-contrast
microscope (Nikon Instruments, Florence, Italy).
Transwell migration assay
Migration assays with DU-145 cells were performed
using a Transwell kit from BD Biosciences (Franklin Lakes, NJ,
USA). Cells were removed from culture plates with trypsin and
washed twice with PBS. Cells (4×104) in 250 µl
Dulbecco's modified Eagle's medium (DMEM) with or without 0.1 and 2
mg/ml GLT were placed in the upper invasion chamber and the lower
compartment was loaded with DMEM supplemented with 10% FBS. The
cell migration chamber was inserted into the lower compartment and
incubated for 24 h at 37°C. Cells on the upper side of the filter
were removed with a cotton swab. Cells attached to the filter were
fixed with 100% methanol for 10 min at room temperature. Cells
attached to the filter were stained with Giemsa stain (5%) for 1 h
at room temperature. Filters were destained by washing with water
and the number of cells attached to the filter was then quantified
by enumerating cells in images captured using a light microscope of
the stained filters.
Transwell invasion assay
An invasion assay with DU-145 cells was performed
using BD BioCoat Matrigel invasion chambers (BD Biosciences),
according to the manufacturer's protocol. DU-145 cells were seeded
at 4×104 cells per well in serum-free DMEM in the upper
compartment, with or without 0.1 and 2 mg/ml GLT, and DMEM
supplemented with 10% FBS was placed in the lower compartment of
the chamber as a chemoattractant. Following a 24-h incubation, the
non-invading cells on the upper side of the chamber were removed
and the membranes were fixed with 100% methanol for 10 min at room
temperature, washed with PBS and stained with Giemsa stain (5%) for
1 h at room temperature. Invasiveness was evaluated by counting the
invading cells under a light microscope.
Flow cytometric assay
Cells were washed with ice-cold PBS, resuspended in
