Introduction
Rapid economic development and changes in human life
style, particularly the dietary structure, have resulted in an
increase in the incidence of colon cancer. According to clinical
data, colon cancer has become a global problem and ranks 3rd in all
common malignant tumors in male and even 2nd in female tumors. Thus
it is a malignant tumor with a high incidence and is extremely
difficult to treat (1). At present,
the treatment for the disease is mainly comprehensive therapy
combined with surgery, chemotherapy and other types of treatment.
The therapeutic effect of early-stage colon cancer is relatively
good and its 5-year survival rate is more than 90%. However, the
therapeutic effect on patients with middle- and advanced stage
colon cancer is not satisfactory. Although early-stage colon cancer
can be successfully found via colonoscopy and more patients can
receive the self-healing treatment, more than 25% of patients have
been accompanied with metastasis when diagnosed (2), and 20–45% patients suffer from
recurrence or distant metastasis after surgery (3). Therefore it is important to investigate
the underlying mechanisms of the pathogenesis of colon cancer, not
only for prevention and prognosis, but also for treatment.
Rhotekin (RTKN) 2, a novel Rho effector, is a member
of the RTKN protein family. The two RTKN proteins, RTKN1 and 2,
have homologues in the majority of animals and mammals, including
horses, dogs, rats, and humans and each of the proteins has an
N-terminal Rho-guanosine triphosphatase (GTPase) binding domain and
pleckstrin homology domain (4). The
similar protein architecture of RTKN proteins indicated that they
may share functional characteristics (4). Previous studies showed that RTKN
proteins regulate critical cell functions including cell
proliferation and cell cycle progression, cytokinesis, apoptosis
and transformation. Wei et al (5) reported the overexpression of RTKN2 in
the majority of hepatocellular carcinoma (HCC) patients and
demonstrated an association between RTKN2 expression and
proliferation, apoptosis and metastatic progression. Similarly,
Liao et al (6) reported the
anti-apoptotic effects of RTKN2 in bladder cancer cells.
Furthermore, the overexpression of RTKN2 reduced viability and
increased sensitivity to 25-OHC (7),
which is directly associated with apoptosis in hematopoietic and
leukemic cells (8–10). Although the RTKN2 gene is
associated with several cancer types, including HCC, bladder and
breast cancer (5,6,11), the
expression pattern and biological functions of RTKN2 in human colon
cancer have yet to be investigated.
In the present study, the role of RTKN2 in human
colon cancer and the associated mechanisms were explored. Firstly,
we found that the gene expression level of RTKN2 was markedly
higher in human colon cancer tissues. Furthermore, we investigated
the role of RTKN2, including cell proliferation, cell cycle and
apoptosis in RTKN2 knockdown, as well as in SW480 and HCT116 colon
cancer cells.
Materials and methods
Patients and tissue samples
Tumor tissues and paired non-cancerous tissues were
collected from 30 patients with colon cancer who were admitted to
the Department of Radiology, The First Affiliated Hospital of
Soochow University (Suzhou, China) between 2010 and 2012. Ethics
approval for the study was provided by the Independent Ethics
Committee of The First Affiliated Hospital of Soochow University.
Informed and written consent was obtained from all the patients or
their advisers according to the Ethics Committee guidelines.
Cell lines
HIEC, SW480 and HCT116 cells were obtained from the
Cell Bank of Shanghai Biology Institute, Chinese Academy of Science
(Shanghai, China). Culture media were supplemented with 10% fetal
bovine serum, 100 mg/ml penicillin G and 50 µg/ml streptomycin
(Life Technologies; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). HIEC, SW480 and HCT116 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Life Technologies; Thermo Fisher
Scientific, Inc.). The cells were maintained at 37°C in 5%
CO2.
Vector construction
pLKO.1, psPAX2 and pMD2.G were purchased from
Addgene, Inc., (Cambridge, MA, USA). Three small hairpin RNAs
(shRNAs; Generay Biotech Co., Ltd., Shanghai, China) targeting
human RTKN2 mRNA were cloned into a lentiviral vector (PLKO.1). A
non-specific scramble shRNA sequence (CCTAAGGTTAAGTCGCCCTCG) was
used as a negative control. The constructs were then transfected
into HIEC cells with lentiviral packaging vectors (psPAX2 and
pMD2.G) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Viruses were collected 48 h subsequent to
transfection and used to infect SW480 and HCT116 cells. After 48 h,
the cells were processed for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blot analysis. The
shRNA target sequence for RTKN2 was CAGGGAAAGAACAATAGAAGTCT
(1967–1989).
