Introduction
Acute myeloid leukemia (AML) is a hematopoietic
malignancy characterized by the accumulation of clonal myeloid
precursor in the bone marrow (1).
Despite improvements in chemotherapy and the development of novel
drugs, the prognosis for most AML subtypes remains poor (1). It is well recognized that abnormalities
in the immune system are involved in the pathogenesis of AML
(2). Previous studies have
demonstrated that aberrant T-helper cells (Th) are involved in in
AML progress. It has been established that the innate immune
response is closely associated with the adaptive immune response;
however, research regarding the innate immune response in AML
pathogenesis is limited (3).
The activation of the innate immune response
requires the recognition of pathogen-associated molecular patterns
(PAMPs) or danger-associated molecular patterns (DAMPs) by pattern
recognition receptors (PRRs) (4). The
nucleotide-binding and oligomerization domain-like receptor (NLR)
family are typical PRRs. Inflammasomes are essential for the
activation of the innate immune response. In recent decades, the
NLR family pyrin domain-containing 3 (NLRP3) inflammasome has
attracted attention as it may be activated by both PAMPs and DAMPs
(5). The NLRP3 inflammasome includes
three main components: The sensor protein, NLRP3, the adaptor
protein, apoptosis-associated speck-like protein (ASC), and
pro-caspase-1. To activate the NLRP3 inflammasome, there are two
steps (5). The first step involves
the recognition of PAMPs and DAMPs by NLRP3. Nuclear factor κB
(NF-κB) is activated, resulting in the increased transcription of
inflammasome-associated molecules, including inactive NLRP3,
pro-interleukin (IL)-18 and pro-IL-1β (6). The second step is the formation of a
molecular platform; NLRP3 recruits ASC and interacts with
pro-caspase-1 (7). This induces the
cleavage of pro-caspase-1 to its active form, caspase-1; in turn,
caspase-1 cleaves pro-IL-1β and pro-IL-18 to their biologically
active forms, IL-1β and IL-18. The NLRP3 inflammasome has been
demonstrated to be involved in the pathogenesis of numerous
diseases, including diabetes, chronic kidney disease and coronary
heart disease (7). At present, to the
best of our knowledge, no study has focused on the role of the
NLRP3 inflammasome in AML.
Accumulating evidence indicates that imbalanced Th
subset proportions are involved in the pathogenesis of a number of
types of tumor, including hematological malignancies; however, the
specific role of Th subsets in tumor pathogenesis is under debate.
Th22 is a recently identified cluster of differentiation (CD)4+ Th
subset, which only secretes IL-22, and does not secrete IL-17 or
interferon (IFN)-γ. Past research has demonstrated that IL-22 is
involved in the pathogenesis of many diseases, including
inflammatory autoimmune disease (8–11) and
hematological diseases, including myelodysplastic syndrome, immune
thrombocytopenia (ITP) and acute lymphoblastic leukemia (12–14). A
study by Lucas et al suggested that imbalanced Th22 and Th1
subset proportions serve important roles in the development of AML;
however, the mechanism for this remains ambiguous (2).
Goergens et al observed that aryl hydrocarbon
receptor (AHR) may be involved in the development of AML (15). It has also been suggested that AHR
serves a pivotal role in the regulation of the immune response,
particularly in Th subset differentiation (16). It has been previously demonstrated
that AHR negatively regulates NLRP3 inflammasome activation by
inhibiting the transcription of NLRP3, as summarized in a review by
Huai (17).
It has been established that NLRP3 inflammasome and
the associated cytokines, IL-1β and −18, modulate the adaptive
immune response via the regulation of Th subset differentiation.
Gris et al demonstrated that the production of IFN-γ in
NLRP3−/− mice was decreased, suggesting that the NLRP3
inflammasome may be associated with the differentiation of the Th1
subset (18).
The present study aimed to investigate the NLRP3
inflammasome, and the associated cytokines IL-1β and −18, in the
development of AML, identify statistical correlations between the
NLRP3 inflammasome and Th subsets in the peripheral blood (PB) and
bone marrow (BM) microenvironments, and explore their clinical
relevance.
Materials and methods
Patients and controls
A total of 90 newly-diagnosed (ND) patients with AML
(42 females and 48 males; age range, 15–75 years; median age, 49
years) and 79 patients exhibiting complete remission (CR) from AML
(31 females and 48 males; age range, 15–75 years; median age, 37
years) were included in the study. AML was diagnosed according to
the French-American-British classification system (19) and CR was defined using the Word Health
Organization Classification (20).
Patients that exhibited hypertension, cardiovascular diseases,
infection, connective tissue diseases or autoimmune diseases were
excluded from the study. A total of 28 healthy controls were
included in the study. Bone marrow mononuclear cells (BMMCs) and
peripheral blood mononuclear cells (PBMCs) were isolated from the
patients with AML and controls. In CR patients, leukemic cells can
only marginally be detected (20).
Therefore, BMMCs of ND patients were used to represent leukemic
cells, whereas BMMCs of the CR patients or controls were used to
represent normal cells. Enrollment in the study was between
September 2014 and September 2015 at the Qilu Hospital of Shandong
University (Jinan, China). Detailed clinical features of the
patients with AML and the control group are described in Table I. The present study received approval
from the Medical Ethics Committee of the Qilu Hospital of Shandong
University. All the patients provided informed consent prior to
inclusion in the study.
 | Table I.Clinical characteristics of patients
with AML and controls. |
Table I.
Clinical characteristics of patients
with AML and controls.
|
Characteristics | ND | CR | Controls |
|---|
| Total | 90 | 79 | 28 |
| Age range,
years | 14–75 | 14–75 | 20–60 |
| Gender,
female/male | 42/48 | 31/48 | 13/15 |
| Serum levels,
g/l |
|
|
|
|
Albumin | 38.11±5.32 | 43.30±4.26 | – |
|
Globulin | 26.00±4.90 | 26.76±4.59 | – |
| Total
protein | 64.11±6.32 | 70.11±5.99 | – |
| Cell counts |
|
|
|
| White
blood cells, median, range; ×1012/l | 17.11,
0.4–218.2 | 4.98, 1.9–14.7 | 5.10,
4.13–10.0 |
| Red
blood cells, median, range; ×1012/l | 2.50,
1.26–4.55 | 3.73,
2.06–5.13 | 3.50, 3.20–5.5 |
|
Platelets, median, range;
×109/l | 35, 4–492 | 269, 49–731 | 307, 105–410 |
| Lactate
dehydrogenase, median, range; U/l | 492, 152–2,906 | 182, 98–459 | – |
|
French-American-British classification
subtype |
|
|
|
| M1 | 1 | 1 | N/A |
| M2 | 5 | 4 | N/A |
| M3 | 20 | 27 | N/A |
| M4 | 12 | 8 | N/A |
| M5 | 47 | 38 | N/A |
| M6 | 5 | 1 | N/A |
Flow cytometric analysis
Intracellular cytokines were detected by flow
cytometry to identify the cytokine-producing cells. Briefly,
heparinized peripheral whole blood (100 µl) with an equal volume of
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), including 25 ng/ml of phorbol myristate acetate
(PMA), 1 µg/ml of ionomycin and 1.7 µg/ml monensin (Alexis
Biochemicals, San Diego, CA, USA), was incubated for 4 h at 37°C
with 5% CO2. PMA and ionomycin are T cell-activating
pharmaceuticals that mimic signals generated by the T-cell receptor
complex and may stimulate T cells of any antigen specificity.
Monensin was used to block intracellular transport mechanisms and
led to an accumulation of cytokines in the cells.
All antibodies were purchased from eBioscience, Inc.
(San Diego, CA, USA). After incubation, 100 µl incubated blood was
placed in each tube, the cells were stained with PE-Cy5-conjugated
anti-CD3 monoclonal antibody (#300420) and anti-CD8
monoclonal antibody (#344714) (both from BioLegend,
Inc., San Diego, CA, USA) at room temperature in the dark for 20
min. Then, 100 µl reagent A (Fixation) was placed in each tube and
incubated at room temperature in the dark for 15 min. Following
this, 3 ml PBS was placed in each tube and centrifugated at 1,000 ×
g for 5 min at 25°C, to wash the cells. A total of 100 µl reagent B
(Permeabilisation) was put in each tube with FITC-conjugated
anti-IFN-γ (cat no. 502506), PE-anti-human IL-17A (cat no. 512306)
(both from BioLegend, Inc.) and APC-conjugated anti-IL-22
monoclonal antibodies (cat no. 50-7229-42; eBioscience, Inc.) in a
total of 100 µl buffe. Fixation and permeabilisation reagents were
purchased from Caltag; Invitrogen; Thermo Fisher Scientific, Inc.,
and used according to the manufacturer's protocol. The cells were
then stained at room temperature in the dark for 20 min. Subsequent
to this, 500 µl PBS was placed in each tube and centrifugated at
1,000 × g for 5 min at 25°C, to wash the cells again.
