Introduction
Gastric cancer (GCa) is one of the most common types
of malignancy, and the third leading cause of cancer-associated
mortality worldwide, with 951,000 incident cases and 723,000
mortalities in 2012 (1). The overall
5-year survival rate is low due to high recurrence rates, nodal
metastasis and poor responses to chemotherapy (2). Differences in lifestyle, environment and
diet may also have a role in the high incidence and mortality
associated with GCa (3). The aryl
hydrocarbon receptor (AhR) is a ligand-activated transcription
factor involved in cell differentiation and carcinogenesis,
including in lung cancer (4), breast
cancer (5) and prostate cancer
(6). There are currently few studies
involving the AhR pathway and GCa (7). Our previous study demonstrated there is
significant AhR expression in human GCa cells (8), and determined that the AhR agonist
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) could inhibit GCa
cell growth (9). Thus, AhR maybe a
promising target for GCa therapy. Due to the toxic and carcinogenic
effects of TCDD in humans, our previous study had suggested that
the selective AhR receptor modulator 3′3-diindolylmethane (DIM)
could inhibit SGC-7901 human GCa cell proliferation by delaying
cell cycle progression and inducing apoptosis (10). DIM is an acid-catalyzed condensation
product of indole-3-carbinol, a constituent of cruciferous
vegetables (11). DIM has been
identified as an anti-cancer agent involved in various solid
malignancies, including ovarian (12), prostate (13), colon (14) and pancreatic cancer (15). The anti-cancer effects of DIM include
suppressing cancer cell proliferation (16–18) and
promoting cancer cell apoptosis (19–21). There
have been few reports to date regarding the effects of DIM on GCa
cells, and the purpose of the present study was to examine the
potential beneficial effect of DIM in the prevention of tumor
development following the subcutaneous transplantation of SGC-7901
cells into mice, in addition to exploring the possible underlying
mechanisms.
Materials and methods
Cell line and culture
The SGC-7901 Human gastric cancer cell line was
obtained from the Cancer Institute of Chinese Academy of Medical
Science (Beijing, China). SGC-7901 cells were maintained in
RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), 1×105 U/l
penicillin and 0.1 g/l streptomycin. The cells were maintained at
37°C in an atmosphere containing 50 ml/l CO2.
Animal model
A total of 32 female Balb/c nude mice (4 weeks old,
weight, 15–18 g) were purchased from Center of Experiment Animal of
Guangdong (Guangzhou, China). The animals were housed in metal
cages (4 mice/cage) and were kept in a room lit for 12 h per day
and maintained at a temperature of 22±1°C. Diet (purchased from
Center of Experiment Animal of Guangdong, Guangzhou, China) and
sterile water were given ad libitum. The mice were randomly
divided into two groups, receiving castor oil (n=8; control) or DIM
(n=8; 5, 10 or 10 mg/kg/day) respectively. Two weeks later,
SGC-7901 cells (1×106 cells) were inoculated
subcutaneously into the right upper flank of the two groups of mice
and were administered castor oil or DIM for another 6 weeks
continuously. At the end of the experiment, all mice were
sacrificed under general anesthesia by isoflurane and tumor tissues
were weighed prior to collection for western blot and TdT-UTP
nick-end labeling (TUNEL) assay. The body weights of mice were
recorded every 4 days for 8 weeks. The tumor volumes were
calculated using the formula V = a × b2 × π/6, where a
is the length, b is the width and V is the volume in
mm3. In addition, blood samples were collected to
evaluate liver and kidney function. The experiment was conducted in
accordance with the Committee for the Supervision of Animal
Experiments, the First Affiliated Hospital of Sun Yat-sen
University (Guangzhou, China), who approved the study protocol.
Western blot analysis
Tissue was extracted from tumors of the control and
treated mice, and then lysed in buffer (20 mmol/l HEPES, 1 mmol/l
EGTA, 50 mmol/l β-glycerophosphate, 2 mmol/l sodium orthovanadate,
100 ml/l glycerol, 10 ml/l Triton X-100, 1 mmol/l DTT, and 1X
protease inhibitor cocktail; Roche Diagnostics, Mannheim, Germany).