RNA extraction and RT-PCR
Total RNA was extracted using TRIzol and the purity
and concentration of the extracted RNA were detected using a
nucleic acid protein detector. The reverse transcription reaction
system was in strict accordance with the instructions of the
reverse transcription M-MLV first strand kit (Invitrogen; Thermo
Fisher Scientific, Inc.). The fluorescence quantitative PCR of mRNA
was performed according to the instructions of the fluorescence
real-time quantitative PCR kit SYBR-Premix Ex Taq (Takara
Biotechnology Co., Ltd., Dalian, China). The reaction parameters of
RT-qPCR were: pre-denaturation at 94°C for 5 min, denaturation at
95°C for 30 sec, annealing at 58°C for 30 sec, extension at 72°C
for 30 sec, a total of 40 cycles, collection of fluorescence at
75–80°C and melting curve analysis at 65–95°C [with glyceraldehyde
3-phosphate dehydrogenase (GAPDH) as the internal reference]. The
primers used for quantitative PCR were designed using Primer 5.0
software and synthesized by Invitrogen (Thermo Fisher Scientific,
Inc.) after homologous alignment. The specific sequences, length of
amplification and annealing temperature are shown in Table I.
 | Table I.Primer sequences of genes. |
Table I.
Primer sequences of genes.
| Gene | Primer sequence
(5′-3′) | Length of
amplification (bp) | Annealing temperature
(°C) |
|---|
| RTKN2 | F:
ACAGTTCGCGTTGGAGATGGAG | 245 | 58 |
|
| R:
GTCGAGCATTGCACACCATGAG |
|
|
| GAPDH | F:
CACCCACTCCTCCACCTTTG | 218 | 58 |
|
| R:
CCACCACCCTGTTGCTGTAG |
|
|
Western blot analysis
Polyacrylamide electrophoresis was performed in a
predetermined order and the loading amount of protein was 150 µg
per well. Electrophoresis was performed under 100 V when the
markers began to be separated until all the markers were separated
and the target bands were obtained. The protein was electronically
transferred onto the polyvinylidene fluoride membrane under the 350
mA current for approximately 2 h. The membrane was sealed using 5%
bovine serum albumin/milk at room temperature for 1 h, and the
rabbit anti-human polyclonal antibody RTKN2 (dilution, 1:500; cat.
no. PA5-25716; Invitrogen, Waltham, MA, USA), Rabbit anti-human
monoclonal antibody (PCNA, β-catenin, Bax, Bcl-2, cyclin D1, c-myc
and GAPDH; dilution, 1:1000; cat. nos. 13110, 8480, 5023, 4223,
2872, 13987 and 5174; Cell Signaling Technology, Inc., Danvers, MA,
USA) were incubatedd at 4°C overnight. The goat anti-rabbit
secondary polyclonal antibodies (dilution, 1:2,000; cat. no. 7074;
Cell Signaling Technology, Inc.) were added for incubation at 37°C
for 1 h on the second day. Finally, the exposure liquid was added,
and images were captured using chemiluminescence apparatus.
Cell Counting kit (CCK)-8 assay
SW480 and HCT116 cells in the logarithmic growth
phase were inoculated onto the 96-well plates and the cell density
was adjusted to 2×103 and 200 µl RPMI-1640 medium
containing 10% fetal bovine serum was added, with 6 control wells
for each group, prior to incubation for 24 h. Then, 10 µl CCK-8 was
added into each well for incubation in an incubator containing
CO2 for 4 h and wells with phosphate-buffered saline
(PBS) were used as controls. The absorbance (A) value at 450 nm was
detected using the microplate reader (Thermo Fisher Scientific,
Waltham, MA, USA). The growth curve was drawn with the mean (A)
value as the ordinate and time as the abscissa.
Cell cycle assay
SW480 and HCT116 cells in the logarithmic growth
phase were digested with trypsin (the digestion time should be as
short as possible and the cells blown and beaten as slightly as
possible), collected and washed twice with PBS to wash away the
trypsin and surface impurities. The cells were incubated and
stained using 10 µg/ml propidium iodide solution (PI) in 500 µl PBS
for 30 min (containing 100 µg/ml RNase). The cell cycle of
chondrocytes treated in each group was analyzed using the Beckman
flow cytometer, followed by statistical analysis using GraphPad
software (GraphPad Software Inc., La Jolla, CA, USA).
Cell apoptosis assay
Adherent cells were digested with trypsin without
ethylene diamine tetraacetic acid (the digestion time should be as
short as possible to avoid false positives). The cells were washed
with PBS twice (centrifuged for 5 min at 2,115 × g) and
1–5×105 cells were collected, prior to the addition of
500 µl binding buffer to suspend cells. Then, 5 µl Annexin V-FITC
and 5 µl PI were added and mixed evenly for reaction for 5–15 min
in the dark at room temperature. The cells were observed and
detected using a flow cytometer (Thermo Fisher Scientific) within 1
h and the excitation wavelength (Ex=488 nm) and emission wavelength
(Em=530 nm) were measured. Green fluorescence of Annexin V was
detected via the FITC channel (FL1) and PI red fluorescence was
detected via FL3. Statistical analysis was performed using the
GraphPad software (GraphPad Software Inc.).