Isotype controls (mouse IgG1κ) were used to enable
the correct compensation and confirm antibody specificity. Stained
cells were analyzed by flow cytometric analysis using a Beckman
gallios cytometer (Beckman Coulter, Brea, CA, USA). For analysis,
CD3+CD8- lymphocytes were gated, then the proportion of Th22
(CD3+CD8-IL-17-IFNr-IL-22+) and Th1 (CD3+CD8-IFN-γ+) cells in
CD3+CD8- lymphocytes was analyzed.
ELISA of IL-18
BM and PB plasma were collected from ND and CR
patients with AML and normal controls and stored at −80°C
immediately after centrifugation (1,000 × g for 5 min at 25°C). The
plasma was used for the detection of NLRP3-related cytokines. The
level of IL-18 in each group was determined using the ELISA method,
according to the manufacturer's protocol (lower detection limit 78
pg/ml; eBioscience, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted with TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Total RNA (~1 µg) from each sample was used to synthesize
complementary (c)DNA with the PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). The RT reaction was
performed at 37°C for 15 min, followed by 85°C for 5 sec.
Quantitative PCR was conducted using an LC480II Real-Time PCR
system (Roche Diagnostics, Basel, Switzerland) in accordance with
the manufacturer's protocol. For amplification, an initial
denaturation step at 95°C for 5 min was followed by 40 cycles at
95°C for 15 sec, 60°C for 15 sec and 72°C for 40 sec. The qPCR
reaction contained, in a final volume of 10 µl, 5 µl of 2X
SYBR-Green Real-Time PCR Master Mix (Toyobo Co., Ltd., Osaka,
Japan), 1 µl of cDNA, 3.2 µl of ddH2O, and 0.4 µl of the
forward and reverse primers. The sequences for all primers are
described in Table II. All
experiments were conducted in triplicate. The PCR products were
analyzed by melt curve analysis and agarose gel electrophoresis to
determine product size and to confirm that no by-products were
formed. The results were expressed relative to the number of
β-actin transcripts, an internal control. Relative gene expression
level was calculated using the 2−ΔΔCq method (21).
 | Table II.Primer sequences. |
Table II.
Primer sequences.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| AHR |
CAAATCCTTCCAAGCGGCATA |
CGCTGAGCCTAAGAACTGAAAG |
| NLRP3 |
CAGACTTCTGTGTGTGGGACTGA |
TCCTGACAACATGCTGATGTGA |
| ASC |
TGGATGCTCTGTACGGGAAG |
CCAGGCTGGTGTGAAACTGAA |
| CASP-1 |
AAATCTCACTGCTTCGGACATG |
GGAACTTGCTGTCAGAGGTCTT |
| IL-18 |
GCTTGAATCTAAATTATCAGTC |
GAAGATTCAAATTGCATCTTAT |
| IL-1β |
ATGATGGCTTATTACAGTGGCAA |
GTCGGAGATTCGTAGCTGGA |
Statistical analysis
Results were expressed as the mean ± standard
deviation, or median (range). The statistical significance of
differences in Th cells (including Th1 as well as Th22 in PB) and
IL-18 (levels in PB plasma) were determined by ANOVA. The
differences in the levels of NLRP3 inflammasome molecules (NLRP3,
ASC, caspase-1, IL-18 and IL-1β) and the transcription factor AHR
were determined using the Kruskal-Wallis Test. The differences
between two groups were determined by the Mann-Whitney U test,
unless data were normally distributed, in which case a T-test was
used. The Pearson or Spearman correlation test was used for
correlation analysis, depending on the data distribution. All tests
were performed with SPSS 13.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
NLRP3 inflammasome molecules were
aberrantly expressed in patients with AML
NLRP3 inflammasome is a multiprotein complex, which
includes NLRP3, ASC and caspase-1. The expression of these proteins
was detected in BMMCs and PBMCs with RT-qPCR. In the BM
microenvironment, the expression of NLRP3 was significantly higher
in the ND AML group (median, 0.0023; range, 0.00029–0.0084) than in
the CR AML group (median, 0.00088; range, 0.00049–0.0019;
P<0.001; Fig. 1A). The data
revealed that the expression of ASC was elevated in the ND group
(median 0.0051, range 0.0014–0.017) compared with the CR group
(median, 0.0037; range, 0.0015–0.012; P=0.011; Fig. 1B). No statistical significance was
found between the expression of caspase-1 in the ND group (median,
0.0088; range, 0.00089–0.045) and the CR group (median, 0.0085;
range, 0.0049–0.023; P=0.457; Fig.
1C).
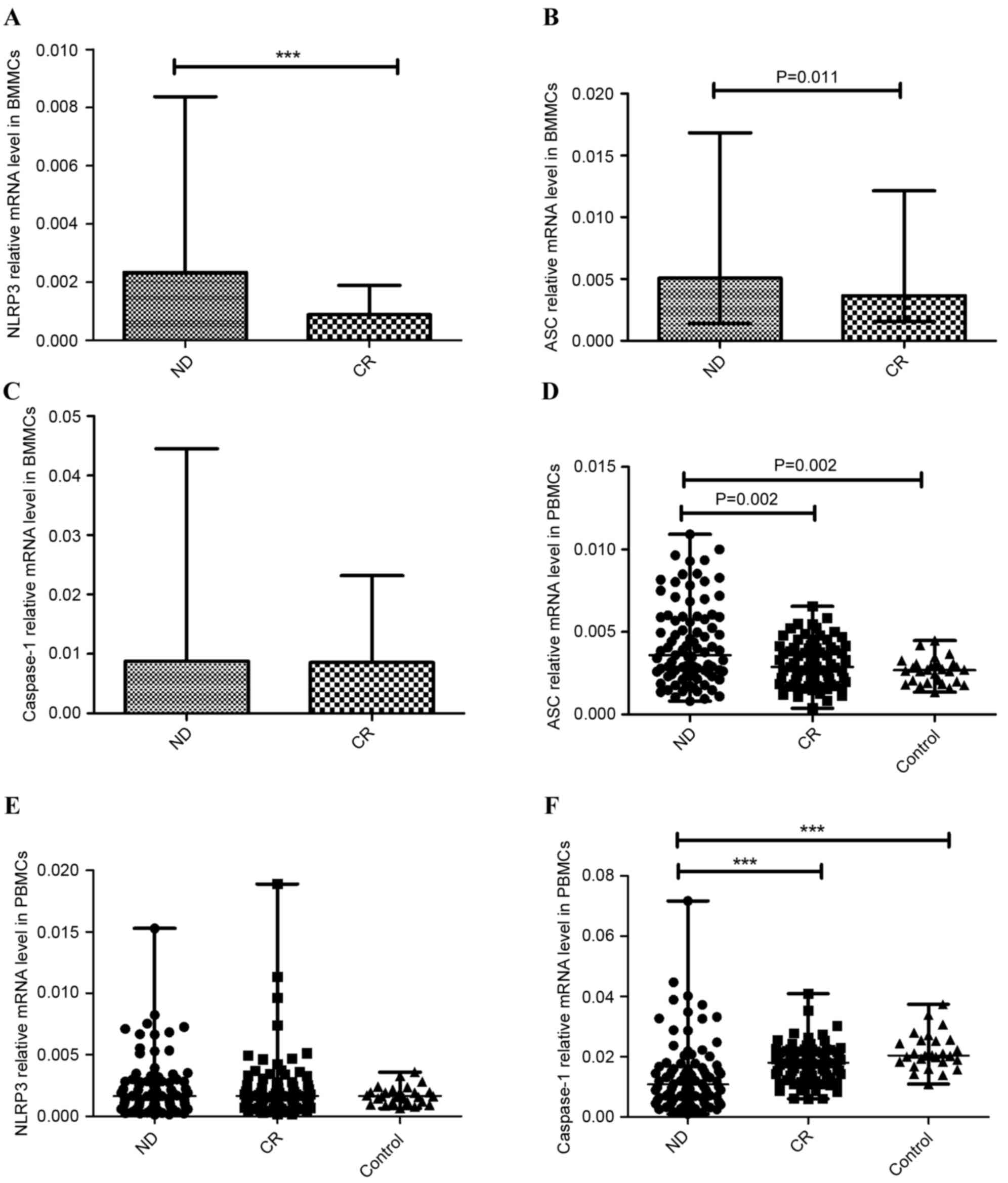 | Figure 1.Relative NLRP3 inflammasome molecule
(NLRP3, ASC, caspase-1) mRNA expression. A significantly increased
expression level of (A) NLRP3, (B) ASC and (C) caspase-1 mRNA in
BMMCs was observed in ND patients compared with CR Patients with
AML. (D) In PBMCs, the expression of ASC in the ND group was
elevated compared with the CR and control groups. (E) No
significant difference in NLRP3 expression between ND patients, CR
patients and normal controls was identified in PBMCs. (F) The
expression of caspase-1 in ND patients was markedly decreased
relative to the CR and control groups in PBMCs. ***P<0.001.