100 µl binding buffer (1% bovine serum albumin (Thermo Fisher
Scientific, Inc.) in PBS) and stained with fluorescein
isothiocyanate (FITC)-annexin V (BD Biosciences). The cells were
incubated for 15 min at 37°C in the dark. Subsequently, 200 µl
binding buffer supplemented with propidium iodide (PI; 20 µg/ml)
was added immediately prior to flow cytometry. The cells were
analyzed using FACSCanto cytometer and FACSDiva software (V.5.0.2;
BD Biosciences). Early and late apoptosis was evaluated. Cells at
early and late apoptosis were counted.
Statistical analysis
Results are presented graphically as the mean ±
standard error of the mean of at least three independent
experiments. The statistical significance of the differences was
analyzed using Student's t-test between two groups and one-way
analysis of variance with Newman-Keuls post-hoc tests for
comparisons among more than two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
GLT extract inhibits the viability of
prostate cancer cells in a dose- and time-dependent manner
To examine the effect of GLT extract on cell
viability, cultured DU-145 cells were treated with increasing
concentrations of GLT (0.1, 0.5, 1 and 5 mg/ml), and the viability
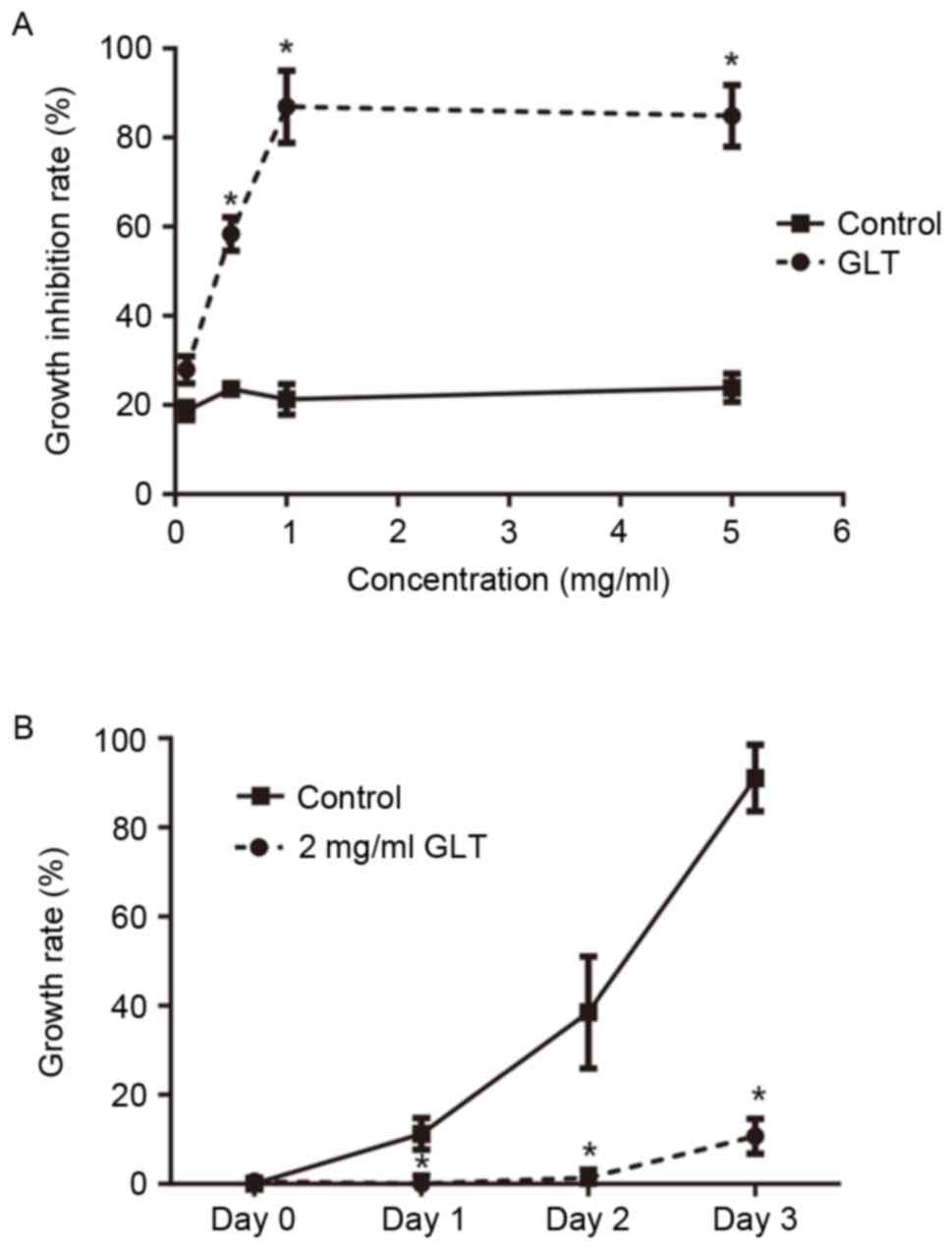
of cells was determinedusing an MTT assay. As presented in Fig. 1A, a low concentration of GLT (0.1
mg/ml) had less effect on the viability of DU-145 cells compared
with the control (P>0.05). Once the concentration reached ≥0.5
mg/ml, the viability of DU-145 cells was significantly inhibited
compared with the control (P<0.05). Furthermore, as presented in
Fig. 1B, 2 mg/ml GLT markedly
inhibited the proliferation of DU-145 cells at 24 h after the
treatment. Subsequently, 2 mg/ml GLT was selected as the treatment
concentration and 0.1 mg/ml GLT as an additional control for
furtherexperiments.