Statistical analysis
Results were analyzed using the GraphPad Prism 5
software (GraphPad Software Inc.). The data were presented as mean
± standard deviation (SD). The independent sample t-test was used
to compare the difference between the two groups, while analysis of
variance was used for comparison of the multi-sample means.
P<0.05 was considered to indicate a statistically significant
difference.
Results
RTKN2 is upregulated in human colon
cancer tissues and cell lines
The mRNA expression levels of RTKN2 were compared
between colon cancer tissues and adjacent non-cancerous tissues
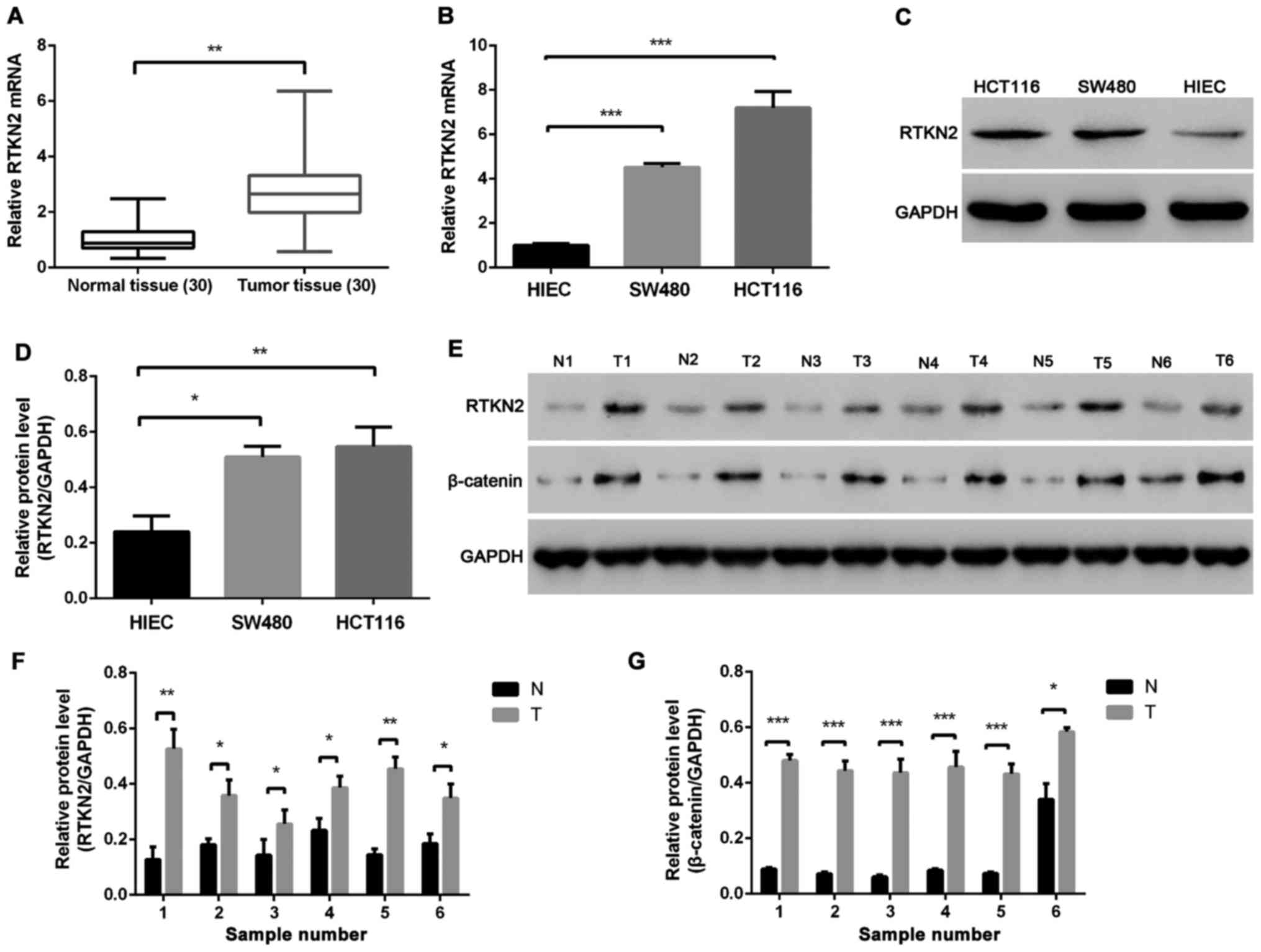
using RT-PCR. Overexpression of RTKN2 was confirmed in 90% (27/30)
of the measured colon cancer tissues (Fig. 1A). The protein levels of RTKN2 were
analyzed in six pairs of colon cancer tissues and matched adjacent
normal tissues using western blot analysis. The results showed that
the protein level of RTKN2 was markedly increased in colon cancer
tissues, compared with the matched adjacent normal tissues
(Fig. 1D and F). The present study
also examined the mRNA and protein levels of RTKN2 in human colon
cancer cell lines. The results demonstrated the mRNA and protein
levels of RTKN2 were significantly higher in SW480 and HCT116 human
colon cancer cell lines, compared with the HIEC cells (Fig. 1B, C and E).