NLRP3, NLR family pyrin domain-containing 3; ASC,
apoptosis-associated speck-like protein; BMMCs, bone marrow
mononuclear cells; ND, newly-diagnosed; CR, complete remission;
AML, acute myeloid leukemia; PBMCs, peripheral blood mononuclear
cells. |
In the PB microenvironment, the expression of ASC
was elevated in the ND group (median 0.0037, range 0.00081–0.026)
compared with the CR group (median 0.0029, range 0.00039–0.0066;
P=0.002) or the normal control group (median 0.0027, range
0.0014–0.0045; P=0.002) (Fig. 1D),
consistent with the results from BMMCs. There was no significant
difference of NLRP3 expression between ND patients (median 0.0016,
range 0.00014–0.015), CR patients (median 0.0017, range
0.00018–0.019) and the control group (median 0.0017, range
0.00064–0.0036; Fig. 1E). However,
caspase-1 level in ND patients (median 0.011, range 0.00098–0.072)
was decreased relative to the CR group (median 0.018, range
0.0060–0.041; P<0.001) and control group (median 0.020, range
0.011–0.037; P<0.001) (Fig.
1F).
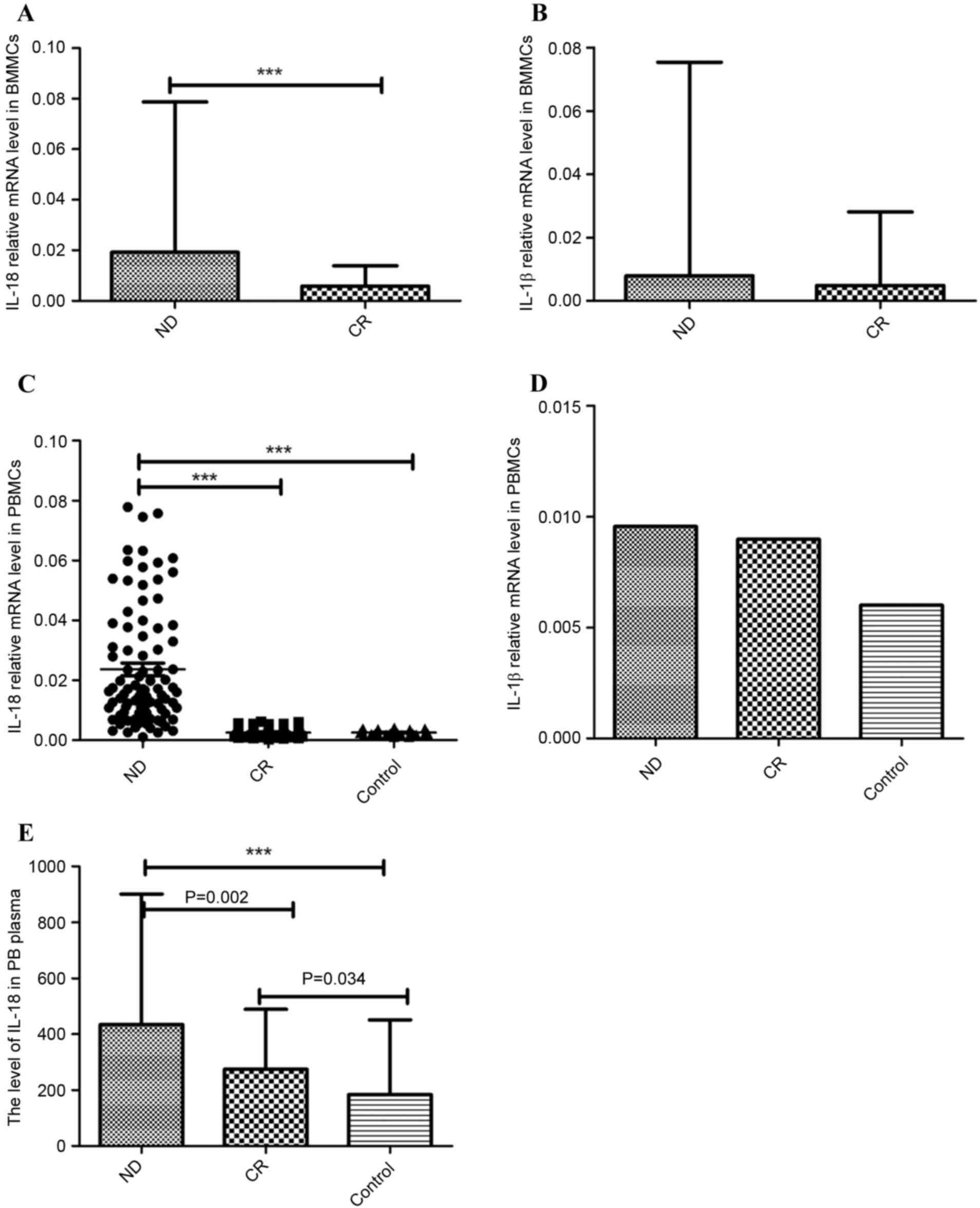
NLRP3 effector cytokines (IL-1β and
IL-18) were abnormal in patients with AML
IL-1β and IL-18 are the main effector cytokines of
the NLRP3 inflammasome. In the BM microenvironment of patients with
AML, IL-18 mRNA expression was significantly increased in the ND
group (median 0.019, range 0.0034–0.079) relative to the CR group
(median 0.0058, range 0.00038–0.014; P<0.001; Fig. 2A). IL-1β mRNA level was also
marginally elevated in the ND patients (median 0.0079, range
0.00018–0.075) compared with the CR patients (median 0.0049, range
0.00090–0.028; P=0.116; Fig. 2B).
In PB, the relative mRNA level of IL-18 in the ND
group (median 0.017, range 0.00104–0.078) was significantly higher
than in the CR group (median 0.0022, range 0.00030–0.0063;
P<0.001) and controls (median 0.0023, range 0.00113–0.0042;
P<0.001; Fig. 2C). IL-1β mRNA
expression was increased in the ND group (median 0.0095, range
0.00012–0.11) and CR group (median 0.0090, range 0.00051–0.33)
compared with the control group (median 0.0060, range
0.0009–0.011), although no statistical significance was identified
(Fig. 2D). No significant difference
in IL-1β level was found between the ND and CR groups.
ELISA was used to detect the level of IL-18 protein
level in PB plasma. IL-18 was identified as significantly increased
in ND patients (444.717±219.420 pg/ml) compared with the CR
patients (272.284±81.776 pg/ml; P=0.002) and controls
(207.296±98.827 pg/ml; P<0.001). IL-18 serum protein in CR
patients was also increased relative to the control group (P=0.034;
Fig. 2E).
Relationships between NLRP3
inflammasome molecules and effector cytokines
To improve the understanding of the NLRP3
inflammasome, correlations between the relative expression level of
NLRP3 inflammasome molecules and effector cytokines were
investigated. The resulting data indicated that, in PBMCs, the
expression levels of NLRP3 inflammasome molecules and effector
cytokines were positively correlated in ND patients (Fig. 3A). NLRP3 level was positively
correlated with ASC (r=0.424, P<0.001), caspase-1 (r=0.327,
P=0.002), IL-1β (r=0.244, P=0.021), and IL-18 (r=0.241, P=0.022)
level. ASC level was positively correlated with caspase-1 (r=0.306,
P=0.003), IL-1β (r=0.211, P=0.046), and IL-18 (r=0.382, P<0.001)
level. Caspase-1 level was positively correlated with IL-1β
(r=0.384, P<0.001) and IL-18 (r=0.300, P=0.004) level. As for
effector cytokines, IL-1β level showed positive correlation with
IL-18 level (r=0.548, P<0.001). The correlations between the
level of NLRP3 inflammasome molecules and effector cytokines in CR
patients varied from in ND patients (Fig.
3B). The level of NLRP3 was positively correlated with ASC
(r=0.329, P=0.003), caspase-1 (r=0.256 P=0.023) IL-1β (r=0.441,
P<0.001) levels. The level of ASC was positively correlated with
caspase-1 (r=0.357, P=0.001) and IL-18 (r=0.223, P=0.048) levels.
No statistically significant correlation was identified between
other molecules. In the controls (Fig.
3C), the only positive correlation identified was between NLRP3
and ASC (r=0.648, P<0.001) expression level.