GLT suppresses the migration and
invasion of prostate cancer cells
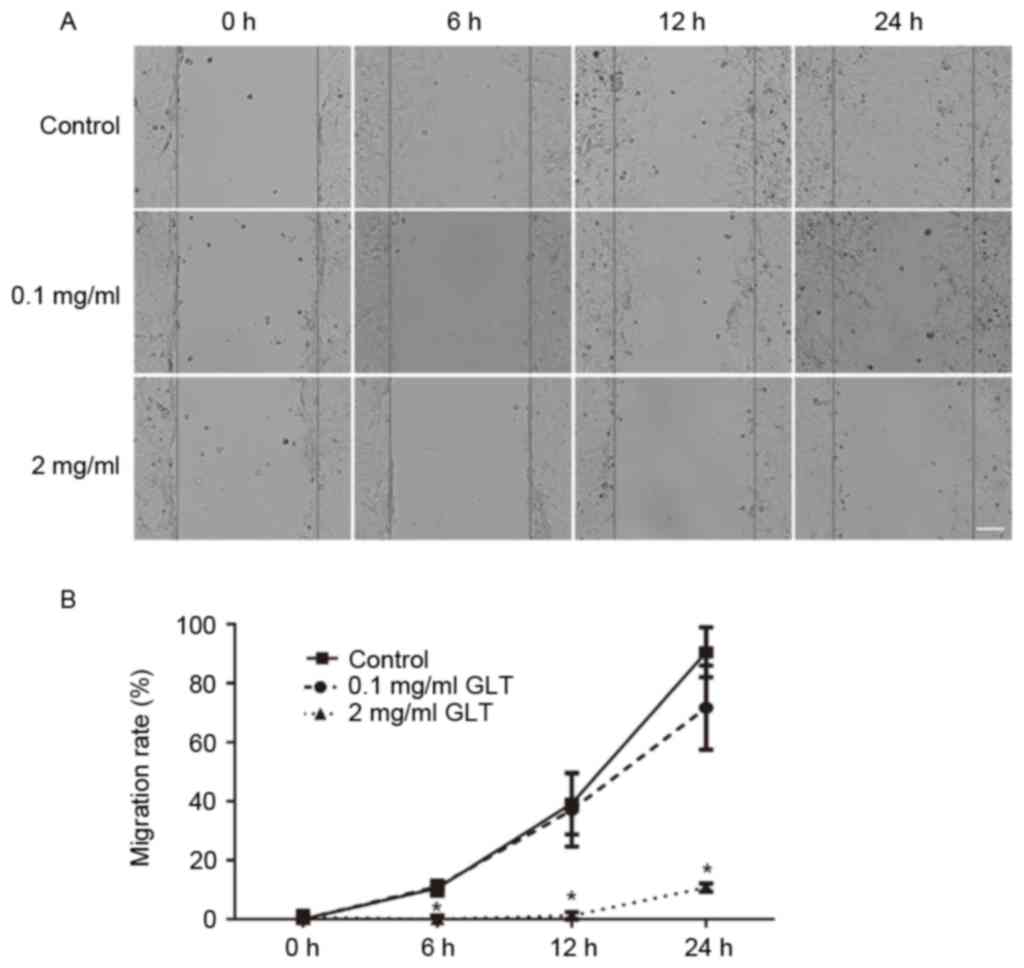
The present study investigated the effect of GLT on
cell migration and invasion. First, a wound-healing assay was
performed. As presented in Fig. 2,
the control group cells migrated across the wound, which was healed
after 24 h; however, 2 mg/ml GLT significantly inhibited this
process and 0.1 mg/ml GLT exhibitedno effect. Furthermore, cell
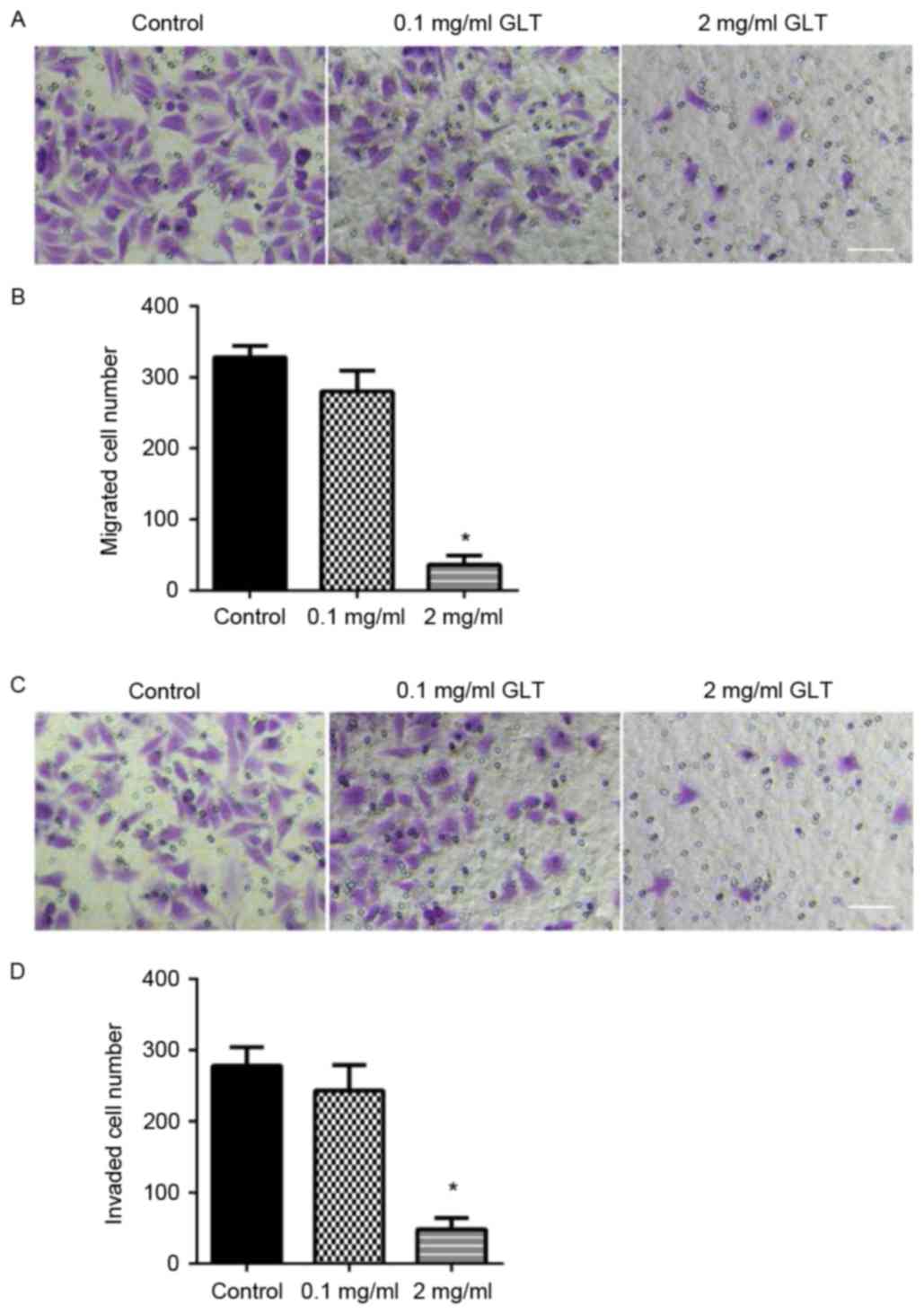
migration and invasion were assessed using a Transwell system.
DU-145 cells treated with or without GLT were allowed to migrate
through a Transwell membrane into complete medium. Equal quantities
of cells were transferred to the membrane surface and cell
