Introduction
Gastric cancer (GC) is one of the most common types
of tumor globally. In 2015, GC had the second highest incidence and
rate of mortality among cancer patients in China (1). Due to the rapid progression of GC and
the absence of early specific symptoms and signs, the majority of
patients are diagnosed at the late stage of gastric carcinoma
(2–5).
At present, surgery is the preferred treatment for patients without
distant metastasis, and postoperative metastasis and recurrence are
the predominant causes of mortality (3,5). Although
disease-free and overall survival (OS) may be improved by
postoperative chemotherapy and radiotherapy, the prognosis of GC
remains poor (3,6). Identifying simple but effective
prognostic markers for GC remains an important aim for
researchers.
Inflammation occurs in various types of tumor, and
serves a crucial function in tumor development and distant
metastasis (7–10). Previous studies have indicated that
inflammation is associated with the formation of the tumor
microenvironment by inflammatory mediators, including vasoactive
amines and cytokines (11–13). The C-reactive protein (CRP)/albumin
(Alb) ratio is used as a novel inflammation-based prognostic
indicator in multiple types of tumor. Previous studies have
demonstrated the prognostic value of CRP/Alb ratio in colorectal
(14) and esophageal cancer (15), hepatocellular carcinoma (16), and renal (17) and pancreatic cancer (18). Another important inflammatory marker
is the neutrophil/lymphocyte ratio (NLR). NLR represents the
balance between inflammatory activation and regulatory factors
(19). Increased NLR may also serve
as an independent prognostic risk factor in multiple types of
cancer, including lung (20) and
breast cancer (21), renal cell
carcinoma (22), and ovarian cancer
(23). The present study evaluated
the individual and combined prognostic value of CRP/Alb and NLR in
Chinese patients with GC.
Patients and methods
Patients
The present study retrospectively reviewed the
clinical data of 395 patients pathologically diagnosed with GC from
Sun Yat-sen University Cancer Center between January 2010 and
December 2010 (Guangzhou, China). The patients were classified and
staged based on the American Joint Committee on Cancer
tumor-node-metastasis (TNM) staging system (24). Patients without pathological diagnosis
and laboratory test information on CRP, Alb, neutrophils and
lymphocytes were excluded from analysis. The present study also
excluded patients with other tumors, autoimmune diseases and those
lost to follow-up. In total, 337 of the 395 patients reviewed were
included for analysis in the present study. Of these, 237 (70.3%)
were male and 100 (29.7%) were female (median age, 59; age range,
19–89). The information on CRP, Alb, neutrophils and lymphocytes
was obtained from the test report from the Department of Clinical
Laboratory at the Sun Yat-sen University Cancer Center. Other
clinicopathological characteristics were collected through medical
records.
Ethics statement
All patients provided written informed consent for
the information to be used in the database of Sun Yat-sen
University Cancer Center. The Ethics Committee at the Sun Yat-sen
University Cancer Center approved the present study. The present
study was conducted in accordance with the ethical standards of the
World Medical Association Declaration of Helsinki.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The significance of association between the CRP/Alb ratio or NLR,
and the clinicopathological characteristics were assessed using the
χ2 test. OS was defined as the time interval between
diagnosis and mortality due to GC or the last follow-up. The
optimal cut-off points for continuous prognostic indices were
determined using the method reported by Budczies et al
(25). Survival curves were
constructed using the Kaplan-Meier method. Differences in survival
were assessed using the log-rank test. The Cox proportional hazards
regression model was used for univariate and multivariate analysis.
The significant prognostic factors identified in univariate
analysis were selected for multivariate analysis. All statistical
analyses were performed using SPSS 13.0 (SPSS, Inc., Chicago, IL,
USA). A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 337 patients were included for analysis
in the present study; however, some of the patients' data of tumor
size, location and differentiation level were lost. Of the
available data, 42 (12.5%), 49 (14.5%), 142 (42.1%) and 104 (30.9%)
were staged I, II, III and IV, respectively. In addition, 89
(34.1%) patients had tumors <4 cm, and 172 (65.9%) had primary
tumors ≥4 cm. The present study identified that 185 (55.2%) and 104
(31.1%) patients with tumors located in the proximal and distal
stomach, respectively, while 46 (13.7%) exhibited tumors in other
locations, including gastric stump cancer and linitis plastica. A
total of 230 (70.1%) patients exhibited poorly or not
differentiated tumors, 96 (29.3%) exhibited moderately
differentiated tumors, and only 2 (0.6%) patients exhibited
well-differentiated tumors.
Association between CRP/Alb ratio, NLR
and clinicopathological characteristics
The association between CRP/Alb ratio and NLR, and
clinicopathological characteristics was assessed (Table I). The CRP/Alb ratio ranged from
0.003–4.77 (median, 0.23), and NLR ranged from 0.47–23.00 (median,
2.95). For OS, the optimal cut-off values of the CRP/Alb ratio and
NLR were 0.38 and 3.41, respectively. The present study revealed
that high CRP/Alb ratio and NLR values were associated with
increased tumor invasion (CRP/Alb, P=0.006; NLR, P=0.005),
increased distant metastasis (CRP/Alb, P<0.001; NLR, P<0.001)
and a more advanced TNM stage (CRP/Alb, P<0.001; NLR,
P<0.001). In addition, a high NLR value was associated with
increased tumor size (P=0.02).
 | Table I.Association between the CRP/ALB ratio
and NLR with clinicopathological characteristics in patients with
gastric cancer. |
Table I.