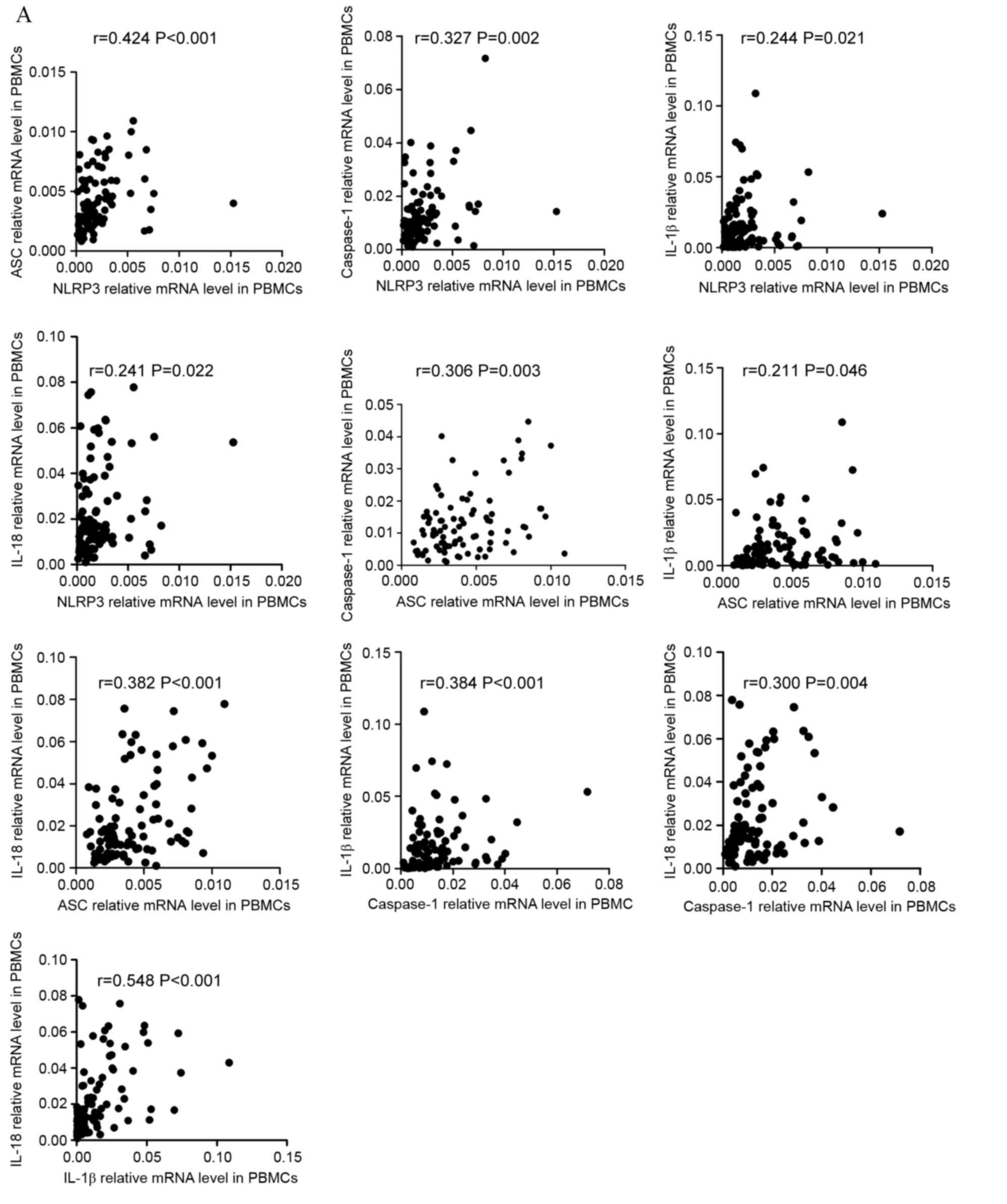 | Figure 3.Correlation between the mRNA
expression of NLRP3 molecules and cytokines. (A) The mRNA
expression of NLRP3 inflammasome molecules in PBMCs and effector
cytokines in PBBCs were positively correlated in ND patients.
Additionally, PBBC IL-1β mRNA levels demonstrated a positive
correlation with IL-18. NLRP3, NLR family pyrin domain-containing
3; PBMCs, peripheral blood mononuclear cells; ND, newly diagnosed;
IL, interleukin; CR, complete remission; ASC, apoptosis-associated
speck-like protein; BMMCs, bone marrow mononuclear cells.
Correlation between the mRNA expression of NLRP3 molecules and
cytokines. (B) In the PBMCs of CR patients, the NLRP3 mRNA level
was positively correlated with ASC and IL-1β mRNA level. The
relative level of ASC mRNA was positively correlated with caspase-1
and IL-18 mRNA levels. (C) In the PBMCs of controls, a positive
correlation between NLRP3 and ASC relative mRNA expression was
identified. NLRP3, NLR family pyrin domain-containing 3; PBMCs,
peripheral blood mononuclear cells; ND, newly diagnosed; IL,
interleukin; CR, complete remission; ASC, apoptosis-associated
speck-like protein; BMMCs, bone marrow mononuclear cells.
Correlation between the mRNA expression of NLRP3 molecules and
cytokines. (D) In BMMCs of ND patients it was identified that NLRP3
expression level was positively correlated with ASC and caspase-1,
and that ASC expression was positively correlated with the
caspase-1 mRNA level. IL-18 mRNA in BMMCs was positively correlated
with the relative ASC mRNA level. IL-1β and IL-18 mRNA levels were
positively correlated with the caspase-1 mRNA level. The IL-1β
level was also positively correlated with the level of IL-18 mRNA.
(E) In the BMMCs of CR patients, the only significant positive
correlation to be identified was between ASC and caspase-1 mRNA
levels. NLRP3, NLR family pyrin domain-containing 3; PBMCs,
peripheral blood mononuclear cells; ND, newly diagnosed; IL,
interleukin; CR, complete remission; ASC, apoptosis-associated
speck-like protein; BMMCs, bone marrow mononuclear cells. |
In BMMCs, it was identified that the expression
level of NLRP3 molecules were positively correlated with each
other. In ND patients, the level of NLRP3 was identified as
positively correlated with ASC (r=0.531, P<0.001) and caspase-1
(r=0.504, P=0.001) level, and ASC level was positively correlated
with caspase-1 level (r=0.504, P=0.001; Fig. 3D). As for correlations between NLRP3
molecules and effector cytokines, the level of IL-18 was positively
correlated with ASC level (r=0.473, P=0.003). IL-1β and Il-18
levels were positively correlated with caspase-1 level (r=0.360,
P=0.03; r=0.356, P=0.03, respectively). IL-1β level was also
identified to be positively correlated with IL-18 level (r=0.460,
P=0.004). In CR patients (Fig. 3E)
the only positive correlation identified was between ASC and
caspase-1 expression levels (r=0.554, P=0.004).
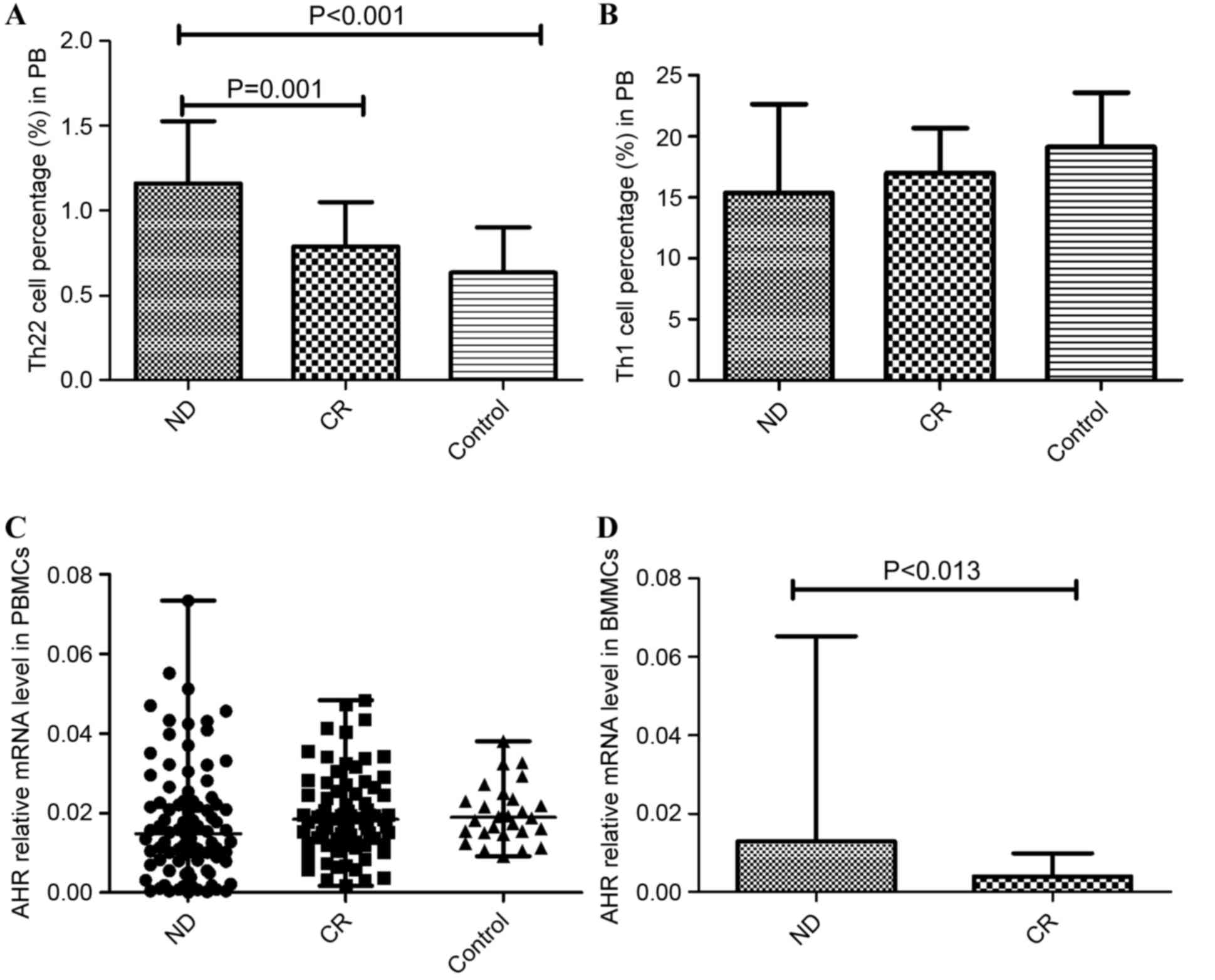
Imbalanced Th cells in patients with
AML
The results of the present study demonstrated immune
deregulation in the PB of patients with AML. The frequency of Th22
was increased in ND patients (1.16±0.37%) relative to CR patients
(0.79±0.26%; P=0.001) and controls (0.635±0.27%; P<0.001;
Fig. 4A). Although the proportion of
Th1 cells was reduced in ND patients (15.38±7.259%) compared with
the CR group (16.98±3.69%) and controls (19.12±4.48%; Fig. 4B), no statistical significance was
identified.