The lysate was centrifuged (18,894 × g) at 4°C for 10 min. The
supernatant was the total cell lysate. Protein concentration was
measured using a BCA protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein (30 µg) was loaded into each lane,
separated by 10% SDS-PAGE and transferred onto an equilibrated
polyvinylidene difluoride membrane via electroblotting. Membranes
were blocked with 5% non-fat milk in 1% TBS-T buffer for 2 h at
room temperature. AhR, cytochrome P450, family 1, subfamily A,
polypeptide 1 (CYP1A1) and GAPDH were detected for 2 h using
antibodies against AhR (#SC-5579; dilution, 1:150; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), CYP1A1 (#AB1258; dilution,
1:500; Chemicon International, Inc., Temecula, CA, USA) and GAPDH
(#2118; dilution, 1:1,000; Cell Signaling Technology, Inc.). After
secondary antibodies (goat anti-rabbit IgG antibody, #7074 and goat
anti-rat IgG antibody, #7077; dilution, 1:2,000; Cell Signaling
Technology, Inc.) incubation for 2 h, protein bands were detected
using an ECL system (Pierce; Thermo Fisher Scientific, Inc.).
Densitometry analysis was performed using Quantity One software
(version 4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
analyzed them using.
TdT-UTP nick end labeling (TUNEL)
assay
The TUNEL assays were performed using the one-step
TUNEL kit (#KGA7072; Nanjing KeyGEN, Inc., Nanjing, China)
according to the manufacturer's instructions. The paraffin-embedded
tissue sections were dewaxed with dimethylbenzene for 15 min at
37°C, dehydrated via an alcohol gradient for 20 min at 37°C, and
then permeabilized using 0.1% Triton X-100 for 8 min on ice,
followed by TUNEL for 1 h at 37°C. The fluorescein
isothiocyanate-labeled TUNEL-positive cells were imaged under a
fluorescent microscope (magnification, ×200) at an excitation
wavelength of 450–500-nm and emission wavelength of 515–565-nm. The
cells with green fluorescence were defined as apoptotic cells. The
percentage of TUNEL-positive cells from images of 10 randomly
selected fields in each group was identified.
Biochemical analysis
All biochemical analyses were performed according to
an automated procedure at the Department of Biochemistry, the First
Affiliated Hospital of Sun Yat-sen University (Guangzhou, China).
Assays of white blood cells (WBC), hemoglobin (HB), platelets (PLT)
in the blood samples were performed using a Sysmex XS-1000i
analyzer (Sysmex Shanghai Ltd., Shanghai, China). Serum glutamic
oxaloacetic transaminase (SGOT), serum glutamic pyruvic
transaminase (SGPT), creatinine and urea were assessed using a
Beckman-Coulter AU5800 analyzer (Beckman Coulter, Inc., Brea, CA,
USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analysis of the data was performed using one-way
analysis of variance and the Student-Newman-Keuls test with the
SPSS statistical software package (version 11.0; SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
DIM activates the AhR pathway
As DIM is a selective AhR receptor modulator, and
our previous study had suggested that DIM could inhibit human GCa
cell (SGC-7901) proliferation by delaying cell cycle progression
and inducing apoptosis, at the end of the experiment the mice were
sacrificed under general anesthesia by isoflurane and the tumor
tissue proteins were evaluated via western blotting to determine
whether the AhR signaling pathway could be activated by DIM. The
results indicated that AhR protein expression gradually decreased
and that the levels of CYP1A1 were increased in a dose-dependent
manner following DIM treatment (Fig.
1).