Association between the CRP/ALB ratio
and NLR with clinicopathological characteristics in patients with
gastric cancer.
|
| CRP/ALB ratio | NLR |
|---|
|
|
|
|
|---|
| Characteristic | <0.3778 n,
(%) | ≥0.3778 n, (%) | P-value | <3.41 n,
(%) | ≥3.41 n, (%) | P-value |
|---|
| Sex |
|
| 0.280 |
|
| 0.130 |
|
Male | 195 (69.1) | 42 (76.4) |
| 176 (68.2) | 61 (77.2) |
|
|
Female | 87 (30.9) | 13 (23.6) |
| 82 (31.8) | 18 (22.8) |
|
| Age, years |
|
| 0.290 |
|
| 0.180 |
|
≤59 | 140 (49.6) | 23 (41.8) |
| 130 (50.4) | 33 (41.8) |
|
|
>59 | 142 (50.4) | 32 (58.2) |
| 128 (49.6) | 46 (58.2) |
|
| TNM
stageb |
|
|
<0.001a |
|
|
<0.001a |
| I | 40 (14.2) | 2 (3.6) |
| 40 (15.5) | 2 (2.5) |
|
| II | 45 (16.0) | 4 (7.3) |
| 42 (16.3) | 7 (8.9) |
|
|
III | 126 (44.7) | 16 (29.1) |
| 117 (45.3) | 25 (31.6) |
|
| IV | 71 (25.2) | 33 (60.0) |
| 59 (22.9) | 45 (57.0) |
|
| N
stageb |
|
| 0.600 |
|
| 0.180 |
| N0 | 72 (30.1) | 6 (25.0) |
| 70 (31.3) | 8 (20.5) |
|
|
N1+N2+N3 | 167 (69.9) | 18 (75.0) |
| 154 (68.8) | 31 (79.5) |
|
| T
stageb |
|
| 0.006a |
|
| 0.005a |
|
T1+T2+T3 | 80 (32.8) | 2 (7.4) |
| 77 (33.6) | 5 (11.9) |
|
| T4 | 164 (67.2) | 25 (92.6) |
| 152 (66.4) | 37 (88.1) |
|
| M
stageb |
|
|
<0.001a |
|
|
<0.001a |
| M0 | 211 (74.8) | 22 (40.0) |
| 199 (77.1) | 34 (43.0) |
|
| M1 | 71 (25.2) | 33 (60.0) |
| 59 (22.4) | 45 (57.0) |
|
| Primary tumor size,
cm |
|
| 0.320 |
|
| 0.021a |
|
<4.0 | 83 (35.0) | 6 (25.0) |
| 82 (36.9) | 7 (17.9) |
|
|
≥4.0 | 154 (65.0) | 18 (75.0) |
| 140 (63.1) | 32 (82.1) |
|
| Tumor location |
|
| 0.230 |
|
| 0.070 |
|
Proximal | 160 (57.1) | 25 (45.5) |
| 146 (56.8) | 39 (50.0) |
|
|
Remote | 84 (30.0) | 20 (36.4) |
| 82 (31.9) | 22 (28.2) |
|
|
Other | 36 (12.9) | 10 (18.2) |
| 29 (11.3) | 17 (21.8) |
|
| Degree of
differentiation |
|
| 0.590 |
|
| 0.390 |
|
Poorly/not differentiated | 195 (70.9) | 35 (66.0) |
| 177 (69.4) | 53 (72.6) |
|
|
Moderately differentiated | 78 (28.4) | 18 (34.0) |
| 77 (30.2) | 19 (26.0) |
|
|
Well-differentiated | 2 (0.7) | 0 (0.0) |
| 1 (0.4) | 1 (1.4) |
|
Prognostic value of the CRP/Alb ratio,
NLR and OS
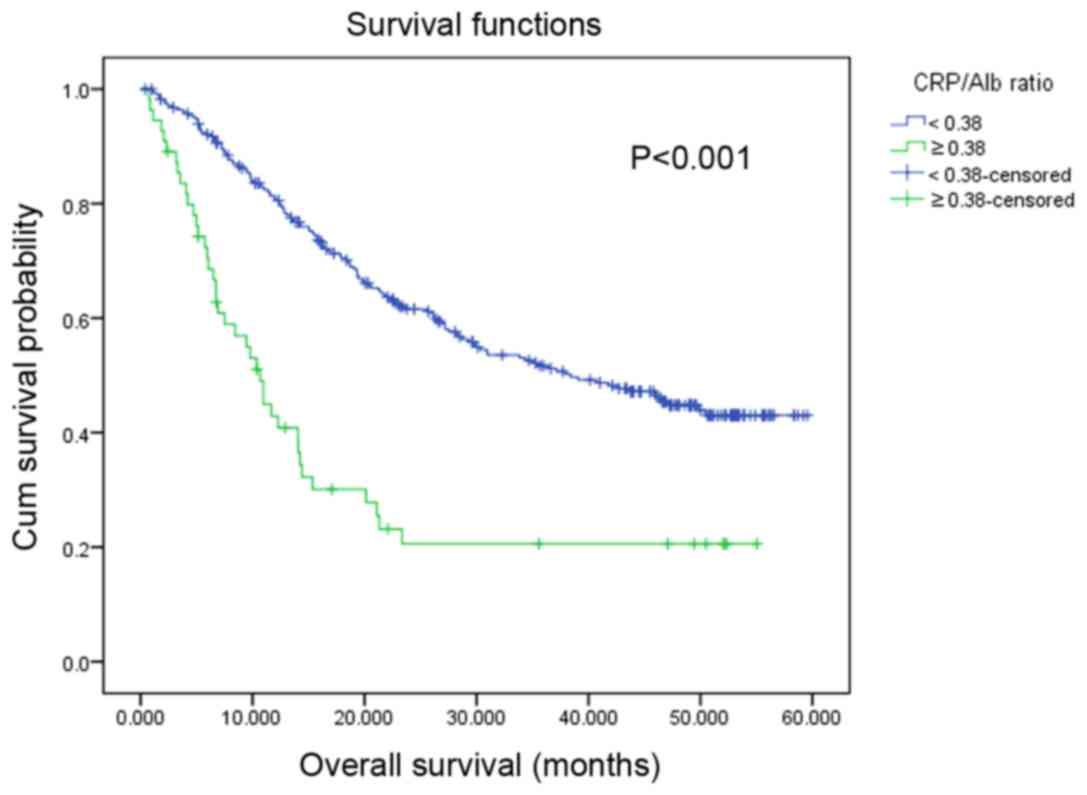
The median survival time for the patients was 29.77
months (range, 0.43–59.57 months). Survival analysis revealed that
patients with a high CRP/Alb ratio were associated with a
significantly decreased OS rate compared with those with a
decreased CRP/Alb ratio (P<0.001; Fig.
