Introduction
Oral squamous cell carcinoma (OSCC), the most common
type of head and neck carcinoma, represents the fifth most
frequently occurring cancer worldwide (1). An estimated 263,900 new cases and
128,000 mortalities occurred globally in 2008 (2). Despite advances in surgery, radiotherapy
and chemotherapy, little improvement in the relative survival has
been observed in OSCC during the past 30 years (3).
Early-stage OSCC (clinical stage I or II) (4) is primarily managed with surgery. The
nodal status of the cervical lymph nodes remains an important
prognostic factor in OSCC (5–6). The presence of cervical lymph node
metastasis reduces the survival of patients with SCC of the upper
aerodigestive tract by up to 50% (7).
Therefore, early detection of cervical lymph node metastasis is
hypothesized to improve survival. However, the diagnostic accuracy
of lymph node metastasis using imaging tools including
ultrasonography (US), computed tomography (CT), magnetic resonance
imaging (MRI) and positron emission tomography (PET) is ~70%
(8). Furthermore, patients with
delayed neck node metastases generally exhibit a poor prognosis
(9).
Sentinel node biopsy (SNB) has been demonstrated to
be an oncologically safe staging modality in patients with early
stage OSCC, allowing for an individualized and minimally invasive
treatment of the neck, and significantly affecting tumor control
and survival (10).
However, patients with tumor recurrence and
metastases generally exhibit poor prognosis, and predictive
biomarkers that identify the risk of tumor relapse may become a
powerful tool for follow-up and development of effective treatment
plans for these patients (11–13). Sera
derived from patients with early stage OSCC were previously
examined for multiple cytokines using a multiplexed measurement
system (14) and serum interleukin-6
(IL-6) level was revealed to negatively correlate with a favourable
outcome in these patients. IL-6 is a multifunctional cytokine that
functions in the regulation of inflammatory and immune responses.
IL-6 is produced by a variety of cells, primarily monocytes,
macrophages and several types of tumor cell during infection and
immunological challenge (15).
Previous studies have revealed that IL-6 is involved in cancer
progression, including proliferation, angiogenesis and
lymphangiogenesis in several types of cancer, including OSCC
(16–19), and worsens cancer prognosis (20–22). The
aim of the present study was to determine the function of serum
IL-6 concentration in patients with early-stage OSCC defined by
SNB.
Materials and methods
Patients
The present study was approved by the medical ethics
committee of Ehime University Hospital (Tōon, Japan) for the
Protection of Human Subjects. Informed written consent was obtained
from all patients, and the collection of samples was approved by
the Institutional Review Board.