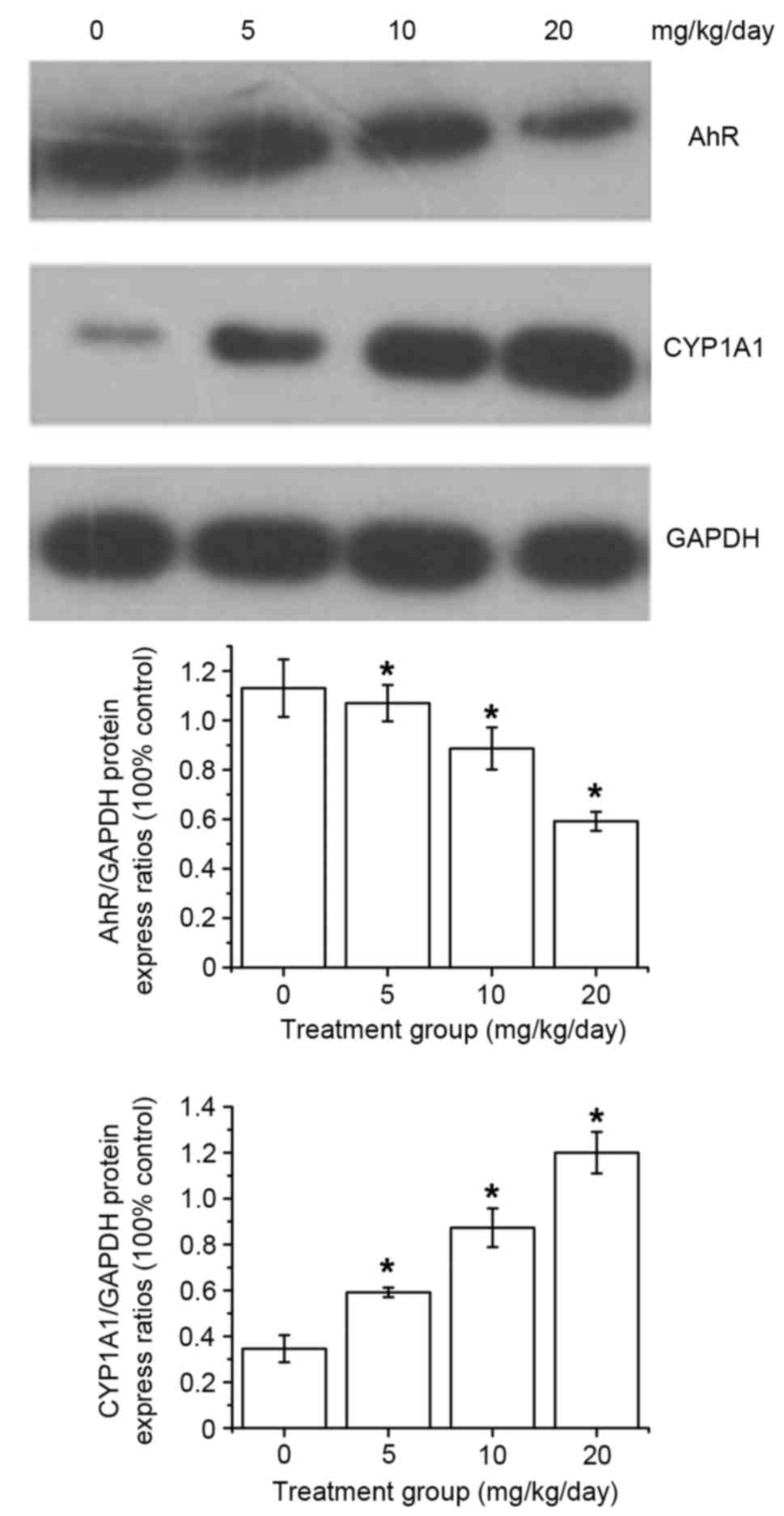 | Figure 1.AhR and CYP1A1 expression in the
SGC-7901 tumors of Balb/c mice following DIM treatment. After DIM
treatment with 0, 5, 10 or 20 mg/kg/day DIM, AhR protein expression
decreased and the levels of CYP1A1 increased dose-dependently.
*P<0.05 in the DIM-treated groups (5, 10 or 20 mg/kg/day) vs.