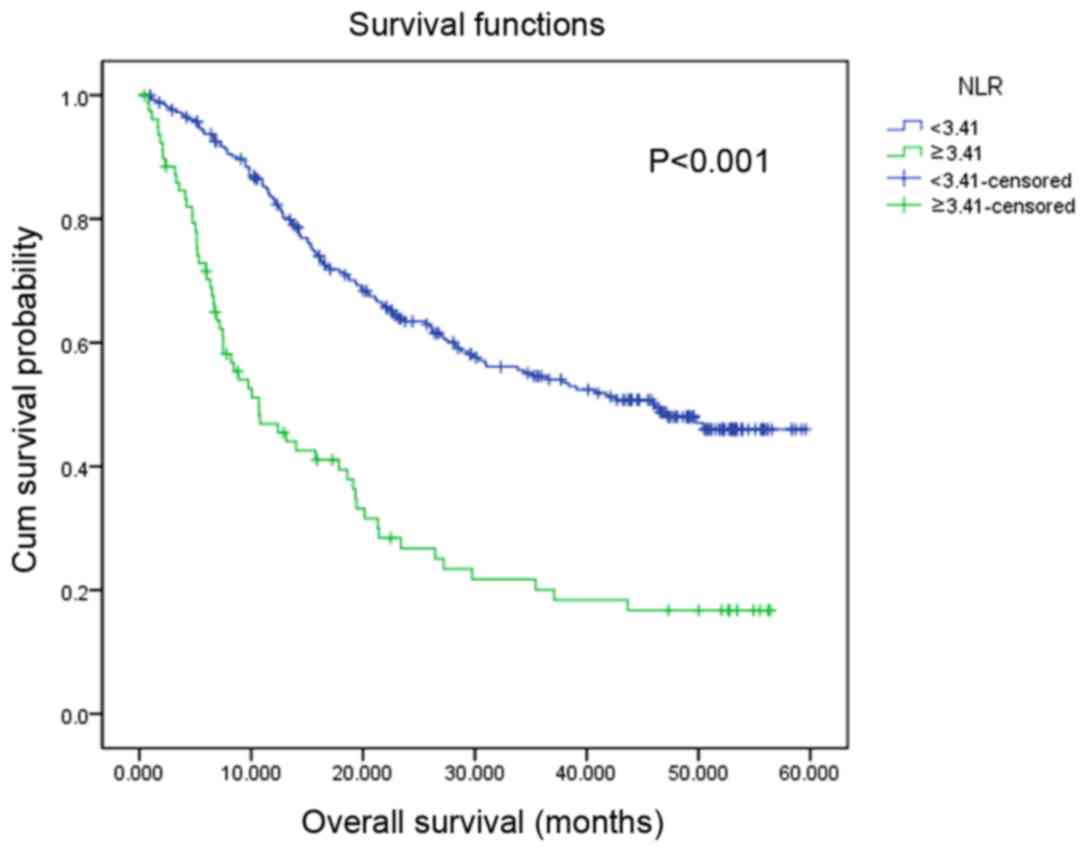
1). Similarly, patients with a high NLR value were associated
with a significantly decreased OS rate compared with those with a
low NLR (P<0.001; Fig. 2). In
univariate analysis, age (P=0.005), tumor location (P<0.001),
TNM stage (P<0.001), distant metastasis (P<0.001), surgery
(P<0.001), CRP/Alb ratio (P<0.001) and NLR (P<0.001) were
revealed to be significantly associated with OS. Multivariate
analysis identified age (P<0.001), TNM stage (P<0.001),
surgery (P=0.002), CRP/Alb ratio (P=0.005) and NLR (P=0.001) as
independent prognostic factors (Table
II).
 | Table II.Prognostic value of the CRP/ALB ratio
and NLR for overall survival in patients with gastric cancer by
univariate and multivariate analysis. |
Table II.