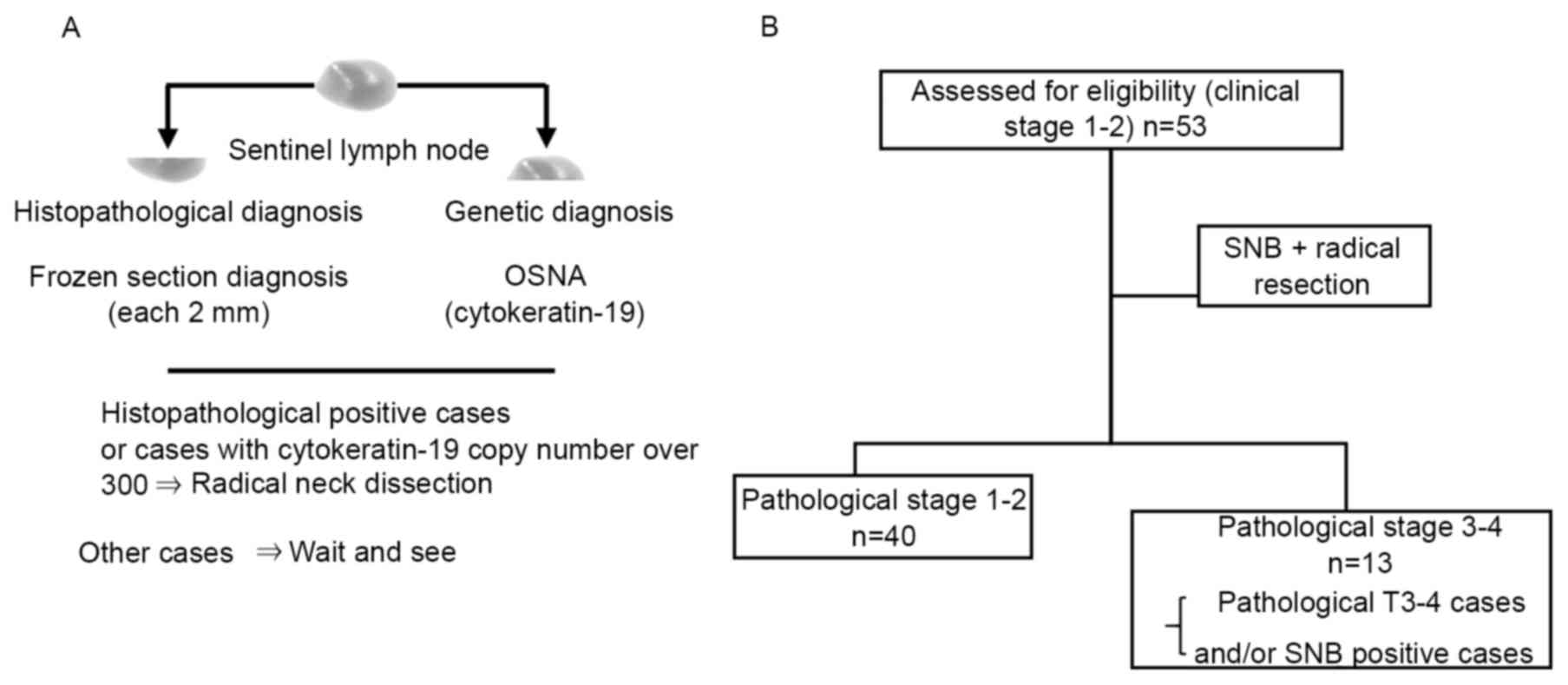
A total of 53 patients with clinically diagnosed
T1-2N0 OSCC (4) scheduled for radical
resection of their tumor(s) and SNB between September 2006 and June
2013 were eligible to participate in the present study. The primary
sites were the tongue, gingiva, oral floor, buccal mucosa and lip
in 50, 36, 8, 5 and 1% of tumors, respectively. The histological
differentiation of the samples was classified in accordance with
the World Health Organization classification (23). The mode of cancer invasion was
classified into five grades according to the classification
proposed by Yamamoto and Kohama (YK classification): YK-1,
well-defined border; YK-2, cords, less marked border; YK-3, groups
of cells, no distinct border; YK-4C, diffuse invasion, cord-like
type; and YK4-D, diffuse invasion, widespread type (24).
Serum collection and IL-6 ELISA. Prior to surgery,
serum was collected and immediately frozen at −80°C until being
used for the IL-6 assay. Serum IL-6 levels were analyzed using a
human IL-6 ELISA kit (cat. no. S6056; BioLegend, Inc., San Diego,
CA, USA) according to the manufacturer's protocol. The cut-off
value of 20.0 pg/ml was selected based on the receiver operating
characteristic (ROC) curve.
SNB
SNB has received considerable attention for its role
in the decision of whether to perform neck dissection (11). The day prior to surgery, a total of
0.4 ml (74 MBq) 99mTc-tin colloid was slowly injected
around the primary tumor at four points. The sentinel lymph node
(SN) was detected by scintigraphy 2 h following this. The middle
and lower portions of the face were covered with a lead plate to
reduce shine-through, and four Eppendorf mini tubes, each
containing 30 µl radioisotope solution, were placed at the chin,
the mandibular angle and the anterior and posterior ends of the
clavicle to serve as point markers. A small incision was made in
the skin above the SN at operation. Radioactivity of the SN was
measured with the gamma probe Neo 2000 (Navidea Biopharmaceuticals,
Dublin, OH, USA). The SN was rinsed quickly in saline solution to
remove attached blood, the peripheral adipose tissue and connective
tissue were carefully stripped off, and the SN was subsequently
divided in two. One half of each lymph node was examined through
sectioning (200 µm thick) of the maximal cut surface of the
specimen, stained with hematoxylin and eosin and diagnosed as
metastatic or non-metastatic. The remaining half of the lymph node
was analyzed by one step nucleic acid amplification (OSNA) to
calculate the cytokeratin-19 (CK-19) mRNA copy number as previously
described (25). A previous study
classified OSNA levels in OSCC as follows: CK19 mRNA copies
<300/µl was designated as negative, and >300/µl as positive
(25). Neck dissection was performed
in metastatic cases, determined by either histopathological or
genetic examination.