control group. AhR, aryl hydrocarbon receptor; DIM,
3′3-diindolylmethane; CYP1A1, cytochrome P450, family 1, subfamily
A, polypeptide 1. |
Effect of DIM on tumor
development
The 8 mice in each group were pretreated for 2 weeks
via gavage of three doses (5, 10 and 20 mg/kg/day) of DIM. These
doses were selected according to other published articles (19,22,23). On
week 2, tumors were initiated by transplanting SGC-7901 cells
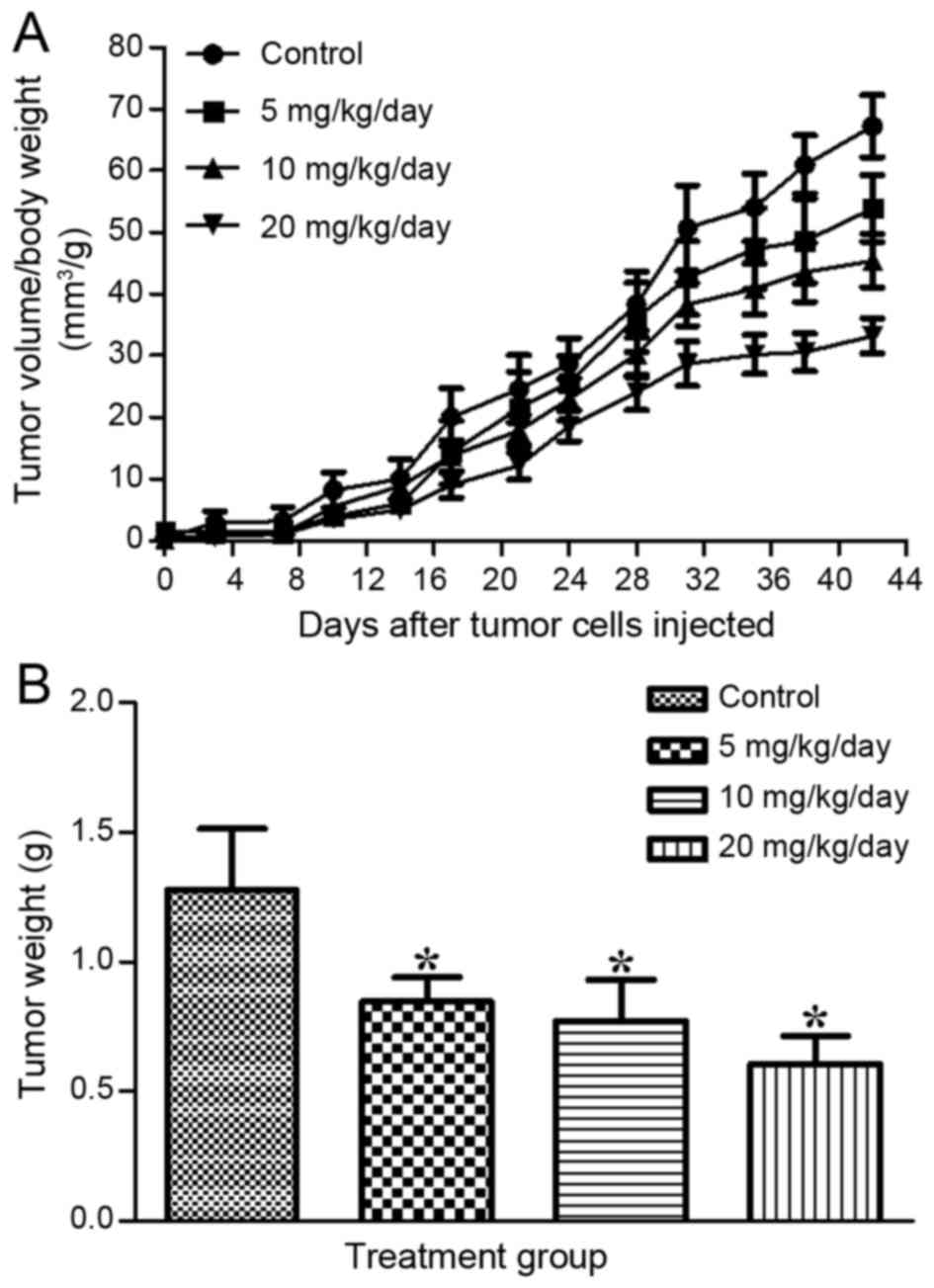
subcutaneously into the flanks of the mice. Graphical
representation of the changes in tumor volume/body weight with time
(Fig. 2A) revealed that the tumor
volume of the DIM-treated groups increased more slowly than that of
the control group. The weights of the tumors presented in the
treated groups were 0.845±0.096, 0.768±0.161 and 0.607±0.106 g for
5, 10 and 20 mg/kg/day, respectively, which was significantly lower
than that observed in the control group (1.275±0.236 g; Fig. 2B). The results indicated that DIM
significantly suppressed tumor development in the mice treated with
higher concentrations.