Imbalanced AHR in patients with
AML
AHR is associated with the differentiation of Th
subsets, particularly Th17 cells. The present study identified that
no significant difference in AHR expression level was found between
the PBMCs of the ND patients (median, 0.0148; range,
0.000131–0.0733), CR patients (median, 0.0184; range,
0.00167–0.0483) and controls (median, 0.0189; range,
0.00912–0.0380; Fig. 4C). In BMMCs,
it was identified that AHR was markedly elevated in ND patients
(median, 0.0129; range, 0.00025–0.0652) compared with CR patients
(median, 0.0040; range, 0.00119–0.00989; P=0.013; Fig. 4D).
Associations between NLRP3-related and
Th-related molecules
The data indicated that there were positive
correlations between NLRP3 inflammasome and Th subsets in BMMCs. In
ND patients, it was identified that NLRP3 (r=0.463, P=0.003),
caspase-1 (r=0.7144, P<0.001) or IL-1β (r=0.571, P<0.001)
were positively correlated with AHR (Fig.
5A). In CR group, caspase-1 (r=0.432, P=0.031) and IL-1β
(r=0.451, P=0.024) demonstrated positive correlation with AHR
(Fig. 5B).
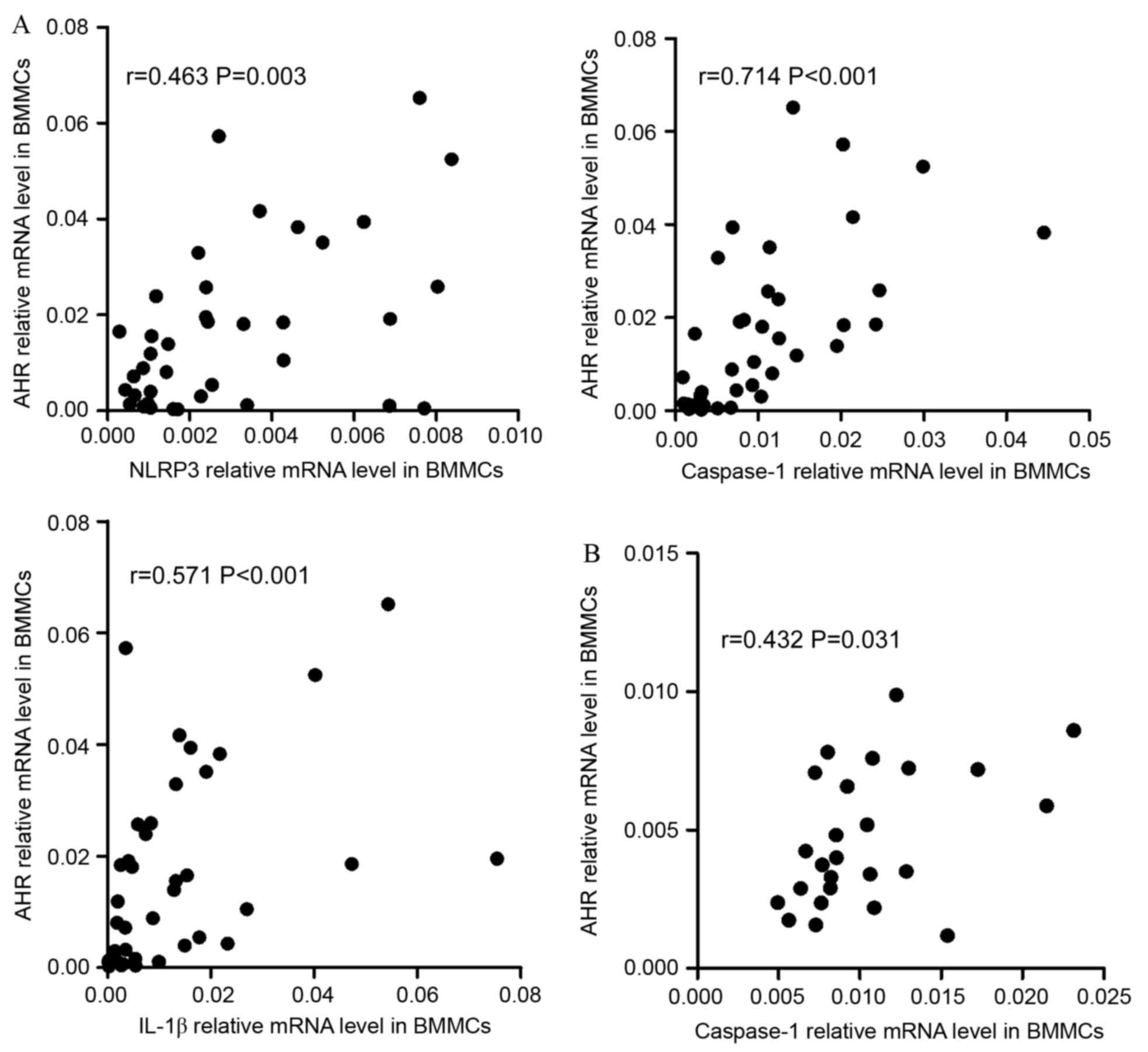 | Figure 5.Correlations between NLRP3-related
molecules and the Th-associated molecule AHR. (A) In the ND group,
NLRP3, caspase-1 and IL-1β mRNA levels were positively correlated
with AHR expression in the BM. (B) In CR patients, caspase-1 and
IL-1β mRNA level were positively correlated with the relative level
of AHR mRNA in the BM. (C) In ND patients, the expression of NLRP3,
ASC, caspase-1, IL-18 and IL-1β mRNA were positively correlated
with the relative AHR mRNA level in PBMCs. NLRP3, NLR family pyrin
domain-containing 3; Th, T-helper cell; AHR, aryl hydrocarbon
receptor; ND, newly diagnosed; IL, interleukin; CR, complete
remission; ASC, apoptosis-associated speck-like protein; PBMCs,
peripheral blood mononuclear cells. Correlations between
NLRP3-related molecules and the Th-associated molecule AHR. (D) In
PBMCs of the CR group, the levels of all NLRP3 inflammasome
molecule and effector cytokine mRNA levels were positively
correlated with the relative AHR mRNA level. (E) In the control
group, the AHR level was observed to be negatively correlated with
the caspase-1 mRNA level in PBMCs. NLRP3, NLR family pyrin
domain-containing 3; Th, T-helper cell; AHR, aryl hydrocarbon
receptor; ND, newly diagnosed; IL, interleukin; CR, complete
remission; ASC, apoptosis-associated speck-like protein; PBMCs,
peripheral blood mononuclear cells. |
In PBMCs, it was identified that the expression
levels of NLRP3 (r=0.409, P<0.001), ASC (r=0.319, P=0.002),
caspase-1 (r=0.568, P<0.001), IL-18 (r=0.415, P<0.001) and
IL-1β (r=0.465, P<0.001) had positive correlations with the
expression level of AHR in ND patients (Fig. 5C). In CR patients (Fig. 5D), it was also identified that the
levels of inflammasome molecules and effector cytokines were
positively correlated with AHR. In controls, it was observed that
AHR was negatively correlated with caspase-1 (r=−0.425, P=0.024;
Fig. 5E).