Statistical analysis
Kaplan-Meier curves and log-rank tests were utilized
to assess any differences in survival times between the treatment
groups. A multivariate analysis was performed to evaluate impact
factors on survival using Cox's proportional hazards regression
model. Differences between the groups of categorical data were
analyzed using two-sided Fisher's exact tests. P<0.05 was
considered to indicate a statistically significant difference.
GraphPad Prism statistical software version 5 (GraphPad Software,
Inc., La Jolla, CA, USA) was used for statistical analysis.
Results
SNB
Table I lists the
patient and tumor characteristics. A total of 53 patients with
clinically diagnosed T1-2N0 OSCC undergoing SNB were included in
the present study. There were 31 male and 22 female patients, with
a mean age of 69 years (range, 40–91 years). The mean number of SNs
identified was 1.9 (range, 1–5). In total, 19 patients had 1 SN
removed, 22 patients had 2 SNs, 11 patients had 3 SNs and 1 patient
had 5 SNs removed. The SNB results are summarized in Fig. 1 and Table
II. The SN metastases were pathologically and genetically
diagnosed by the SNB specimens in 18.9% of cases (10/53 cases). The
identification rate by SNB was 100% (53/53 cases), accuracy was
92.5% (49/53 cases), the SN positive rate was 18.9% (10/53) and the
false negative rate was 28.6% (4/14 cases). In 46 patients with
pathological T1-2, the identification rate by SNB was 100% (46/46
cases), accuracy was 90.3% (42/46 cases), SN positive rate was 13%
(6/46) and the false negative rate was 29% (1-sensitivity). In
patients with pathological T3-4, the identification rate of SNB was
100% (7/7 cases), the accuracy was 100% (7/7 cases), the SN
positive rate was 57.1% (4/7) and the false negative rate was 0%.
Of these 53 patients, 13 (24.5%) were excluded from the
pathological stage 1–2 group due to presence of pathological T 3–4
stage (including bone invasion) tumor and SN metastasis (Tables II and III).
 | Table I.Patient and tumor characteristics. |
Table I.
Patient and tumor characteristics.
| Clinicopathological
characteristic | Number (%) |
|---|
| Number of
patients | 53 |
| Age, median
(range) | 68.5 (40–91) |
| Sex |
|
| Male | 31 (58.5) |
|
Female | 22 (41.5) |
| WHO (grade) |
|
| 1 | 38 (71.7) |
| 2 | 12 (22.6) |
| 3 | 3 (5.7) |
| Stage |
|
| 1 | 24 (45.3) |
| 2 | 27 (50.9) |
| 3 | 0 |
| 4 | 2 (3.8) |
| SNB |
|
| + | 10 (18.9) |
| − | 43 (81.1) |
| Pathological T4 (bone
invasion) | 7
(13.2) |
| Mode of cancer
invasion |
|
| YK-1 | 2 (3.8) |
| YK-2 | 7
(13.2) |
| YK-3 | 27 (50.9) |
|
YK-4C | 16 (30.2) |
|
YK-4D | 1 (1.9) |
| Primary sites |
|
|
Tongue | 24 (45.3) |
|
Gingiva | 23 (43.4) |
| Floor of
the mouth | 4 (7.5) |
| Buccal
mucosa | 1 (1.9) |
| Lip | 1 (1.9) |
 | Table II.Summary of SNB results. |
Table II.
Summary of SNB results.
| Cases | n | Identification rate
(%) | SN positive rate
(%) | False negative rate
(%) | Accuracy (%) |
|---|
| All | 53 |
100 (53/53) | 18.9 (10/53) | 28.6 (4/14) | 92.5 (49/53) |
| pT1–2 | 46 |
100 (46/46) | 13 (6/46) | 40 (4/10) | 91.3 (42/46) |
| pT3-4 | 7 | 100 (7/7) | 57.1 (4/7) | 0 (0/4) | 100 (7/7) |
 | Table III.Metastasis in SNB positive and
negative cases. |
Table III.