migration was assessed within 24 h. Compared with the control or
0.1 mg/ml GLT-treated cells, 2 mg/ml GLT led to a decreased level
of cell migration (Fig. 3A and B). To
evaluate cell invasion, DU-145 cells were plated on the Matrigel
surface. As presented in Fig. 3C and
D, 2 mg/ml GLT treatment significantly decreased DU-145 cell
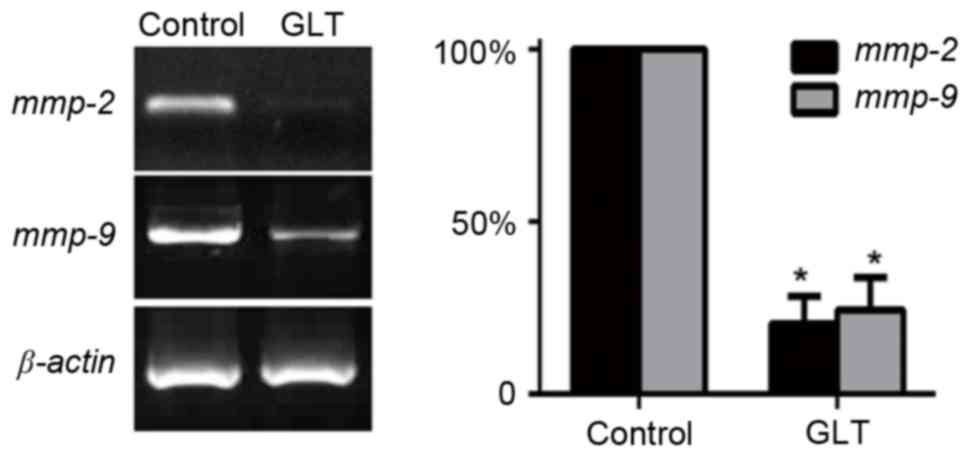
invasion. In addition, migration/invasion-associated genes, MMPs,
have previously been implicated in prostate cancer metastasis
(17,18). Thus, the mRNA expression level of
MMP-2 and MMP-9 were investigated. As presented in Fig. 4, the results of RT-PCR indicated that
2 mg/ml GLT significantly suppressed the expression levels of MMPs.