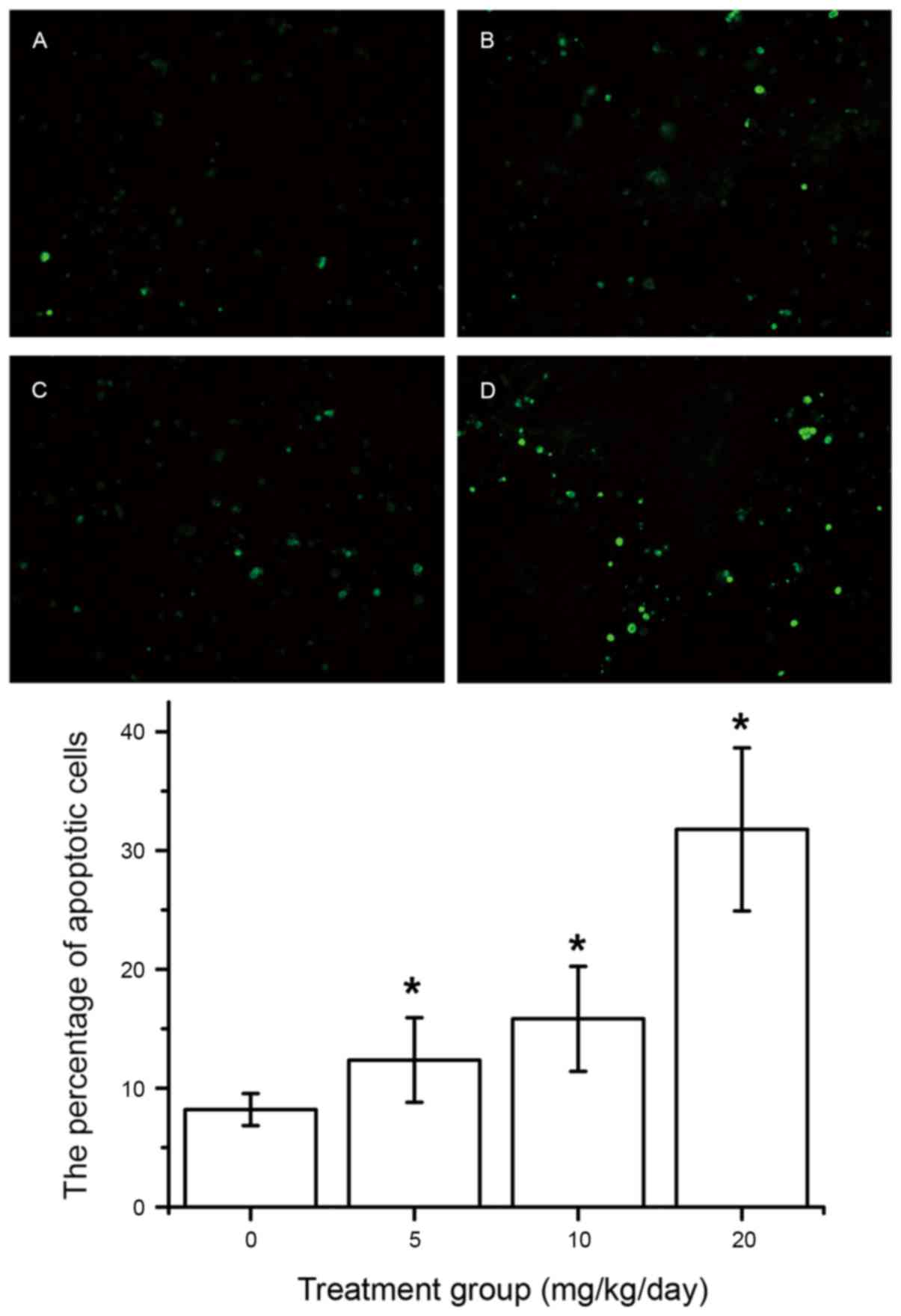
Effect of DIM on cell apoptosis
The mechanism underlying the inhibition of tumor
cell viability was further studied by evaluating the apoptotic
effects of various treatments using a TUNEL assay. It was
identified that DIM can markedly induce apoptosis in SGC-7901 tumor
cells. Additionally, the level of apoptosis among SGC-7901 tumor
cells increases in a dose-dependent manner. The data are presented
in Fig. 3A-E.