Metastasis in SNB positive and
negative cases.
| Cases | Metastasis (+) | Metastasis (−) | Total |
|---|
| All | 14 | 39 | 53 |
| SNB
(+) | 10 | 0 | 10 |
| SNB
(−) | 4 | 39 | 43 |
| pT1-2 | 10 | 36 | 46 |
| SNB
(+) | 6 | 0 | 6 |
| SNB
(−) | 4 | 36 | 40 |
| pT3-4 | 4 | 3 | 7 |
| SNB
(+) | 4 | 0 | 4 |
| SNB
(−) | 0 | 3 | 3 |
Association between the SNB status of
patients with OSCC and serum IL-6 levels
The cut-off value of 20.0 pg/ml was selected based
on the ROC curve. ELISA was performed for IL-6 in the 53 patients
exhibiting cT1-T2 and evaluated for association with lymph node
metastasis. Analysis revealed no association between SN metastasis
and IL-6 level (P=0.73; Table
IV).
 | Table IV.Serum IL-6 levels in SNB negative and
positive cases. |
Table IV.
Serum IL-6 levels in SNB negative and
positive cases.
| Serum IL-6 | SNB negative | SNB positive |
|---|
| High | 25 | 5 |
| Low | 18 | 5 |
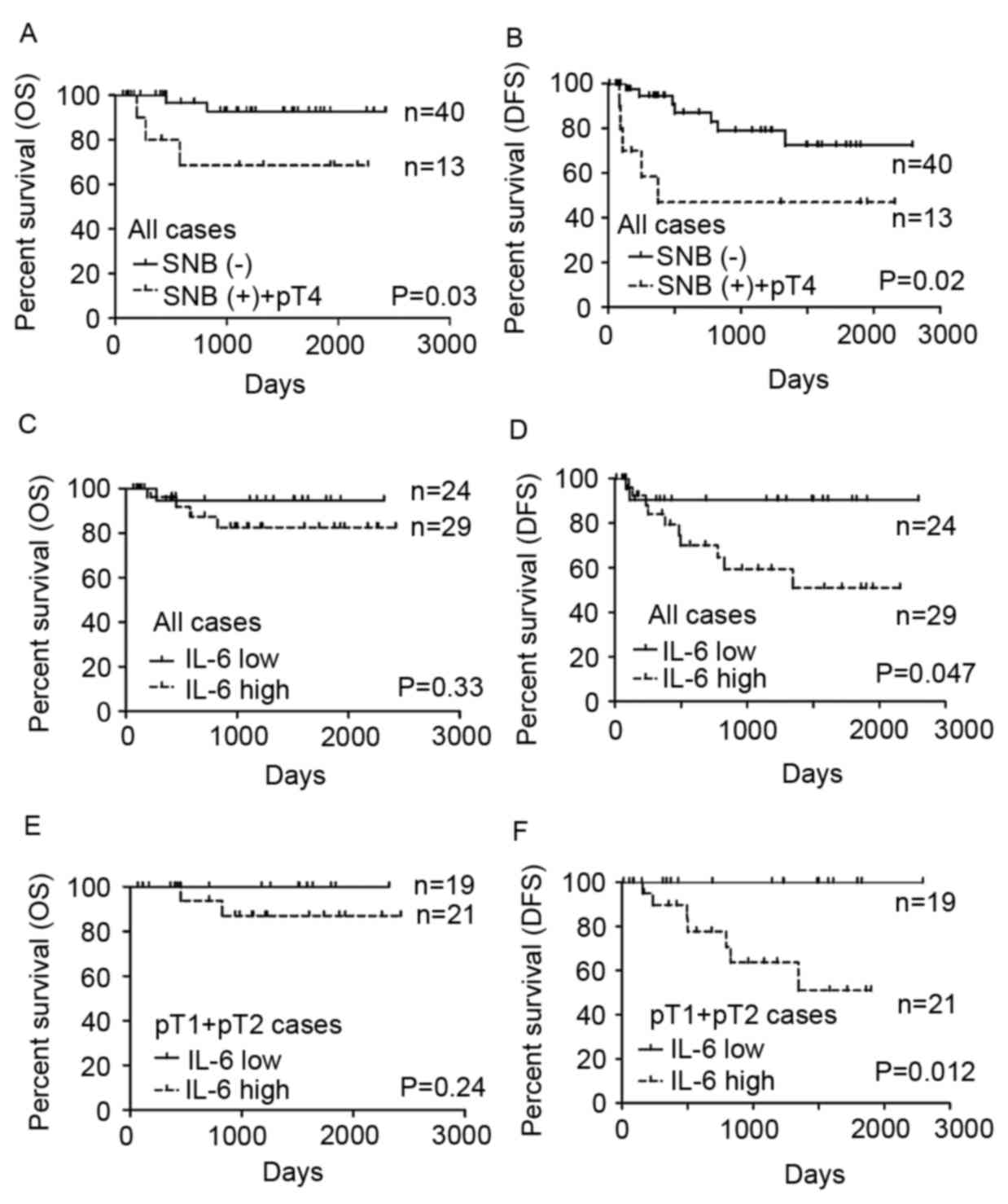
Kaplan-Meier survival plots comparing
SNB and serum IL-6 levels
Kaplan-Meier survival plots comparing the overall
survival (OS) and disease-free survival (DFS) fractions of each
group are depicted in Fig. 2. OS (Log
rank P=0.03) and DFS (Log rank P=0.02) were significantly lower
among the patients whose SNB status and tumor bone invasion status
were positive (Fig. 2A and B). Among
the 53 patients, the 24 patients of the low serum IL-6 group tended
to survive longer (P=0.33 in OS, P=0.047 in DFS), but the
difference was not significant for OS (Fig. 2C and D). A total of 13 patients were
diagnosed with lymph node metastasis by SNB or were reclassified to
pathological stage T4 from evaluation of the surgically resected
specimen. Thus, 40 patients were strictly defined as pathological
stage 1–2 OSCC. Among them, the OS of patients with low serum IL-6
levels was not statistically significant different compared with
those with high serum levels (P=0.24; Fig. 2E) but DFS of the patients with low
serum IL-6 levels was significantly longer compared with those with
high serum IL-6 (P=0.012; Fig. 2F).
For patients with pathological stage l-2 tumors strictly defined by
SNB and pathological examination, OS and DFS were 100% in the low
serum IL-6 group in the follow-up period (Fig. 2E and F).
Discussion
The present study demonstrated that staging by SNB
and preoperative serum IL-6 level exhibited a high prognostic value
in patients with OSCC. In patients with early-stage OSCC, the nodal
status of the cervical lymph nodes remained an important prognostic
factor. Advances in imaging technology have made additional
techniques available, including US, CT, MRI and PET imaging.
However, the diagnostic ability of US, CT and MRI is primarily
based on node morphology and size criteria, with nodes <10 mm
generally considered to not harbour metastasis. Attempts with
functional imaging using PET scanning were also revealed not to be
efficient in detecting occult cervical metastasis. Since the early