Correlation of NLRP3 inflammasome
protein mRNA levels with clinicopathological characteristics of
patients with AML
Correlations between clinicopathological
characteristics and NLRP3 inflammasome protein expression levels
were investigated. The data indicated that IL-18 expression level
was negatively correlated with the level of serum albumin
(r=−0.508, P<0.001; Fig. 6A) and
positively correlated with lactate dehydrogenase (r=0.609,
P<0.001; Fig. 6B) in all patients
including ND and CR patients. The association between NLRP3
inflammasome molecules and white blood cell (WBC) count in ND
patients with AML was additionally assessed. Significant positive
correlations between NLRP3 inflammasome molecule expression level
(NLRP3, ASC, IL-1β, and IL-18) and WBC count (r=0.296, P=0.005;
r=0.219, P=0.038; r=0.321, P=0.002; r=0.358 P=0.001; respectively;
Fig. 6C-F) were identified. AHR
expression level was also positively correlated with WBC count in
ND patients (r=0.257, P=0.015; Fig.
6G).
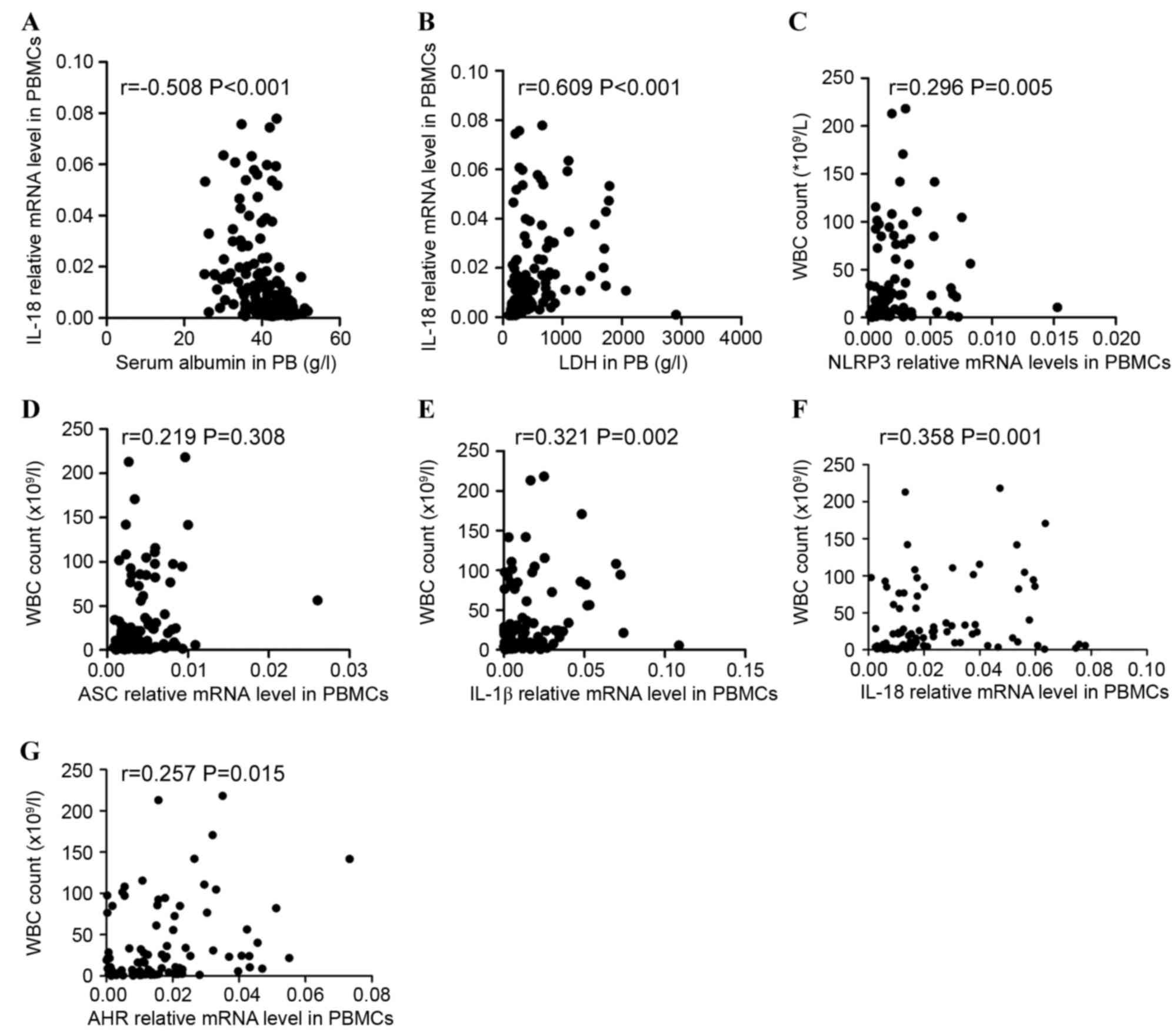 | Figure 6.Correlations between the relative
levels of NLRP3 inflammasome-associated molecules and clinical
characteristics. The expression of IL-18 was (A) negatively
correlated with the level of serum albumin and (B) positively
correlated with the serum level of LDH in ND and CR patients.
Significantly positive correlations between the relative mRNA
levels of the NLRP3 inflammasome molecules [(C) NLRP3, (D) ASC, (E)
IL-1β, (F) IL-18, (G) AHR] and WBC count were identified in the ND
group. NLRP3, NLR family pyrin domain-containing 3; IL,
interleukin; LDH, lactate dehydrogenase; ND, newly diagnosed; CR,
complete remission; ASC, apoptosis-associated speck-like protein;
AHR, aryl hydrocarbon receptor; WBC, white blood cell. |
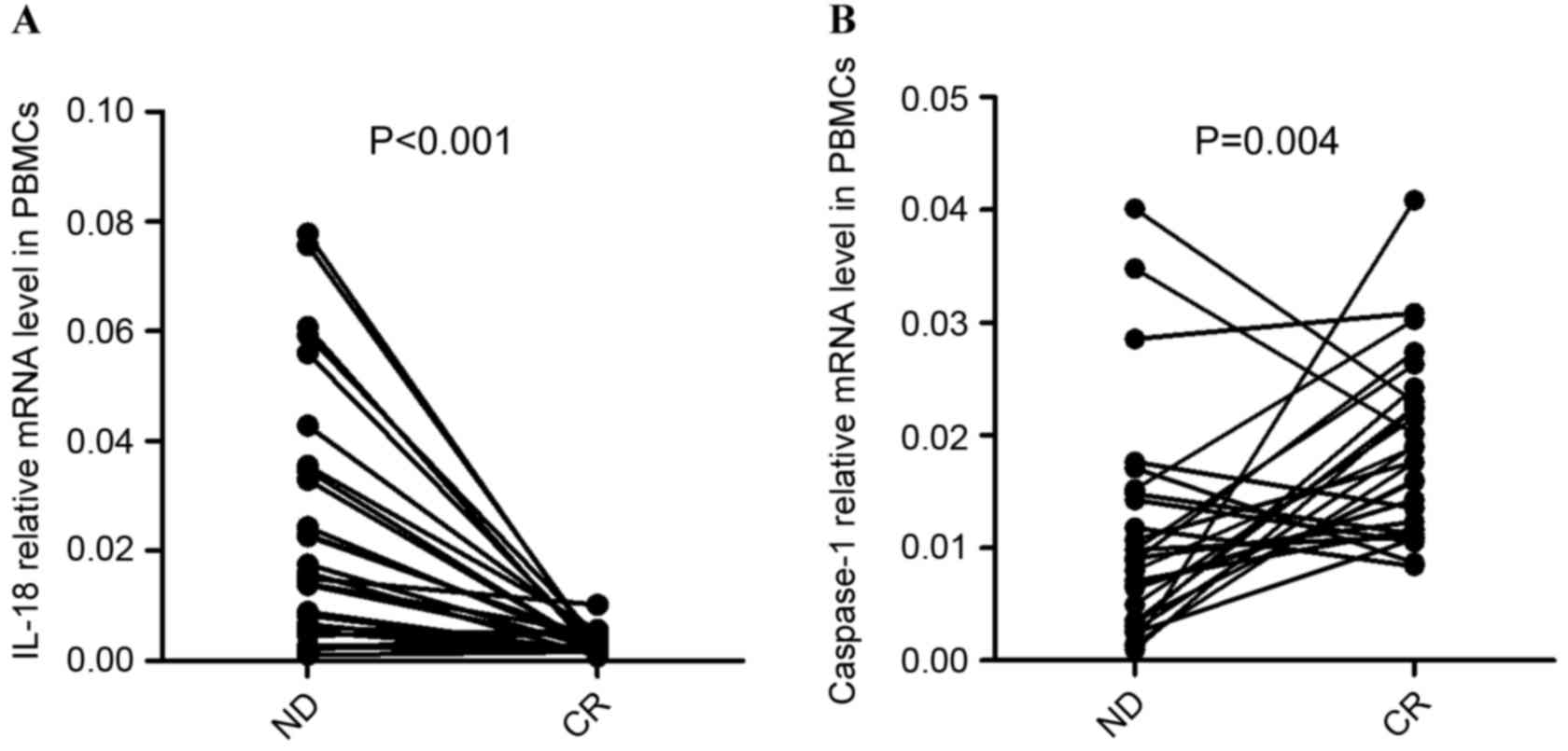
Chemotherapy recovered the aberrant
expression of IL-18 and caspase-1 in the PBMCs of patients with
AML
To further the understanding of the influence of
chemotherapy on the immune system in AML, the complete treatment
process of 28 patients with AML (PBMCs were obtained from 28/90
patients, including ND and CR patients) who obtained CR subsequent
to standard induction chemotherapy was observed. It was identified
that the expression of IL-18 in PBMCs was significantly decreased
once the patients achieved CR (P<0.001; Fig. 7A). In the majority of ND patients (out
of the 28 patients that PBMCs were obtained from), the expression
of Caspase-1 was observed to be elevated after achieving CR
(P=0.004; Fig. 7B).