RTKN2 is downregulated by siRNA in
SW480 and HCT116 cell lines
To investigate the functions of RTKN2 in human colon
cancer, we designed the shRNA targeting RTKN2 and packaged RTKN2
shRNA virus. The mRNA and protein expression levels of RTKN2 in
response to the specific RTKN2 shRNA virus were then assessed using
RT-PCR and western blot analysis, respectively. The results showed
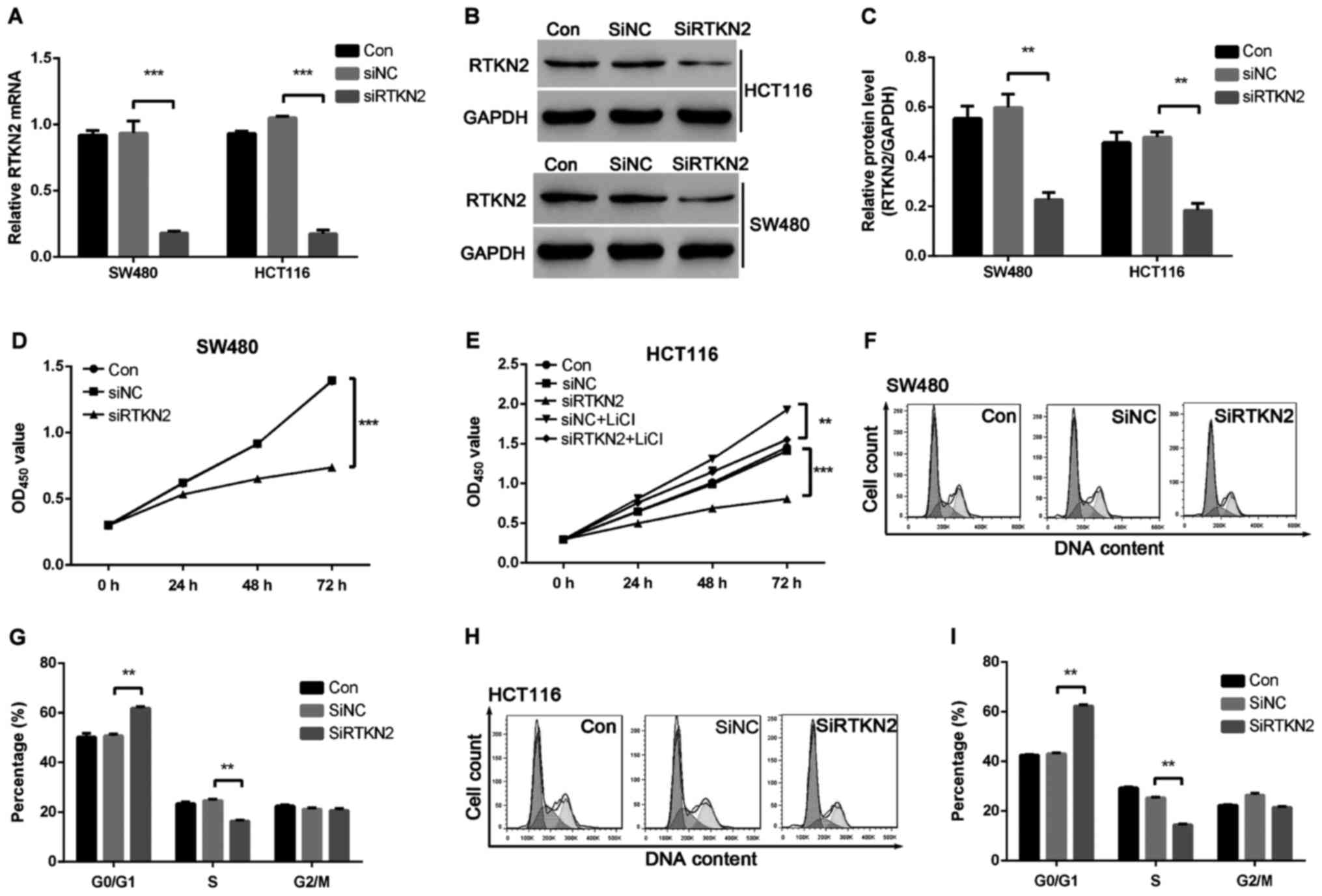
that the shRNA virus targering RTKN2 was able to efficiently
inhibit endogenous RTKN2 in SW480 and HCT116 cells (Fig. 2A-C). Therefore, the RTKN2 shRNA virus
was stably infections into SW480 and HCT116 cells for further
functional analysis.