1980s, treatment of patients with clinical N0 OSCC has evolved from
observation to elective neck dissection for all but the
earliest-stage types of cancer of the oral cavity, with elective
neck dissection becoming the standard of care at the majority of
institutions treating a large number of patients with cancer in the
1990s (7). However, elective neck
dissection is a surgical procedure that was revealed to be
overtreatment for ~70% of patients with cN0, who were revealed to
have a pathological node-negative neck (8). Observation was the primary therapeutic
modality employed in the management of patients with N0 neck
tumors, yet it soon became evident that ~30% of observed patients
developed cervical node metastasis despite tumor control at the
primary site (26–28).
An SN is the first lymph node(s) to which cancer
cells are most likely to spread from a primary tumor. SNB may be
used to help determine the extent of the disease, and to stage and
guide the use of adjuvant radiation and chemotherapy to reduce
disease recurrence. As SNB involves less extensive surgery and the
removal of fewer lymph nodes compared with neck dissection, the
risk of adverse effects is lower. In the present study, the SNB
positive rate was 18.9% (10/53), but the delayed neck metastasis
was 7.5% (4/53). Consistent with previous reports of cervical
metastasis, the present study revealed a lymph node metastasis rate
of 26.4%.
The present results suggested that SNB may improve
the prognosis of patients with OSCC, with a significantly higher OS
(P=0.03) and DFS (P=0.02) in patients whose SNB status was
negative. However, a few cases of delayed neck metastasis and local
recurrence were observed, even in SNB negative cases. A possible
cause may be the lack of rapid and accurate intraoperative
detection of metastatic disease in the SN(s) (29,30). In
addition, the occurrence of a second primary tumor is 3–7% higher
per year in patients with OSCC compared with other malignancies
(31). Therefore, the identification
of suitable and reliable biomarkers is essential for achieving
early detection and treatment, which may reduce mortality rates in
patients with OSCC. However, several serum cytokines were
previously examined in patients with OSCC by using a multiplexed
measurement system, revealing that the serum IL-6 level tended to
negatively correlate with favorable outcome in these patients, with
OS and DFS rates of 100% in patients with early-stage OSCC
diagnosed by SNB with low levels of serum IL-6. Furthermore,
adjuvant and/or neo-adjuvant therapies may be administered to
patients with high levels of serum IL-6, despite having only
early-stage OSCC. In this previous study, there were 4 false
negative cases (delayed neck metastasis). It was impossible to
analyze the statistical significance with respect to correlation
between the serum IL-6 level and frequency of the delayed neck
metastasis in SNB negative cases as there were few applicable
cases. Therefore, this requires additional investigation.