These results revealed that a high dose of GLT inhibited prostate
cancer cell migration and invasion via the suppression of MMPs.
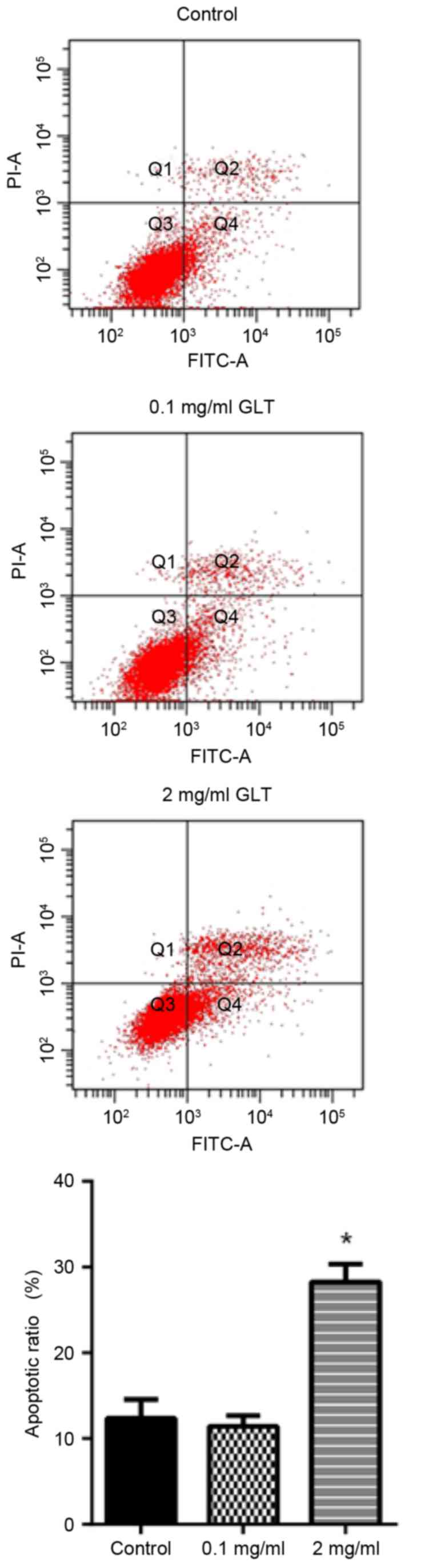
GLT induces prostate cancer cell
apoptosis
It was investigated whether GLT was able to induce
the apoptosis of prostate cancer cells. The apoptosis rate was
determined by annexin V-FITC and PI double staining. Compared with
the control group, 0.1 mg/ml GLT administration revealed no effect,
whereas the apoptosis rate with 2 mg/ml GLT treatment was markedly
increased (Fig. 5). These results
suggested that GLT inhibited viability, migration and invasion, and
also induces the apoptosis of prostate cancer cells in
vitro.
Discussion
The results of the present study demonstrated that
GLT inhibits the growth of prostate cancer cells, suppresses the
migration and invasion and induces apoptosis via the inhibition of
MMP expression. The elucidation of the anticancer mechanism
underlying GLT may contribute to the clinical usage of active
compounds isolated from G. lucidum.
G. lucidum has been used as a preventive
medicine in Asia for centuries (19).
Various biologically active compounds have been isolated from G.
lucidum, including polysaccharides, phenols, lipids and
triterpenes. Polysaccharides possess antioxidant (20), immunomodulatory (21) and antitumor characteristics (22). Also, polysaccharides activate the
immune response via the stimulation of production of inflammation
mediators (5,23). Phenols have antioxidant properties
(24) and lipids are able to inhibit
the growth of hepatoma and sarcoma (25). Triterpenes demonstrate cytotoxicity
towards hepatoma, cervical cancer and lung carcinoma cells
(26–28). Besides the antitumor activity, GLT
serve a role in a variety of other biological process, including
anti-human immunodeficiency virus (HIV) and anti-HIV-1 protease
activity (29,30), neurotrophic activity (31) and anti-obesity activity (32). The results of the present study
identified that GLT inhibited the metastasis and induced the
apoptosis of prostate cancer cells. These results are consistent
with those of previous studies that identified the anti-prostate
cancer effect of G. lucidum (15,16). The
identification of biologically active components of G.
lucidum is important for the mechanistic characterization of
their specific activity and the results of the present study
further revealed that triterpenes may be the active compounds
responsible for the antitumor effect of G. lucidum. In
addition, there is certain evidence that specific components in the
natural herbal products interact with each other to function as the
whole product (33). The present
study did not identify other compounds extracted from G.
lucidum, which requires further investigation.