Prognostic value of the CRP/ALB ratio
and NLR for overall survival in patients with gastric cancer by
univariate and multivariate analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | N, (%) | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Sex |
| 0.500 |
|
|
|
|
Male | 237 (70.3) |
|
|
|
|
|
Female | 100 (29.7) |
|
|
|
|
| Age, years |
| 0.005a |
|
|
<0.001a |
|
≤59 | 163 (48.4) |
| 1.00 | Reference |
|
|
>59 | 174 (51.6) |
| 1.92 | 1.39–2.65 |
|
| Tumor location |
|
<0.001a |
|
|
|
|
Proximal | 185 (55.2) |
|
|
|
|
|
Remote | 104 (31.0) |
|
|
|
|
|
Other | 46 (13.7) |
|
|
|
|
| Degree of
differentiation |
| 0.060 |
|
|
|
| Poorly
or not differentiated | 230 (70.1) |
|
|
|
|
|
Moderately differentiated | 96 (29.3) |
|
|
|
|
|
Well-differentiated | 2 (0.6) |
|
|
|
|
| TNM
stageb |
|
<0.001a |
|
|
<0.001a |
| I | 42 (12.5) |
| 1.00 | Reference |
|
| II | 49 (14.5) |
| 1.43 | 0.55–3.69 | 0.470 |
|
III | 142 (42.1) |
| 4.24 | 1.95–9.22 |
<0.001a |
| IV | 104 (30.9) |
| 8.17 | 3.53–18.91 |
<0.001a |
| Distant
metastasis |
|
<0.001a |
|
|
|
| No | 233 (69.1) |
|
|
|
|
|
Yes | 104 (30.9) |
|
|
|
|
| Surgery |
|
<0.001a |
|
| 0.002a |
| No | 74 (22.0) |
| 1.00 | Reference |
|
|
Yes | 263 (78.0) |
| 0.46 | 0.28–0.75 |
|
| CRP/ALB ratio |
|
<0.001a |
|
| 0.005a |
|
<0.3778 | 282 (83.7) |
| 1.00 | Reference |
|
|
≥0.3778 | 55 (16.3) |
| 1.78 | 1.20–2.65 |
|
| NLR |
|
<0.001a |
|
| 0.001a |
|
<3.41 | 258 (76.6) |
| 1.00 | Reference |
|
|
≥3.41 | 79 (23.4) |
| 1.74 | 1.25–2.44 |
|
Prognostic value of the CRP/Alb ratio
and NLR classification
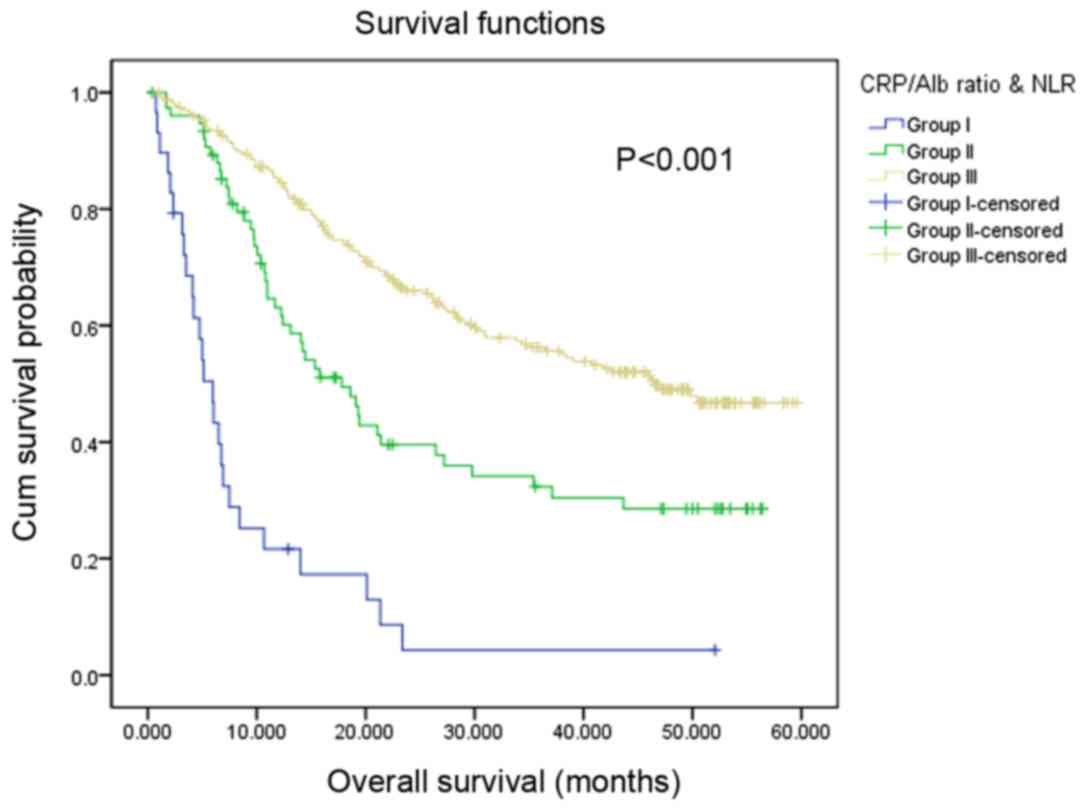
According to the optimal cut-off values of the
CRP/Alb ratio (<0.38/≥0.38) and NLR (<3.41/≥3.41), the
present study classified the patients into subgroups: Group I,
patients with increased CRP/Alb ratio (≥0.38) and NLR (≥3.14);
group III, patients with decreased CRP/Alb ratio (<0.38) and NLR
(<3.14); and group II, all remaining patients. Among the 337
patients, 29 (8.60%) patients were located in group I, 76 (22.55%)
patients were located in group II and 232 (68.84%) patients were
located in group III. The median OS values for patients in group I,
II and III were 5.10, 17.80, and 46.50 months, respectively. Using
univariate analysis, the present study identified that patients in
group I exhibited the lowest OS of the subgroups (P<0.001).