Silencing of RTKN2 inhibits cell
growth and induces G1 cell cycle arrest in SW480 and HCT116 cell
lines
To investigate the role of RTKN2 on the
proliferation of human colon cancer cells, the proliferation of
RTKN2 silencing cells was examined using a CCK-8 assay. As shown in
Fig. 2D and E, cell growth was
significantly suppressed in RTKN2 shRNA virus-infected cells
(SiRTKN2) compared with scramble shRNA virus-infected cells (SiNC)
and wild-type cells (Con) in SW480 and HCT116 cells. These results
suggested that RTKN2 promoted colon cancer cells proliferation.
The present study then examined the possible
inhibitory effect of RTKN2 silencing on cell cycle progression. As
presented in Fig. 2F-I, cell cycle
analysis suggested that the population of RTKN2 knockdown in SW480
and HCT116 cells in G0/G1 phase was markedly increased by 20 and
41.1%, respectively (P<0.05), and the population of S-phase
cells was significantly reduced, by 33.3 and 43.8%, respectively
(P<0.05), compared with scramble shRNA SiNC cells. The data
indicated a role for RTKN2 in the promotion of colon cancer G1/S
cell cycle transition.
Suppression of RTKN2 expression
induces apoptosis of SW480 and HCT116 cells
To determine whether RTKN2 affected the apoptosis
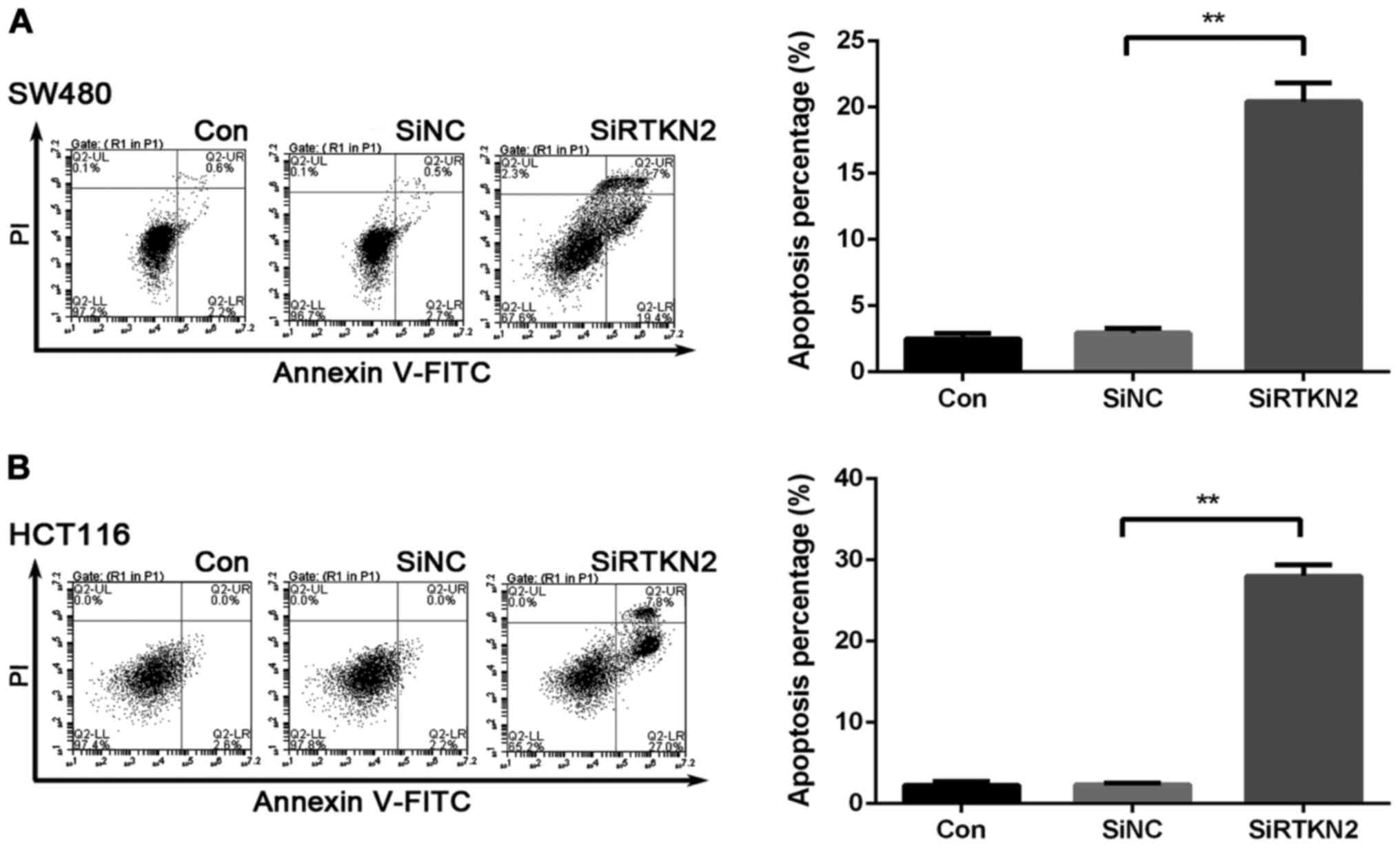
ability of the human colon cancer cells, Annexin V/PI staning and
flow cytometric analysis were performed. The ratio of cells
undergoing apoptosis was markedly increased, by 21.1±0.9% in the
RTKN2 shRNA virus-infected SW480 cells, compared with SiNC (2.7%)
and Con (2.3%) (Fig. 3A). Similar
results were observed in HCT116 cells. HCT116 cells were increased
by 29±1.2% in the SiRTKN2 group, compared with the SiNC group
(2.1%) and Con group (2.5%) (Fig.