The inhibition of the viability of prostate cancer
cells by GLT may be caused by the induction of apoptosis. Apoptosis
is a physiological process where cells are removed when they
experience critical DNA damage (34,35).
Inhibition of apoptosis, rather than enhanced cell proliferation,
is particularly important for the development of cancer (36,37). The
results of the present study revealed that GLT significantly
induced apoptosis, confirmed by annexin V staining and flow
cytometry. Generally, apoptosis can be divided into early and late
stages. During the late stage of apoptosis, DNA fragmentation
occurs following reactive oxygen species generation, caspase-3
activation and mitochondrial dysfunction (38). Additionally, apoptosis can be divided
into the extrinsic and intrinsic pathways. The extrinsic apoptotic
pathway can be initiated by death receptors (39) and the intrinsic pathway is also called
the mitochondrion-dependent pathway (40). The majority of stimuli induce
apoptosis via the mitochondrial pathway, which induces
mitochondrial outer membrane permeabilization and these processes
are regulated by members of the B cell lymphoma-2 (Bcl-2) protein
family. This family may be subdivided into pro-apoptotic
(Bcl-2-associated X protein and Bcl-2 homologous antagonist
killer), pro-Bcl-2 homology 3-only proteins (including
BH3-interacting domain death agonist, Bcl-2-like protein 11 and
p53-upregulated modulator of apoptosis) and anti-apoptotic proteins
[including Bcl-2 and B cell lymphoma extra-large (Bcl-xL)]
(35). Decreased expression levels of
anti-apoptotic proteins and increased expression levels or
activation of pro-apoptotic proteins have been demonstrated to be
critical for the induction of apoptosis (35,41).
DU-145 prostate cancer cells have increased expression levels of
Bcl-2 and Bcl-xL, protecting cells from apoptosis (42,43). The
results of the present study revealed that GLT induced apoptosis;
however, the specific underlying molecular mechanisms remain under
investigation. The results of the present study suggested that GLT
may decrease the expression of anti-apoptotic proteins and increase
the expression levels of, or activate, pro-apoptotic proteins, thus
inducing cell death.
The results of the present study revealed the
antitumor effect of GLT. GLT administration inhibits the
proliferation of human prostate cancer cells and induced apoptosis.
Additional detailed signaling mechanisms and in vivo studies
are required to establish GLT as a potential clinical agent for the
prevention and/or treatment of prostate cancer.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for the Central Universities (grant no. 21615498),
Medical Scientific Research Foundation of Guangdong Province (grant
no. A2015463) and Administration of Traditional Chinese Medicine of
Guangdong Province, China (grant no. 20151186).
References
|
1
|
Jemal A, Murray T, Samuels A, Ghafoor A,
Ward E and Thun MJ: Cancer statistics, 2003. CA Cancer J Clin.
53:5–26. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taichman RS, Loberg RD, Mehra R and Pienta
KJ: The evolving biology and treatment of prostate cancer. J Clin
Invest. 117:2351–2361. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Norgaard M, Jensen AØ, Jacobsen JB, Cetin
K, Fryzek JP and Sørensen HT: Skeletal related events, bone
metastasis and survival of prostate cancer: A population based
cohort study in Denmark (1999 to 2007). J Urol. 184:162–167. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cheung WM, Hui WS, Chu PW, Chiu SW and Ip
NY: Ganoderma extract activates MAP kinases and induces the
neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS
Lett. 486:291–296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS,
Shiao MS and Ho CK: The anti-tumor effect of Ganoderma lucidum is
mediated by cytokines released from activated macrophages and T
lymphocytes. Int J Cancer. 70:699–705. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wasser SP and Weis AL: Therapeutic effects
of substances occurring in higher Basidiomycetes mushrooms: a
modern perspective. Crit Rev Immunol. 19:65–96. 1999.PubMed/NCBI
|
|
7
|
Ji Z, Tang Q, Zhang J, Yang Y, Jia W and
Pan Y: Immunomodulation of RAW264.7 macrophages by GLIS, a
proteopolysaccharide from Ganoderma lucidum. J Ethnopharmacol.