Effect of DIM on the weight and
biochemical state of the mice
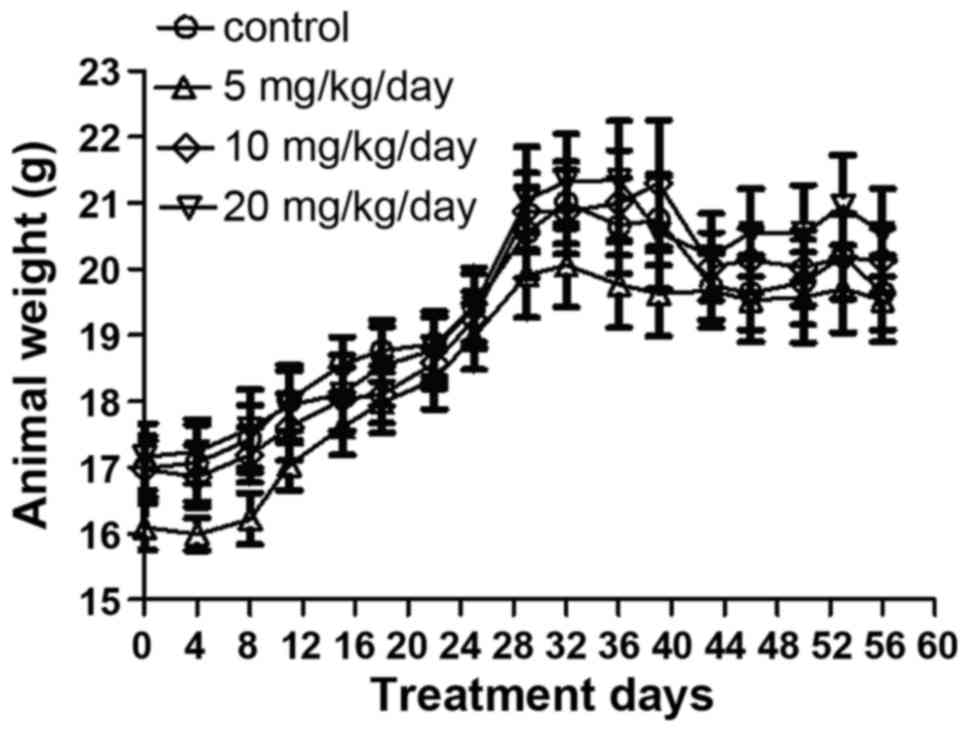
The weights of the mice were measured every 4 days
during the experiment. It was identified that treatment of the mice
with DIM via gavage for 8 weeks had no significant effect on animal
weight (Fig. 4). The data also
indicated that DIM has no notable effect on liver or kidney
function, as determined by quantifying WBC, HB, PLT, SGOT, SGPT,
creatinine and urea in the blood samples (Table I).
 | Table I.Liver and kidney function in the
various groups. |
Table I.
Liver and kidney function in the
various groups.
|
|
| DIM |
|---|
|
|
|
|
|---|
| Test | Control | 5 mg/kg/day | P-value | 10 mg/kg/day | P-value | 20 mg/kg/day | P-value |
|---|
| WBC
(109/l) | 4.25±1.14 | 3.66±1.13 | 0.32 | 3.66±1.11 | 0.31 | 3.39±0.81 | 0.14 |
| Hemoglobin (g/l) | 164.75±8.41 | 159.75±7.55 | 0.23 | 164.38±5.48 | 0.92 | 158.83±8.54 | 0.22 |
| Platelet
(109/l) | 133.50±94.24 | 132.50±115.56 | 0.99 | 250.38±200.11 | 0.16 | 227.00±179.04 | 0.23 |
| SGOT (U/l) | 228.50±60.54 | 246.75±95.48 | 0.69 | 233.00±77.99 | 0.91 | 195.00±20.74 | 0.27 |
| SGPT (U/l) | 92.50±107.46 | 55.00±12.71 | 0.34 | 62.50±6.02 | 0.51 | 52.00±19.43 | 0.43 |
| Urea (mmol/l) | 6.33±0.55 | 7.24±1.96 | 0.30 | 7.35±1.10 | 0.07 | 6.82±0.97 | 0.32 |
| Creatinine
(µmol/l) | 22.50±5.13 | 23.75±7.57 | 0.73 | 25.43±7.41 | 0.43 | 19.00±5.15 | 0.29 |
Discussion
DIM is an anticancer agent present in cruciferous
vegetables. It has been reported that DIM can modulate the
expression of VEGF, IL-8, uPA and MMP-9 through inhibiting the
NF-κB pathway in prostate cancer cells, suggesting an inhibitory
effect on cancer cell proliferation (24). Jin (25)
reported that the growth of MCF-7 breast cancer cells could be
inhibited by downregulating the expression of Cdc25A. Our previous
study determined that DIM activates the AhR pathway and induces the
expression of CYP1A1 in human GCa cells (SGC-7901), which results
in the inhibition cell proliferation, delaying of cell cycle
progression and the induction of cell apoptosis in vitro
(10). Based on this prior study, the
inhibitory effect of DIM was evaluated using the SGC-7901 mouse
model.