3B). These data obtained in the present study indicated that
RTKN2 may play an important anti-apoptosis role in human colon
cancer cells.
Silencing of RTKN2 inhibits the
expression levels of cell apoptosis-associated proteins and
anti-apoptosis protein
To investigate the possible mechanism of RTKN2
knockdown that inhibits colon cell proliferation, induces G1 cell
cycle arrest and apoptosis, western blot analysis was performed to
detect PCNA, cell cycle-associated proteins and cell
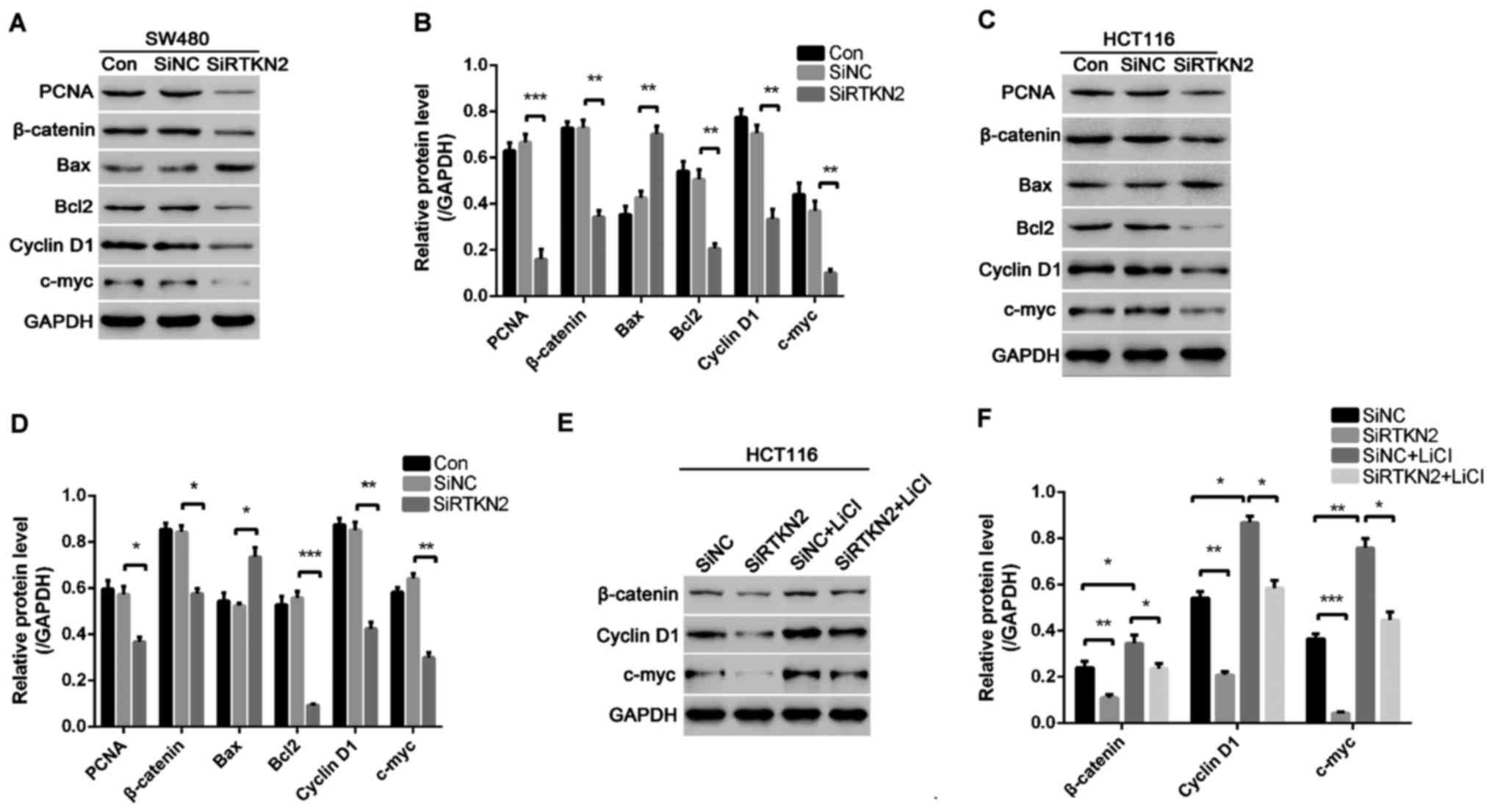
apoptosis-associated proteins in SW480 and HCT116 cells. RTKN2
silencing resulted in marked downregulation in the levels of PCNA,
Cyclin D1 and c-myc in the SW480 and HCT116 cells, compared with
the SiNC group of cells (Fig. 4A-D).