Discussion
The importance of the immune system in AML is well
recognized. Previous studies have suggested that Th1 and Th22 cells
are involved in the development of AML (1,2). Although
the contribution of the NLRP3 inflammasome has been widely studied
in many diseases, the specific role of the NLRP3 inflammasome in
tumorigenesis is poorly understood. In the present study, it was
demonstrated that the expression levels of NLRP3 molecules were
significantly increased in BMMCs from ND patients compared with CR
patients and that this was accompanied with elevated AHR. Aberrant
levels of NLRP3 inflammasome molecules were also identified in
PBMCs from ND patients. The results suggested that in patients with
AML, the expression level of AHR was closely correlated with the
expression of proteins from the NLRP3 inflammasome in BMMCs and
PBMCs. Furthermore, abnormal proportions of Th subsets were
identified in patients with AML, which was consistent with a
previous study (1). Taken together,
the data demonstrated that aberrant NLRP3 inflammasome and AHR
expression were associated with the development of AML, and may
contribute to the imbalance of Th subsets. The results indicated
that an abnormal immune response was associated with the
pathogenesis of AML.
The NLRP3 inflammasome is involved in autoimmune and
inflammatory diseases, including ITP, multiple sclerosis, primary
glomerular diseases and rheumatoid arthritis (RA) (22). The NLRP3 inflammasome was also
previously demonstrated to serve an important role in metabolic
diseases, including type II diabetes, gout and coronary artery
disease (23). Currently,
accumulating evidence indicates that the NLRP3 inflammasome is
involved in the pathogenesis of tumors, the NLRP3 inflammasome
correlated with the generation of tumor by regulating immune
system; however, the effect of the NLRP3 inflammasome and
associated cytokines on tumor development remains unclear (24). Numerous studies showed that the NLRP3
inflammasome and its associated cytokines served a
tumor-suppressive role in the development of cancer, whereas other
findings have shown that NLRP3 inflammasome facilitated
tumorigenesis (25,26). In hepatocellular cancer (HCC), Wei
et al demonstrated that patients with low expression levels
of NLRP3 inflammasome components had a worse prognosis (27). Contradicting this, Terlizzi et
al argued that patients with cancer with increased serum
concentrations of IL-18 and IL-1β had a reduced disease-free
survival time (28). The present
study demonstrated that the NLRP3 inflammasome and associated
cytokines were aberrantly expressed in ND patients with AML, which
may be associated with the development of AML.
In the present study, the mRNA of the inflammasome
molecules NLRP3, ASC and IL-18 was significantly increased in the
BMMCs of ND patients compared with CR patients. Furthermore, in the
ND group, the expression levels of NLRP3 inflammasome molecules
were positively correlated with each other, whereas a decreased
extent of correlation was identified in the CR group. These data
suggest that the NLRP3 inflammasome plays a role in the pathology
of AML in the BM microenvironment. In PBMCs, aberrant NLRP3
inflammasome protein expression levels were also identified in ND
patients, but there was no significant difference in BMMCs. AML is
a disease originating from the BM; the clinical symptoms of AML
become apparent in the BM first, and after a period of time, are
demonstrated in the peripheral blood (14). This may explain the difference between
the results for BMMCs and PBMCs.
The result in PBMCs suggested that the expression of
the NLRP3 inflammasome molecules NLRP3, ASC and caspase-1 were
positively correlated with each other in ND and CR patients,
whereas in controls, only a positive correlation between NLRP3 and
ASC was identified. Additionally, the expression levels of the
effective cytokines IL-1β and IL-18 were observed to be positively
correlated with the levels of NLRP3 inflammasome molecules in ND
and CR patients with AML. However, in controls, no correlation
between NLRP3 inflammasome molecules and the effective cytokines
was identified.
AHR has been hypothesized to negatively regulate the
NLRP3 inflammasome by inhibiting the transcription of NLRP3
(17). The results of the present
study suggested that the relative AHR expression level differed
between BMMCs and PBMCs. In BMMCs, AHR was found to be markedly
elevated in ND patients when compared with CR patients.
Furthermore, the AHR expression level was positively correlated
with the level of NLRP3 inflammasome molecules in the BMMCs and
PBMCs of patients with AML. A balance must be maintained between
the activation and inhibition of the inflammasome to avoid
detrimental effects (17). The
abnormally elevated expression of AHR and NLRP3 may contribute to
the pathogenesis of AML.
AHR serves a critical role in the regulation of the
immune response, including in the innate and adaptive immune
responses (16). Emerging evidence
suggests that AHR expression level is correlated with the
differentiation of Th subsets (29).
Negishi et al demonstrated that AHR participated in the
modulation of the Th1/Th2 balance in vivo (30). Accumulating reports have also
demonstrated that AHR serves a pivotal role in the development of
autoimmune disorders, including inflammatory bowel diseases, RA and
systemic lupus erythematosus by impairing the balance of Th1, Th17
and regulatory T cells (Tregs) (31–33).
Moreover, emerging studies have demonstrated that the activation of
AHR aberrantly induced Th17/Tregs though prompting the generation
of Tregs and suppressing Th17 cells (34). Quintana et al (35) demonstrated that AHR modulated the
differentiation of Tregs and Th17 in a ligand-specific manner. AHR
activation by its ligand, 2,3,7,8-tetrachlorodibenzo-p-dioxin,
induces functional T(reg) cells, however, AHR activation by
6-formylindolo [3,2-b] carbazole promotes T(H)17 cell
differentiation and accelerates the severity of experimental
autoimmune encephalomyelitis in animal experiments (35). The result of the present study
revealed that AHR expression in BMMCs from ND patients with AML was
markedly increased compared with CR patients.
Previous studies have demonstrated that imbalanced
Th subsets were involved in the pathologies of hematological
malignancies (1,14). The present study has identified that
Th22 in PB was markedly increased in ND patients compared with CR
patients or controls. The frequency of Th1 cells was also reduced
in ND patients. Therefore, we hypothesize that the expression of
AHR in patients with AML may result in the aberrant Th subsets.
Tregs serve a key role in the maintenance of immune
homeostasis and are elevated in tumors (36,37). In a
previous study, Tregs were observed to be at an increased level in
an ND group compared with a CR group although the mechanism was
unclear (2). In a mouse model, it was
identified that DC-derived IL-18 promoted the differentiation of T
cells towards CD4+CD25+ Tregs (38).
The result of the present study demonstrated that IL-18 was
increased in ND patients with AML, which may facilitate the
polarization of Tregs. Tregs in patients with AML may have
suppressed the immune response and promoted the development of
AML.
A recent study associated the NLRP3 inflammasome
with Th differentiation. Peelen et al identified that
inflammasome activity promoted naive CD4+ T-cell differentiation
into pro-inflammatory subsets, particularly Th17 (39). Th22 is a newly identified Th subset
that is associated not only with the immune response, but also
inflammation. It was recently demonstrated that Th22 may also be
involved in the pathogenesis of a number of tumor types, including
HCC (40) and cervical cancer
(41). In the present study, it was
identified that the relative proportion of Th22 was significantly
higher in ND patients than in CR patients and controls.
Furthermore, AHR expression level was positively correlated with
NLRP3 inflammasome molecule expression level, and the associated
cytokines IL-1β and IL-18, in patients with AML. The results
suggest that aberrant NLRP3 inflammasome protein and AHR expression
may influence the differentiation of Th subsets in the development
of AML.
In conclusion, the results of the present study
suggested that the NLRP3 inflammasome, which was associated with
AHR, played a role in the pathogenesis of AML, contributing to the
imbalance of Th subset proportion. Based on this observation,
targeting the NLRP3 inflammasome may be considered as a novel
potential treatment option against AML. Further studies are awaited
in order to clarify the specific role and mechanism of the NLRP3
inflammasome in the immunopathology of AML.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81470319)
and the Natural Science Foundation of Shandong Province (grant no.
ZR2015PH060).
References
|
1
|
Yu S, Liu C, Zhang L, Shan B, Tian T, Hu
Y, Shao L, Sun Y, Ji C and Ma D: Elevated Th22 cells correlated
with Th17 cells in peripheral blood of patients with acute myeloid
leukemia. Int J Mol Sci. 15:1927–1945. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lucas CM, Wang L, Austin GM, Knight K,
Watmough SJ, Shwe KH, Dasgupta R, Butt NM, Galvani D, Hoyle CF, et
al: A population study of imatinib in chronic myeloid leukaemia
demonstrates lower efficacy than in clinical trials. Leukemia.