Patients in group II [hazard ratio (HR), 95%; confidence interval
(CI), 0.33 (0.19–0.57); P<0.001] and group III [HR, 95%; CI,
0.25 (0.15–0.42); P<0.001] exhibited significantly increased OS
compared with patients in group I (Fig.
3; Table III). Univariate
analysis revealed that age (P=0.005), tumor location (P<0.001),
TNM stage (P<0.001), distant metastasis (P<0.001), surgery
(P<0.001), and CRP/Alb ratio and NLR classification (P<0.001)
were significant prognostic factors. Multivariate analysis also
demonstrated that these parameters, with the exception of tumor
location, were significant prognostic factors. Notably, the CRP/Alb
ratio and NLR classification (P<0.001) was identified as an
independent prognostic factor in univariate and multivariate
analyses (Table III).
 | Table III.Prognostic value of the CRP/ALB ratio
and NLR for overall survival in patients with gastric cancer by
univariate and multivariate analysis. |
Table III.
Prognostic value of the CRP/ALB ratio
and NLR for overall survival in patients with gastric cancer by
univariate and multivariate analysis.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Characteristic | N, (%) | P-value | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Sex |
| 0.500 |
|
|
|
|
Male | 237 (70.3) |
|
|
|
|
|
Female | 100 (29.3) |
|
|
|
|
| Age, years |
| 0.005a |
|
| <0.001 |
|
≤59 | 163 (48.4) |
| 1.00 | Reference |
|
|
>59 | 174 (51.6) |
| 2.01 | 1.45–2.78 |
|
| Tumor location |
|
<0.001a |
|
|
|
|
Proximal | 185 (55.2) |
|
|
|
|
|
Remote | 104 (31.0) |
|
|
|
|
|
Other | 46 (13.7) |
|
|
|
|
| Degree of
differentiation |
| 0.060 |
|
|
|
| Poorly
or not differentiated | 230 (70.1) |
|
|
|
|
|
Moderately differentiated | 96 (29.3) |
|
|
|
|
|
Well-differentiated | 2 (0.6) |
|
|
|
|
| TNM
stageb |
|
<0.001a |
|
|
<0.001a |
| I | 42 (12.5) |
| 1.00 | Reference |
|
| II | 49 (14.5) |
| 1.40 | 0.54–3.61 | 0.500 |
|
III | 142 (42.1) |
| 4.38 | 2.01–9.51 |
<0.001a |
| IV | 104 (30.9) |
| 8.50 | 3.68–19.63 |
<0.001a |
| Distant
metastasis |
|
<0.001a |
|
|
|
| No | 233 (69.1) |
|
|
|
|
|
Yes | 104 (30.9) |
|
|
|
|
| Surgery |
|
<0.001a |
|
|
<0.001a |
| No | 74 (22.0) |
| 1.00 | Reference |
|
|
Yes | 263 (78.0) |
| 0.49 | 0.30–0.80 |
|
| CRP/ALB ratio and
NLR classification |
|
<0.001a |
|
|
<0.001a |
| Group
I | 29 (8.6) |
| 1.00 | Reference |
|
| Group
II | 76 (22.6) |
| 0.33 | 0.19–0.57 |
<0.001a |
| Group
III | 232 (68.8) |
| 0.25 | 0.15–0.42 |
<0.001a |
Discussion
GC is a common malignant tumor of the upper
digestive tract. In 2015, GC exhibited the second highest incidence
and mortality rate among various types of cancer in China (1). In previous studies, plasma fibrinogen
(26), α-fetoprotein (27), carcinoembryonic antigen-related cell
adhesion molecule 5 (28) and the
platelet/lymphocyte ratio (29) were
demonstrated to be prognostic indicators for GC with the same TNM
stage. The levels of CRP, Alb, neutrophils and lymphocytes are all
routinely tested in clinical practice, which means that data on
these parameters are readily available. The present study revealed
that CRP/Alb ratio and NLR served as independent prognostic factors
for OS in patients with GC. The combination of these indexes was
associated with significant prognostic value and may further
stratify prognosis.
The association between inflammation and cancer is
complex. During the inflammatory response, cytokines [e.g.,
interleukin (IL)-6] and chemokines (e.g., C-C motif chemokine
ligand 2 and C-X-C motif chemokine ligand 8) are produced, which
may activate signal transducer and activator of transcription 3 and
nuclear factor κB, and recruit an increased number of inflammatory
cells, including neutrophilic granulocytes and mononuclear
macrophages, to the lesion site and thereby assist in forming an
inflammatory microenvironment (13,30,31). The
interaction between tumor cells and inflammatory factors may
suppress the immunosurveillance of T and natural killer cells
(32), enhance the permeability of
blood and lymphatic vessels and degrade the extracellular matrix,
thereby potentiating tumor development and metastasis (12,33).
Furthermore, tumor cells may secrete inflammatory mediators and
thereby assist in forming an inflammatory microenvironment
(34).