These results showed that RTKN2 knockdown suppressed the expression
of PCNA and cell cycle-associated proteins, which may have
contributed to the inhibition of cell proliferation and induction
of G1 phase cell cycle arrest. In addition, it was found that RTKN2
silencing inhibited the expression of Wnt/β-catenin protein
pathway. To prove that RTKN2 silencing inhibited colon cancer cell
proliferation by inhibiting the activity of Wnt/β-catenin pathway,
HCT116 cells were treated with Wnt/β-catenin pathway agonist LiCI
and RTKN2 was silenced. The results showed that RTKN2 silencing
inhibited the activation effect of LiCI on the Wnt/β-catenin
pathway and decreased the expressions of cell cycle-associated
proteins induced by LiCI (Fig. 4E and
F), thereby inhibiting cell proliferation.
In addition, the data indicated the silencing of
RTKN2 inhibited the expression of anti-apoptosis protein Bcl2 and
increased the expression of pro-apoptosis protein Bax (Fig. 4A and B), which resulted in cell
apoptosis.
Discussion
RTKN belongs to the group of proteins containing a
Rho-binding domain, which are target effectors for Rho-GTPases
(4). The RTKN gene was only
identified recently and its physiological functions remain largely
unknown (12). RTKN has been reported
to be associated with several cancer types including HCC (5), breast cancer (10), gastric cancer (13), and bladder cancer (11). However, the molecular mechanisms
underlying the development and progression of human colon cancer
have yet to be reported. In the present study, RT-qPCR and western
blot analysis showed that the expression levels of RTKN2 mRNA and
protein were markedly higher in human colon cancer tissues,
compared with adjacent normal tissues. In addition, the data showed
RTKN2 was expressed at high levels in SW480 and HCT116 colon cancer
cells.
Cell cycle progression is frequently abnormal in the
majority of cancer types, leading to aberrant cell growth (14,15). In
the present study, the effects of RTKN2 on proliferation, cell
cycle progression and apoptosis were examined by silencing its
expression in SW480 and HCT116 cells. It was observed that the
suppression of RTKN2 markedly inhibited cell proliferation.
Furthermore, flow cytometric analysis revealed that knockdown of
RTKN notably induced G1 phase cell cycle arrest in SW480 and HCT116
cells, which suggested that the suppression of cell growth in colon
cancer cells was due to the arrest of cell cycle progression. The
expression of the cell cycle regulators, PCNA, Cyclin D1 and c-myc,
was additionally confirmed. The results of the present study
suggested that knockdown of RTKN2 inhibited the expression levels
of PCNA, Cyclin D1 and c-myc in SW480 and HCT116 cells, which was
consistent with the results of cell growth and the cell cycle
analysis.
Wnt signaling pathways can be divided into classical
(Wnt/β-catenin signaling) and non-classical (Wnt/PCP signaling).
The classical typical pathway, Wnt/β-catenin signaling pathway,
plays an important role in tumor cell proliferation,
differentiation, metastasis and invasion (16–18). The
changes in the transcriptional activity and protein stability of
β-catenin are important indexes of Wnt/β-catenin signaling pathway
activation. The results of our research showed that Wnt/β-catenin
pathway was activated in colon cancer and RTKN2 silencing inhibited
the β-catenin expression. Cyclin D1 is a key regulatory protein in
the G1 phase and a downstream target gene of Wnt/β-catenin pathway.
To confirm whether there was any inhibitory effect of RTKN2 on
colon cancer cell proliferation following inhibition of the
Wnt/β-catenin signaling pathway, HCT116 cells were treated with
Wnt/β-catenin pathway agonist LiCI and RTKN2 expression was
silenced in this study. The results revealed that RTKN2 silencing
reduced the activation effect of LiCl on the Wnt/β-catenin
signaling pathway and reduced the expression of Cyclin D1 and c-myc
induced by LiCI. The CCK-8 results showed that RTKN2 silencing
reduced the promoting effect of LiCI on colon cancer cell
proliferation in HCT116 cells. These results suggested that TRKN2
silencing inhibits colon cancer cell proliferation by inhibiting
the Wnt/β-catenin signaling pathway.
Previous studies have confirmed the anti-apoptotic
effects of RTKN2 on several cancer types including HCC and bladder
cancer (5,6). Consistent with these observations, the
results of the present study demonstrated that silencing of RTKN2
markedly induced the apoptosis of human colon cancer cells.