Previous studies have revealed that stromal
fibroblasts isolated from various types of cancer produced a
significant amount of IL-6, which induced tumor proliferation,
invasion, migration and angiogenesis (19,32,33). An
on-going prospective study may further elucidate the prognostic and
predictive significance of IL-6, which may warrant future clinical
trials of an IL-6 inhibitor for patients with high-risk OSCC.
In conclusion, the present study revealed that SNB
staging and preoperative serum IL-6 level have a high prognostic
value in patients with OSCC, and IL-6 may be associated with poor
clinical outcome. Future studies are required to increase
understanding of the biological significance underlying the
association of IL-6 with OSCC and the tumor microenvironment,
including how these findings may be combined with other treatment
approaches, including radiation and chemotherapy, which may
contribute to the continued improvement of OSCC treatments.
Acknowledgements
The present study was supported by a Grant-in-Aid
for Scientific Research from the Ministry of Education, Culture,
Sports, Science and Technology of Japan.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer Statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global Cancer Statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
James DB, Mary KG and Christian W: UICC:
TNM classification of malignant tumors. 8th edition.
Wiley-Blackwell; Hoboken, NJ: pp. 2722016
|
|
5
|
Kunishi M, Kayada Y and Yoshiga K:
Down-regulated expression of CD44 variant 6 in oral squamous cell
carcinomas and its relationship to regional lymph node metastasis.
Int J Oral Maxillofac Surg. 26:280–283. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okada Y, Mataga I, Katagiri M and Ishii K:
An analysis of cervical lymph nodes metastasis in oral squamous
cell carcinoma. Relationship between grade of histopathological
malignancy and lymph nodes metastasis. Int J Oral Maxillofac Surg.
32:284–288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Myers EN and Fagan J: Treatment of the N+
neck in squamous cell carcinoma of the upper aerodigestive tract.
Otolaryngol Clin North Am. 31:671–686. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stuckensen T, Kovács AF, Adams S and Baum
RP: Staging of the neck in patients with oral cavity squamous cell
carcinomas: a prospective comparison of PET, ultrasound, CT and
MRI. J Craniomaxillofac Surg. 28:319–324. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cunningham MJ, Johnson JT, Myers EN,
Schramm VL Jr and Thearle PB: Cervical lymph node metastasis after
local excision of early squamous cell carcinoma of the oral cavity.
Am J Surg. 152:361–366. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Broglie MA, Haerle SK, Huber GF, Haile SR
and Stoeckli SJ: Occult metastases detected by sentinel node biopsy
in patients with early oral and oropharyngeal squamous cell
carcinomas: impact on survival. Head Neck. 35:660–666. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Almadori G, Bussu F and Paludetti G:
Should there be more molecular staging of head and neck cancer to
improve the choice of treatments and thereby improve survival? Curr
Opin Otolaryngol Head Neck Surg. 16:117–126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Woolgar JA and Hall GL: Determinants of
outcome following surgery for oral squamous cell carcinoma. Future
Oncol. 5:51–61. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kreppel M, Drebber U, Rothamel D, Eich HT,
Kübler A, Scheer M and Zöller JE: Prognostic impact of different
TNM-based stage groupings for oral squamous cell carcinoma. Head
Neck. 33:1467–1475. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Biancotto A, Feng X, Langweiler M, Young
NS and McCoy JP: Effect of anticoagulants on multiplexed
measurement of cytokine/chemokines in healthy subjects. Cytokine.
60:438–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kishimoto T, Akira S, Narazaki M and Taga
T: Interleukin-6 family of cytokines and gp130. Blood.
86:1243–1254. 1995.PubMed/NCBI
|
|
16
|
Shinriki S, Jono H, Ota K, Ueda M, Kudo M,
Ota T, Oike Y, Endo M, Ibusuki M, Hiraki A, et al: Humanized
anti-interleukin-6 receptor antibody suppresses tumor angiogenesis
and in vivo growth of human oral squamous cell carcinoma. Clin
Cancer Res. 15:5426–5434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Q, Li G, Li R, Shen J, He Q, Deng L,
Zhang C and Zhang J: IL-6 promotion of glioblastoma cell invasion
and angiogenesis in U251 and T98 G cell lines. J Neurooncol.