112:445–450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mizushina Y, Takahashi N, Hanashima L,
Koshino H, Esumi Y, Uzawa J, Sugawara F and Sakaguchi K: Lucidenic
acid O and lactone, new terpene inhibitors of eukaryotic DNA
polymerases from a basidiomycete, Ganoderma lucidum. Bioorg Med
Chem. 7:2047–2052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferreira IC, Heleno SA, Reis FS, Stojkovic
D, Queiroz MJ, Vasconcelos MH and Sokovic M: Chemical features of
Ganoderma polysaccharides with antioxidant, antitumor and
antimicrobial activities. Phytochemistry. 114:38–55. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lakshmi B, Ajith TA, Sheena N, Gunapalan N
and Janardhanan KK: Antiperoxidative, anti-inflammatory and
antimutagenic activities of ethanol extract of the mycelium of
Ganoderma lucidum occurring in South India. Teratog Carcinog
Mutagen. Suppl 1:S85–S97. 2003. View Article : Google Scholar
|
|
11
|
Lin ZB and Zhang HN: Anti-tumor and
immunoregulatory activities of Ganoderma lucidum and its possible
mechanisms. Acta Pharmacol Sin. 25:1387–1395. 2004.PubMed/NCBI
|
|
12
|
Pan K, Jiang Q, Liu G, Miao X and Zhong D:
Optimization extraction of Ganoderma lucidum polysaccharides and
its immunity and antioxidant activities. Int J Biol Macromol.
55:301–306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao W, Jiang X, Deng W, Lai Y, Wu M and
Zhang Z: Antioxidant activities of Ganoderma lucidum
polysaccharides and their role on DNA damage in mice induced by
cobalt-60 gamma-irradiation. Food Chem Toxicol. 50:303–309. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Min BS, Gao JJ, Nakamura N and Hattori M:
Triterpenes from the spores of Ganoderma lucidum and their
cytotoxicity against meth-A and LLC tumor cells. Chem Pharm Bull
(Tokyo). 48:1026–1033. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stanley G, Harvey K, Slivova V, Jiang J
and Sliva D: Ganoderma lucidum suppresses angiogenesis through the
inhibition of secretion of VEGF and TGF-beta1 from prostate cancer
cells. Biochem Biophys Res Commun. 330:46–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang J, Slivova V, Valachovicova T,
Harvey K and Sliva D: Ganoderma lucidum inhibits proliferation and
induces apoptosis in human prostate cancer cells PC-3. Int J Oncol.
24:1093–1099. 2004.PubMed/NCBI
|
|
17
|
Qi M, Liu Z, Shen C, Wang L, Zeng J, Wang
C, Li C, Fu W, Sun Y and Han B: Overexpression of ETV4 is
associated with poor prognosis in prostate cancer: Involvement of
uPA/uPAR and MMPs. Tumour Biol. 36:3565–3572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sehgal I, Forbes K and Webb MA: Reduced
secretion of MMPs, plasminogen activators and TIMPS from prostate
cancer cells derived by repeated metastasis. Anticancer Res.
23:39–42. 2003.PubMed/NCBI
|
|
19
|
Shiao MS: Natural products of the
medicinal fungus Ganoderma lucidum: Occurrence, biological
activities, and pharmacological functions. Chem Rec. 3:172–180.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu W, Wang H, Pang X, Yao W and Gao X:
Characterization and antioxidant activity of two
low-molecular-weight polysaccharides purified from the fruiting
bodies of Ganoderma lucidum. Int J Biol Macromol. 46:451–457. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao X, Liu C, Fang J and Li X: Structural
and immunological studies of a major polysaccharide from spores of
Ganoderma lucidum (Fr.) Karst. Carbohydr Res. 332:67–74. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao QZ and Lin ZB: Ganoderma lucidum
polysaccharides peptide inhibits the growth of vascular endothelial
cell and the induction of VEGF in human lung cancer cell. Life Sci.