Taken together, the present results indicated that
RTKN2 was upregulated in human colon cancer tissues and cells.
Knockdown of RTKN2 suppressed cell proliferation and induced cell
apoptosis. Furthermore, suppression of RTKN2 expression induced the
G1 phase arrest. In addition, the Wnt/β-catenin signaling pathway
was activated in human colon cancer tissues, and silencing of RTKN2
suppressed colon cell proliferation through inhibition of the
activation of Wnt/β-catenin. However, whether RTKN2 can be used as
a diagnostic marker or therapeutic target for colon cancer remains
to be further investigated.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E, Forman D, et al: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilkes GM: Therapeutic options in the
management of colon cancer: 2005 update. Clin J Oncol Nurs.
9:31–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winawer S, Fletcher R, Rex D, Bond J, Burt
R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, et al:
Gastrointestinal consortium panel: Colorectal cancer screening and
surveillance: Clinical guidelines and rationale-Update based on new
evidence. Gastroenterology. 124:544–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Collier FM, Gregorio-King CC, Gough TJ,
Talbot CD, Walder K and Kirkland MA: Identification and
characterization of a lymphocytic Rho-GTPase effector: Rhotekin-2.
Biochem Biophys Res Commun. 324:1360–1369. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei W, Chen H and Liu S: Knockdown of
Rhotekin 2 expression suppresses proliferation and invasion and
induces apoptosis in hepatocellular carcinoma cells. Mol Med Rep.
13:4865–4871. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liao YX, Zeng JM, Zhou JJ, Yang GH, Ding K
and Zhang XJ: Silencing of RTKN2 by siRNA suppresses proliferation,
and induces G1 arrest and apoptosis in human bladder cancer cells.
Mol Med Rep. 13:4872–4878. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gregorio-King CC, Gough T, Van Der Meer
GJ, Hosking JB, Waugh CM, McLeod JL, Collier FM and Kirkland MA:
Mechanisms of resistance to the cytotoxic effects of oxysterols in
human leukemic cells. J Steroid Biochem Mol Biol. 88:311–320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aupeix K, Weltin D, Mejia JE, Christ M,
Marchal J, Freyssinet JM and Bischoff P: Oxysterol-induced
apoptosis in human monocytic cell lines. Immunobiology.
194:415–428. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang J, Chen JX, Chen L, Tang JY, Cui Z,
Liu CH and Wang Z: Metastasis associated in colon cancer 1 (MACC1)
promotes growth and metastasis processes of colon cancer cells. Eur
Rev Med Pharmacol Sci. 20:2825–2834. 2016.PubMed/NCBI
|
|
10
|
Chen M, Bresnick AR and O'Connor KL:
Coupling S100A4 to Rhotekin alters Rho signaling output in breast
cancer cells. Oncogene. 32:3754–3764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fan J, Ma LJ, Xia SJ, Yu L, Fu Q, Wu CQ,
Huang XH, Jiang JM and Tang XD: Association between clinical
characteristics and expression abundance of RTKN gene in human
bladder carcinoma tissues from Chinese patients. J Cancer Res Clin
Oncol. 131:157–162. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu Q, Yu L, Liu Q, Zhang J, Zhang H and
Zhao S: Molecular cloning, expression characterization, and mapping
of a novel putative inhibitor of rho GTPase activity, RTKN, to
D2S145-D2S286. Genomics. 66:328–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yildiz B, Etiz D, Dal P, Junushova B,
Pasaoglu O, Yilmaz E, Erkasap S and Dincer M: Tumor deposits:
Prognostic significance in gastric cancer patients. J BUON.
21:1476–1481. 2016.PubMed/NCBI
|
|
14
|
Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng
L, Zhou H and Zhao RC: miR-145 inhibits breast cancer cell growth
through RTKN. Int J Oncol. 34:1461–1466. 2009.PubMed/NCBI
|
|
15
|
Sevli S, Uzumcu A, Solak M, Ittmann M and
Ozen M: The function of microRNAs, small but potent molecules, in
human prostate cancer. Prostate Cancer Prostatic Dis. 13:208–217.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi L, Wu YX, Yu JH, Chen X, Luo XJ and
Yin YR: Research of the relationship between β-catenin and
c-myc-mediated Wnt pathway and laterally spreading tumors
occurrence. Eur Rev Med Pharmacol Sci. 21:252–257. 2017.PubMed/NCBI
|
|
17
|
Hua F, Liu S, Zhu L, Ma N, Jiang S and
Yang J: Highly expressed long non-coding RNA NNT-AS1 promotes cell
proliferation and invasion through Wnt/β-catenin signaling pathway
in cervical cancer. Biomed Pharmacother. 92:1128–1134. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Lou Y, Bai X and Liang T: EPAS1
promote tumor progression by interacting with Wnt/β-catenin
signaling in pancreatic cancer. HPB. 18:e350–e351. 2016. View Article : Google Scholar
|