100:165–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shinriki S, Jono H, Ueda M, Ota K, Ota T,
Sueyoshi T, Oike Y, Ibusuki M, Hiraki A, Nakayama H, et al:
Interleukin-6 signalling regulates vascular endothelial growth
factor-C synthesis and lymphangiogenesis in human oral squamous
cell carcinoma. J Pathol. 225:142–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nagasaki T, Hara M, Nakanishi H, Takahashi
H, Sato M and Takeyama H: Interleukin-6 released by colon
cancer-associated fibroblasts is critical for tumour angiogenesis:
Anti-interleukin-6 receptor antibody suppressed angiogenesis and
inhibited tumour-stroma interaction. Br J Cancer. 110:469–478.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Z, Malhotra PS, Thomas GR, Ondrey FG,
Duffey DC, Smith CW, Enamorado I, Yeh NT, Kroog GS, Rudy S, et al:
Expression of proinflammatory and proangiogenic cytokines in
patients with head and neck cancer. Clin Cancer Res. 5:1369–1379.
1999.PubMed/NCBI
|
|
21
|
Duffy SA, Taylor JM, Terrell JE, Islam M,
Li Y, Fowler KE, Wolf GT and Teknos TN: Interleukin-6 predicts
recurrence and survival among head and neck cancer patients.
Cancer. 113:750–757. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang KP, Kao HK, Wu CC, Fang KH, Chang
YL, Huang YC, Liu SC and Cheng MH: Pretreatment interleukin-6 serum
levels are associated with patient survival for oral cavity
squamous cell carcinoma. Otolaryngol Head Neck Surg. 148:786–791.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO Classification of tumours of the digestive system,
fourth edition. WHO Classification of Tumours. 3:4172010.
|
|
24
|
Yamamoto E, Kohama G, Sunakawa H, Iwai M
and Hiratsuka H: Mode of invasion, bleomycin sensitivity, and
clinical course in squamous cell carcinoma of the oral cavity.
Cancer. 51:2175–2180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goda H, Nakashiro K, Oka R, Tanaka H,
Wakisaka H, Hato N, Hyodo M and Hamakawa H: One-step nucleic acid
amplification for detecting lymph node metastasis of head and neck
squamous cell carcinoma. Oral Oncol. 48:958–963. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Whitehurst JO and Droulias CA: Surgical
treatment of squamous cell carcinoma of the oral tongue: Factors
influencing survival. Arch Otolaryngol. 103:212–215. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuen AP, Wei WI, Wong YM and Tang KC:
Elective neck dissection versus observation in the treatment of
early oral tongue carcinoma. Head Neck. 19:583–588. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kowalski LP: Results of salvage treatment
of the neck in patients with oral cancer. Arch Otolaryngol Head
Neck Surg. 128:58–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iii RPZ, Todd DW, Renner GJ and Singh A:
Intraoperative radiolymphoscintigraphy for detection of occult
nodal metastasis in patients with head and neck squamous cell
carcinoma. Otolaryngol Head Neck Surg. 122:662–666. PubMed/NCBI
|
|
30
|
Hyde NC, Prvulovich E, Newman L,
Waddington WA, Visvikis D and Ell P: A new approach to
pre-treatment assessment of the N0 neck in oral squamous cell
carcinoma: The role of sentinel node biopsy and positron emission
tomography. Oral Oncol. 39:350–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Day GL and Blot WJ: Second primary tumors
in patients with oral cancer. Cancer. 70:14–19. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Al-Rakan MA, Colak D, Hendrayani SF,
Al-Bakheet A, Al-Mohanna FH, Kaya N, Al-Malik O and Aboussekhra A:
Breast stromal fibroblasts from histologically normal surgical
margins are pro-carcinogenic. J Pathol. 231:457–465. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen SX, Xu XE, Wang XQ, Cui SJ, Xu LL,
Jiang YH, Zhang Y, Yan HB, Zhang Q, Qiao J, et al: Identification
of colonic fibroblast secretomes reveals secretory factors
regulating colon cancer cell proliferation. J Proteomics.
110:155–171. 2014. View Article : Google Scholar : PubMed/NCBI
|