CRP and Alb are produced by liver cells. CRP is an
acute phase protein regulated by IL-6, tumor necrosis factor and
other inflammatory factors (35).
Therefore, CRP may indicate inflammation. Previous studies have
demonstrated that an increased level of CRP may be associated with
poorer prognosis in patients with tumors, including ovarian cancer,
penile cancer and non-small cell lung cancer (36–38). In
addition, malabsorption and malnutrition may be associated with
decreased survival time in various tumors, including esophageal
squamous cell carcinomas and endometrial cancer (39,40). In a
previous study, Alb was used to estimate nutritional status. For
patients with GC, protein digestion and absorption were decreased,
whereas protein metabolism was increased, resulting in a negative
nitrogen balance (18,39). The CRP/Alb ratio is a useful
prognostic indicator that was initially used to identify patients
with serious conditions on an acute medical ward by Fairclough
et al (41). Subsequently,
multiple studies have demonstrated that the CRP/Alb ratio exhibited
prognostic value in numerous types of cancer (15–18,42). The
present study revealed that a high CRP/Alb ratio indicated
increased tumor invasion and metastasis and poorer prognosis
compared with a low CRP/Alb ratio.
NLR serves as an index in routine blood tests.
Multiple studies have confirmed that NLR is an easily available and
reliable marker, which is associated with poor prognosis in various
malignancies (19–23). The present study revealed that NLR was
significantly associated with TNM stage, tumor invasion, tumor
size, distant metastasis and poor prognosis, which was consistent
with a previous study (43). The
balance between the inflammatory and immune response is also
reflected by NLR. Neutrophils contribute to inflammation by
activating pro-angiogenic factors, including vascular endothelial
growth factor or inflammatory cytokines (e.g., IL-1β) (44,45). In
addition, these neutrophils may also secrete agents that promote
tumor cellular proliferation, angiogenesis, invasion and metastasis
(46), and they may inhibit T cells
(47). As important components of the
non-specific and adaptive immune response, lymphocytes are able to
destroy tumor cells via cytotoxic cells and cytokine secretion
(48). A decreased lymphocyte count
results in reduced tumor resistance (49). These mechanisms support the results of
the present study.
The multivariate analysis of the present study
demonstrated that the CRP/Alb ratio and NLR exhibited significant
prognostic value in GC. In the present study, the patients were
classified according to CRP/Alb ratio (<0.38/≥0.38) and NLR
(<3.41/v3.41). In groups I, II, and III, the median OS was 5.10,
17.80 and 46.50 months, respectively. The survival difference among
the subgroups may be due to the CRP/Alb ratio and NLR, which
reflect a combination of inflammation, immune and nutrition status
caused by tumor progression Therefore, the combination of the
CRP/Alb ratio and NLR was associated with significant prognostic
value in GC.
Although the prognostic value of the CRP/Alb ratio
and NLR in GC has been reported previously (42,50), to
the best of our knowledge, the present study is the first to report
that the that the combination of the CRP/Alb ratio and NLR is
associated with significant prognostic value and may further
stratify prognosis compared with using single indicators.
However, the present study has a number of
limitations. The present study was a retrospective analysis, and
the conclusions require confirmation by perspective studies.
Secondly, the present study did not use a uniform cut-off value for
the CRP/Alb ratio and NLR. Therefore, different statistical methods
may obtain different thresholds. However, the method used by the
present study was based on R, which has been reliably used by other
studies (15,51,52). To
conclude, the present study revealed that the combination of the
CRP/Alb ratio and NLR represents a potential inflammation-based
prognostic marker. This marker was associated with invasive
clinicopathological characteristics and was able to predict
prognosis in patients with GC. Larger, prospective studies are
required to evaluate the results of the present study in the
context of other types of malignancy.
Acknowledgements
The authors would like to thank the staff of the
Biochemical Laboratory of the Department of Clinical Laboratory of
Sun Yat-sen University Cancer Center for providing biochemical
markers.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
OS
|
overall survival
|
|
TNM
|
tumor-node-metastasis
|
|
CRP/Alb ratio
|
C-reactive protein/albumin ratio
|
|
NLR
|
neutrophil/lymphocyte ratio
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jass JR, Sobin LH and Watanabe H: The
World Health Organization's histologic classification of
gastrointestinal tumors. A commentary on the second edition.
Cancer. 66:2162–2167. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Waddell T, Verheij M, Allum W, Cunningham
D, Cervantes A and Arnold D: European Society for Medical Oncology
(ESMO); European Society of Surgical Oncology (ESSO); European
Society of Radiotherapy and Oncology (ESTRO): Gastric cancer:
ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis,
treatment and follow-up. Eur J Surg Oncol. 40:584–591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tomasello G, Ghidini M, Liguigli W, Ratti
M, Toppo L and Passalacqua R: Targeted therapies in gastric cancer
treatment: Where we are and where we are going. Invest New Drugs.
34:378–393. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roukos DH: Current status and future
perspectives in gastric cancer management. Cancer Treat Rev.
26:243–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demaria S, Pikarsky E, Karin M, Coussens
LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo
E, et al: Cancer and inflammation: Promise for biologic therapy. J
Immunother. 33:335–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lou Y, Diao L, Cuentas ER, Denning WL,
Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens
C, et al: Epithelial-mesenchymal transition is associated with a
distinct tumor microenvironment including elevation of inflammatory
signals and multiple immune checkpoints in lung adenocarcinoma.