78:1457–1463. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wachtel-Galor S, Choi SW and Benzie IF:
Effect of Ganoderma lucidum on human DNA is dose dependent and
mediated by hydrogen peroxide. Redox Rep. 10:145–149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mau JL, Lin HC and Chen CC: Antioxidant
properties of several medicinal mushrooms. J Agric Food Chem.
50:6072–6077. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Yuan JP, Chung CK and Chen XJ:
Antitumor activity of the sporoderm-broken germinating spores of
Ganoderma lucidum. Cancer Lett. 182:155–161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruan W, Wei Y and Popovich DG: Distinct
responses of cytotoxic ganoderma lucidum triterpenoids in human
carcinoma cells. Phytother Res. 29:1744–1752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li P, Deng YP, Wei XX and Xu JH:
Triterpenoids from Ganoderma lucidum and their cytotoxic
activities. Nat Prod Res. 27:17–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cheng CR, Yue QX, Wu ZY, Song XY, Tao SJ,
Wu XH, Xu PP, Liu X, Guan SH and Guo DA: Cytotoxic triterpenoids
from Ganoderma lucidum. Phytochemistry. 71:1579–1585. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El Dine RS, El Halawany AM, Ma CM and
Hattori M: Anti-HIV-1 protease activity of lanostane triterpenes
from the vietnamese mushroom Ganoderma colossum. J Nat Prod.
71:1022–1026. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Min BS, Nakamura N, Miyashiro H, Bae KW
and Hattori M: Triterpenes from the spores of Ganoderma lucidum and
their inhibitory activity against HIV-1 protease. Chem Pharm Bull
(Tokyo). 46:1607–1612. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang XQ, Ip FC, Zhang DM, Chen LX, Zhang
W, Li YL, Ip NY and Ye WC: Triterpenoids with neurotrophic activity
from Ganoderma lucidum. Nat Prod Res. 25:1607–1613. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee I, Seo J, Kim J, Kim H, Youn U, Lee J,
Jung H, Na M, Hattori M, Min B and Bae K: Lanostane triterpenes
from the fruiting bodies of Ganoderma lucidum and their inhibitory
effects on adipocyte differentiation in 3T3-L1 Cells. J Nat Prod.
73:172–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wilasrusmee C, Kittur S, Siddiqui J, Bruch
D, Wilasrusmee S and Kittur DS: In vitro immunomodulatory effects
of ten commonly used herbs on murine lymphocytes. J Altern
Complement Med. 8:467–475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen D, Peng F, Cui QC, Daniel KG, Orlu S,
Liu J and Dou QP: Inhibition of prostate cancer cellular proteasome
activity by a pyrrolidine dithiocarbamate-copper complex is
associated with suppression of proliferation and induction of
apoptosis. Front Biosci. 10:2932–2939. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Y, Kaul S, Zhang D, Anantharam V and
Kanthasamy AG: Suppression of caspase-3-dependent proteolytic
activation of protein kinase C delta by small interfering RNA
prevents MPP+-induced dopaminergic degeneration. Mol Cell Neurosci.
25:406–421. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dickens LS, Powley IR, Hughes MA and
MacFarlane M: The ‘complexities’ of life and death: Death receptor
signalling platforms. Exp Cell Res. 318:1269–1277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tait SW and Green DR: Mitochondria and
cell death: Outer membrane permeabilization and beyond. Nat Rev Mol
Cell Biol. 11:621–632. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vogler M, Walter HS and Dyer MJS:
Targeting anti-apoptotic BCL2 family proteins in haematological
malignancies-from pathogenesis to treatment. Br J Haematol.
178:364–379. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ibrado AM, Huang Y, Fang G, Liu L and
Bhalla K: Overexpression of Bcl-2 or Bcl-xL inhibits Ara-C-induced
CPP32/Yama protease activity and apoptosis of human acute
myelogenous leukemia HL-60 cells. Cancer Res. 56:4743–4748.
1996.PubMed/NCBI
|
|
43
|
Lebedeva I, Rando R, Ojwang J, Cossum P
and Stein CA: Bcl-xL in prostate cancer cells: Effects of
overexpression and down-regulation on chemosensitivity. Cancer Res.
60:6052–6060. 2000.PubMed/NCBI
|