22:1963–1966. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neill DR, Wong SH, Bellosi A, Flynn RJ,
Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al:
Nuocytes represent a new innate effector leukocyte that mediates
type-2 immunity. Nature. 464:1367–1370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shao BZ, Xu ZQ, Han BZ, Su DF and Liu C:
NLRP3 inflammasome and its inhibitors: A review. Front Pharmacol.
6:2622015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sutterwala FS, Haasken S and Cassel SL:
Mechanism of NLRP3 inflammasome activation. Ann NY Acad Sci.
1319:82–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bauernfeind FG, Horvath G, Stutz A,
Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks
BG, Fitzgerald KA, et al: Cutting edge: NF-kappaB activating
pattern recognition and cytokine receptors license NLRP3
inflammasome activation by regulating NLRP3 expression. J Immunol.
183:787–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozaki E, Campbell M and Doyle SL:
Targeting the NLRP3 inflammasome in chronic inflammatory diseases:
Current perspectives. J Inflamm Res. 8:15–27. 2015.PubMed/NCBI
|
|
8
|
Zhang N, Pan HF and Ye DQ: Th22 in
inflammatory and autoimmune disease: Prospects for therapeutic
intervention. Mol Cell Biochem. 353:41–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng F, Guo Z, Xu H, Yan D and Li Q:
Decreased plasma IL22 levels, but not increased IL17 and IL23
levels, correlate with disease activity in patients with systemic
lupus erythematosus. Ann Rheum Dis. 68:604–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nickoloff BJ: Cracking the cytokine code
in psoriasis. Nat Med. 13:242–244. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brand S, Beigel F, Olszak T, Zitzmann K,
Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A,
et al: IL-22 is increased in active Crohn's disease and promotes
proinflammatory gene expression and intestinal epithelial cell
migration. Am J Physiol Gastrointest Liver Physiol. 290:G827–G838.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shao LL, Zhang L, Hou Y, Yu S, Liu XG,
Huang XY, Sun YX, Tian T, He N, Ma DX, et al: Th22 cells as well as
Th17 cells expand differentially in patients with early-stage and
late-stage myelodysplastic syndrome. PLoS One. 7:e513392012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu Y, Li H, Zhang L, Shan B, Xu X, Li H,
Liu X, Xu S, Yu S, Ma D, et al: Elevated profiles of Th22 cells and
correlations with Th17 cells in patients with immune
thrombocytopenia. Hum Immunol. 73:629–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian T, Sun Y, Li M, He N, Yuan C, Yu S,
Wang M, Ji C and Ma D: Increased Th22 cells as well as Th17 cells
in patients with adult T-cell acute lymphoblastic leukemia. Clin
Chim Acta. 426:108–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goergens A, Frericks M and Esser C: The
arylhydrocarbon receptor is only marginally involved in the
antileukemic effects of its ligand curcumin. Anticancer Res.
29:4657–4664. 2009.PubMed/NCBI
|
|
16
|
Zhu C, Xie Q and Zhao B: The role of AhR
in autoimmune regulation and its potential as a therapeutic target
against CD4 T cell mediated inflammatory disorder. Int J Mol Sci.
15:10116–10135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huai W, Zhao R, Song H, Zhao J, Zhang L,
Zhang L, Gao C, Han L and Zhao W: Aryl hydrocarbon receptor
negatively regulates NLRP3 inflammasome activity by inhibiting
NLRP3 transcription. Nat Commun. 5:47382014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gris D, Ye Z, Iocca HA, Wen H, Craven RR,
Gris P, Huang M, Schneider M, Miller SD and Ting JP: NLRP3 plays a
critical role in the development of experimental autoimmune
encephalomyelitis by mediating Th1 and Th17 responses. J Immunol.
185:974–981. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheson BD, Cassileth PA, Head DR, Schiffer
CA, Bennett JM, Bloomfield CD, Brunning R, Gale RP, Grever MR,
Keating MJ, et al: Report of the national cancer
institute-sponsored workshop on definitions of diagnosis and
response in acute myeloid leukemia. J Clin Oncol. 8:813–819. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vardiman JW: The Word Health Organization
(WHO) classification of tumors of the hematopoietic and lymphoid
tissues: An overview with emphasis on the myeloid neoplasms. Chem
Biol Interact. 184:16–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathews RJ, Robinson JI, Battellino M,
Wong C and Taylor JC: Biologics in Rheumatoid Arthritis Genetics
and Genomics Study Syndicate (BRAGGSS), Eyre S, Churchman SM,
Wilson AG, Isaacs JD, et al: Evidence of NLRP3-inflammasome
activation in rheumatoid arthritis (RA); genetic variants within
the NLRP3-inflammasome complex in relation to susceptibility to RA
and response to anti-TNF treatment. Ann Rheum Dis. 73:1202–1210.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Satoh M, Tabuchi T, Itoh T and Nakamura M:
NLRP3 inflammasome activation in coronary artery disease: Results
from prospective and randomized study of treatment with
atorvastatin or rosuvastatin. Clin Sci (Lond). 126:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karki R, Man SM and Kanneganti TD:
Inflammasomes and cancer. Cancer Immunol Res. 5:94–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Terlizzi M, Colarusso C, Popolo A, Pinto A
and Sorrentino R: IL-1α and IL-1β producing macrophages populate
lung tumor lesions in mice. Oncotarget. 7:58181–58192. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petrilli V: The multifaceted roles of
inflammasome proteins in cancer. Curr Opin Oncol. 29:35–40. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei Q, Mu K, Li T, Zhang Y, Yang Z, Jia X,
Zhao W, Huai W, Guo P and Han L: Deregulation of the NLRP3
inflammasome in hepatic parenchymal cells during liver cancer
progression. Lab Invest. 94:52–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Terlizzi M, Casolaro V, Pinto A and
Sorrentino R: Inflammasome: Cancer's friend or foe? Pharmacol Ther.
143:24–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Busbee PB, Rouse M, Nagarkatti M and
Nagarkatti PS: Use of natural AhR ligands as potential therapeutic
modalities against inflammatory disorders. Nutr Rev. 71:353–369.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Negishi T, Kato Y, Ooneda O, Mimura J,
Takada T, Mochizuki H, Yamamoto M, Fujii-Kuriyama Y and Furusako S:
Effects of aryl hydrocarbon receptor signaling on the modulation of
TH1/TH2 balance. J Immunol. 175:7348–7356. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Arsenescu R, Arsenescu V, Zhong J, Nasser
M, Melinte R, Dingle RW, Swanson H and de Villiers WJ: Role of the
xenobiotic receptor in inflammatory bowel disease. Inflamm Bowel
Dis. 17:1149–1162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kobayashi S, Okamoto H, Iwamoto T, Toyama
Y, Tomatsu T, Yamanaka H and Momohara S: A role for the aryl
hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis.
Rheumatology (Oxford). 47:1317–1322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang J, Yang X, Zou H, Chu Y and Li M:
Recovery of the immune balance between Th17 and regulatory T cells
as a treatment for systemic lupus erythematosus. Rheumatology
(Oxford). 50:1366–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hanieh H: Toward understanding the role of
aryl hydrocarbon receptor in the immune system: Current progress
and future trends. Biomed Res Int. 2014:5207632014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quintana FJ, Basso AS, Iglesias AH, Korn
T, Farez MF, Bettelli E, Caccamo M, Oukka M and Weiner HL: Control
of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon
receptor. Nature. 453:65–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wing JB and Sakaguchi S: Foxp3+
T(reg) cells in humoral immunity. Int Immunol. 26:61–69. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nishikawa H and Sakaguchi S: Regulatory T
cells in cancer immunotherapy. Curr Opin Immunol. 27:1–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Oertli M, Sundquist M, Hitzler I, Engler
DB, Arnold IC, Reuter S, Maxeiner J, Hansson M, Taube C,
Quiding-Järbrink M and Müller A: DC-derived IL-18 drives Treg
differentiation, murine Helicobacter pylori-specific immune
tolerance, and asthma protection. J Clin Invest. 122:1082–1096.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peelen E, Damoiseaux J, Muris AH,
Knippenberg S, Smolders J, Hupperts R and Thewissen M: Increased
inflammasome related gene expression profile in PBMC may facilitate
T helper 17 cell induction in multiple sclerosis. Mol Immunol.
63:521–529. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qin S, Ma S, Huang X, Lu D, Zhou Y and
Jiang H: Th22 cells are associated with hepatocellular carcinoma
development and progression. Chin J Cancer Res. 26:135–141.
2014.PubMed/NCBI
|
|
41
|
Zhang W, Tian X, Mumtahana F, Jiao J,
Zhang T, Croce KD, Ma D, Kong B and Cui B: The existence of Th22,
pure Th17 and Th1 cells in CIN and cervical cancer along with their
frequency variation in different stages of cervical cancer. BMC
Cancer. 15:7172015. View Article : Google Scholar : PubMed/NCBI
|