Clin Cancer Res. 22:3630–3642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Markowitz GJ, Michelotti GA, Diehl AM and
Wang XF: Inflammatory models drastically alter tumor growth and the
immune microenvironment in hepatocellular carcinoma. Sci Bull
(Beijing). 60:762–772. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanguinetti A, Santini D, Bonafè M,
Taffurelli M and Avenia N: Interleukin-6 and pro inflammatory
status in the breast tumor microenvironment. World J Surg Oncol.
13:1292015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishizuka M, Nagata H, Takagi K, Iwasaki Y,
Shibuya N and Kubota K: Clinical significance of the C-reactive
protein to albumin ratio for survival after surgery for colorectal
cancer. Ann Surg Oncol. 23:900–907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei XL, Wang FH, Zhang DS, Qiu MZ, Ren C,
Jin Y, Zhou YX, Wang DS, He MM, Bai L, et al: A novel
inflammation-based prognostic score in esophageal squamous cell
carcinoma: The C-reactive protein/albumin ratio. BMC Cancer.
15:3502015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kinoshita A, Onoda H, Imai N, Iwaku A,
Oishi M, Tanaka K, Fushiya N, Koike K, Nishino H and Matsushima M:
The C-reactive protein/albumin ratio, a novel inflammation-based
prognostic score, predicts outcomes in patients with hepatocellular
carcinoma. Ann Surg Oncol. 22:803–810. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Shao Y, Fan M, Zhuang Q, Wang K,
Cao W, Xu X and He X: Prognostic significance of preoperative
C-reactive protein: Albumin ratio in patients with clear cell renal
cell carcinoma. Int J Clin Exp Pathol. 8:14893–14900.
2015.PubMed/NCBI
|
|
18
|
Haruki K, Shiba H, Shirai Y, Horiuchi T,
Iwase R, Fujiwara Y, Furukawa K, Misawa T and Yanaga K: The
C-reactive protein to albumin ratio predicts long-term outcomes in
patients with pancreatic cancer after pancreatic resection. World J
Surg. 40:2254–2260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HS and Ku JH: Systemic inflammatory
response based on neutrophil-to-lymphocyte ratio as a prognostic
marker in bladder cancer. Dis Markers. 2016:83452862016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu G, Yao Y, Bai C, Zeng J, Shi D, Gu X,
Shi X and Song Y: Combination of platelet to lymphocyte ratio and
neutrophil to lymphocyte ratio is a useful prognostic factor in
advanced non-small cell lung cancer patients. Thorac Cancer.
6:275–287. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pistelli M, De Lisa M, Ballatore Z,
Caramanti M, Pagliacci A, Battelli N, Ridolfi F, Santoni M,
Maccaroni E, Bracci R, et al: Pre-treatment neutrophil to
lymphocyte ratio may be a useful tool in predicting survival in
early triple negative breast cancer patients. BMC Cancer.
15:1952015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bazzi WM, Tin AL, Sjoberg DD, Bernstein M
and Russo P: The prognostic utility of preoperative
neutrophil-to-lymphocyte ratio in localized clear cell renal cell
carcinoma. Can J Urol. 23:8151–8154. 2016.PubMed/NCBI
|
|
23
|
Williams KA, Labidi-Galy SI, Terry KL,
Vitonis AF, Welch WR, Goodman A and Cramer DW: Prognostic
significance and predictors of the neutrophil-to-lymphocyte ratio
in ovarian cancer. Gynecol Oncol. 132:542–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mao MJ, Wei XL, Sheng H, Wang XP, Li XH,
Liu YJ, Xing S, Huang Q, Dai SQ and Liu WL: Clinical significance
of preoperative albumin and globulin ratio in patients with gastric
cancer undergoing treatment. Biomed Res Int. 2017:30832672017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff Finder: A
comprehensive and straightforward Web application enabling rapid
biomarker cutoff optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamamoto M, Kurokawa Y, Miyazaki Y, Makino
T, Takahashi T, Yamasaki M, Nakajima K, Takiguchi S, Mori M and
Doki Y: Usefulness of preoperative plasma fibrinogen versus other
prognostic markers for predicting gastric cancer recurrence. World
J Surg. 40:1904–1909. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Qu H, Jian M, Sun G and He Q: High
level of serum AFP is an independent negative prognostic factor in
gastric cancer. Int J Biol Markers. 30:e387–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deng K, Yang L, Hu B, Wu H, Zhu H and Tang
C: The prognostic significance of pretreatment serum CEA levels in
gastric cancer: A meta-analysis including 14651 patients. PLoS One.
10:e1241512015.
|
|
29
|
Li S, Xu X, Liang D, Tian G, Song S and He
Y: Prognostic value of blood neutrophil-to-lymphocyte ratio (NLR)
and platelet-to-lymphocyte ratio (PLR) in patients with gastric
cancer. Zhonghua Zhong Liu Za Zhi. 36:910–915. 2014.(In Chinese).
PubMed/NCBI
|
|
30
|
Mohamed MM, El-Ghonaimy EA, Nouh MA,
Schneider RJ, Sloane BF and El-Shinawi M: Cytokines secreted by
macrophages isolated from tumor microenvironment of inflammatory
breast cancer patients possess chemotactic properties. Int J
Biochem Cell Biol. 46:138–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ye Y, Liu S, Wu C and Sun Z: TGFβ
modulates inflammatory cytokines and growth factors to create
premetastatic microenvironment and stimulate lung metastasis. J Mol
Histol. 46:365–375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y and Cao X: Immunosuppressive cells
in tumor immune escape and metastasis. J Mol Med (Berl).
94:509–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mocellin MC, Pastore e Silva Jde A,
Camargo Cde Q, Fabre ME, Gevaerd S, Naliwaiko K, Moreno YM, Nunes
EA and Trindade EB: Fish oil decreases C-reactive protein/albumin
ratio improving nutritional prognosis and plasma fatty acid profile
in colorectal cancer patients. Lipids. 48:879–888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dobrzycka B, Mackowiak-Matejczyk B,
Terlikowska KM, Kulesza-Bronczyk B, Kinalski M and Terlikowski SJ:
Serum levels of IL-6, IL-8 and CRP as prognostic factors in
epithelial ovarian cancer. Eur Cytokine Netw. 24:106–113.
2013.PubMed/NCBI
|
|
37
|
Steffens S, Al Ghazal A, Steinestel J,
Lehmann R, Wegener G, Schnoeller TJ, Cronauer MV, Jentzmik F,
Schrader M, Kuczyk MA and Schrader AJ: High CRP values predict poor
survival in patients with penile cancer. BMC Cancer. 13:2232013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Szturmowicz M, Rudziński P, Kacprzak A,
Langfort R, Bestry I, Broniarek-Samson B and Orłowski T: Prognostic
value of serum C-reactive protein (CRP) and cytokeratin 19
fragments (Cyfra 21-1) but not carcinoembryonic antigen (CEA) in
surgically treated patients with non-small cell lung cancer.
Pneumonol Alergol Pol. 82:422–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Heneghan HM, Zaborowski A, Fanning M,
McHugh A, Doyle S, Moore J, Ravi N and Reynolds JV: Prospective
study of malabsorption and malnutrition after esophageal and
gastric cancer surgery. Ann Surg. 262:803–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ryan AM, Power DG, Daly L, Cushen SJ,
NíBhuachalla Ē and Prado CM: Cancer-associated malnutrition,
cachexia and sarcopenia: The skeleton in the hospital closet 40
years later. Proc Nutr Soc. 75:pp. 199–211. 2016, View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fairclough E, Cairns E, Hamilton J and
Kelly C: Evaluation of a modified early warning system for acute
medical admissions and comparison with C-reactive protein/albumin
ratio as a predictor of patient outcome. Clin Med (Lond). 9:30–33.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu X, Sun X, Liu J, Kong P, Chen S, Zhan
Y and Xu D: Preoperative C-reactive protein/albumin ratio predicts
prognosis of patients after curative resection for gastric cancer.
Transl Oncol. 8:339–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Duan H, Zhang X, Wang FX, Cai MY, Ma GW,
Yang H, Fu JH, Tan ZH, Meng YQ, Fu XY, et al: Prognostic role of
neutrophil-lymphocyte ratio in operable esophageal squamous cell
carcinoma. World J Gastroenterol. 21:5591–5597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Croker BA, Lewis RS, Babon JJ, Mintern JD,
Jenne DE, Metcalf D, Zhang JG, Cengia LH, O'Donnell JA and Roberts
AW: Neutrophils require SHP1 to regulate IL-1β production and
prevent inflammatory skin disease. J Immunol. 186:1131–1139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nozawa H, Chiu C and Hanahan D:
Infiltrating neutrophils mediate the initial angiogenic switch in a
mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA.
103:pp. 12493–12498. 2006, View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Brandau S, Moses K and Lang S: The kinship
of neutrophils and granulocytic myeloid-derived suppressor cells in
cancer: Cousins, siblings or twins? Semin Cancer Biol. 23:171–182.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hao S, Andersen M and Yu H: Detection of
immune suppressive neutrophils in peripheral blood samples of
cancer patients. Am J Blood Res. 3:239–245. 2013.PubMed/NCBI
|
|
48
|
Shi F, Shi M, Zeng Z, Qi RZ, Liu ZW, Zhang
JY, Yang YP, Tien P and Wang FS: PD-1 and PD-L1 upregulation
promotes CD8(+) T-cell apoptosis and postoperative recurrence in
hepatocellular carcinoma patients. Int J Cancer. 128:887–896. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu S, Lachapelle J, Leung S, Gao D,
Foulkes WD and Nielsen TO: CD8+ lymphocyte infiltration
is an independent favorable prognostic indicator in basal-like
breast cancer. Breast Cancer Res. 14:R482012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim JH, Han DS, Bang HY, Kim PS and Lee
KY: Preoperative neutrophil-to-lymphocyte ratio is a prognostic
factor for overall survival in patients with gastric cancer. Ann
Surg Treat Res. 89:81–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Choi WJ, Cleghorn MC, Jiang H, Jackson TD,
Okrainec A and Quereshy FA: Preoperative neutrophil-to-lymphocyte
ratio is a better prognostic serum biomarker than
platelet-to-lymphocyte ratio in patients undergoing resection for
nonmetastatic colorectal cancer. Ann Surg Oncol 22 Suppl.
3:S603–613. 2015. View Article : Google Scholar
|
|
52
|
Zhou P, Erfani S, Liu Z, Jia C, Chen Y, Xu
B, Deng X, Alfáro JE, Chen L, Napier D, et al: CD151-α3β1 integrin
complexes are prognostic markers of glioblastoma and cooperate with
EGFR to drive tumor cell motility and invasion. Oncotarget.
6:29675–29693. 2015. View Article : Google Scholar : PubMed/NCBI
|