Herein, a subcutaneously transplanted SGC-7901 mouse
model was developed. Western blot analysis revealed that AhR
protein was gradually decreased and the protein expression of
CYP1A1 was increased in a dose-dependent manner following DIM
treatment (Fig. 1) (26). As a classic target gene of the AhR
pathway, CYP1A1 was selected as an indicator of AhR signal pathway
activation. In the current study, the expression of CYP1A1 was
significantly increased in a dose-dependent manner following DIM
treatment, indicating AhR pathway activation. It has been
established by numerous prior studies that the AhR is a
ligand-dependent transcription factor and is retained in the
cytoplasm in an inactive form. Upon ligand binding, AhR is presumed
to undergo a conformational change that exposes certain nuclear
localization sequences, resulting in the translocation of the
complex into the nucleus and subsequently activating downstream
gene expression. Consequently, the results suggested that DIM
modulates the AhR pathway and causes the translocation of AhR from
the cytoplasm to the nucleus.
The anticancer effects of DIM include not only the
inhibition of cell proliferation, but also the induction of
apoptosis. It has been verified that DIM can induce apoptosis in
colon (19), breast (20), pancreatic (21,22) and
lung cancer cells (27), among other
types. In prostate cancer cells, DIM inhibits the AKT signaling
pathway and induces cell apoptosis (28). In breast cancer cell lines with high
expression of uPA/uPAR, the anticancer effects of DIM involve the
inhibition of the uPA/uPAR pathway. Furthermore, DIM also induces
apoptosis by modulating the Bcl-2 and IKK/IκB-α/NF-κB pathways in
nasopharyngeal cancer cells. (29).
To further explore the apoptosis-promoting effects of DIM in GCa,
cell apoptosis was assessed using a TUNEL assay. The results
demonstrated that the number of TUNEL-positive cells was
significantly increased in the treated group (DIM 5, 10 or 20
mg/kg/day) compared with in the control group (Fig. 3), suggesting that DIM can promote GCa
cell apoptosis.
Additionally, the volume and weight of the tumors in
the DIM-treated mice were significantly lower than those in the
control group (Fig. 2). The AhR
pathway serves an important role in the proliferation and invasion
of GCa. Our prior study determined that TCDD could also suppress
the growth of GCa cells (9), while
TCDD itself is carcinogenic and toxic. Compared with TCDD, DIM may
be a safer and more potent drug. However, there have been few
studies to date regarding the safety of oral DIM. Roh et al
(30) reported that there may be
gastrointestinal toxicity associated with oral DIM in neonatal
mice, due to it inhibiting the elimination of rotavirus. The
results of the present study indicated that DIM has no toxic effect
on body weight or liver and kidney function in mice (Fig. 4; Table
I).
However, the current study neglected to measure the
actual concentrations of DIM in the blood and tissues of the mice
treated with DIM (0–20 mg/kg/day). Anderton et al (31) developed a PBPK model to characterize
the pharmacokinetic properties of DIM, and the plasma
pharmacokinetics and biodistribution of DIM following oral
administration, in mice (31). Future
studies are required to determine the serum DIM concentrations in
mice fed various doses of DIM. Combined, the data demonstrated that
DIM can promote the apoptosis of GCa cells via the downregulation
of AhR and the increased expression of CYP1A1. Exposure to DIM
produced no observable toxicity with regard to animal weight, liver
or kidney function. Thus, the results indicate that DIM could be a
promising therapeutic drug for gastric cancer in the future.
However, this study was limited to only one cell line, SGC-7901,
and the mechanisms underlying the effects of DIM on GCa cell
apoptosis still require further study.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81072048).
Glossary
Abbreviations
Abbreviations:
|
AhR
|
aryl hydrocarbon receptor
|
|
DIM
|
3′3-diindolylmethane
|
|
GCa
|
gastric cancer
|
|
TCDD
|
2,3,7,8-tetrachlorodibenzo-p-dioxin
|
|
TUNEL
|
TdT-UTP nick-end labeling
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zabaleta J: Multifactorial etiology of
gastric cancer. Methods Mol Biol. 863:411–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su JM, Lin P, Wang CK and Chang H:
Overexpression of cytochrome P450 1B1 in advanced non-small cell
lung cancer: A potential therapeutic target. Anticancer Res.
29:509–515. 2009.PubMed/NCBI
|
|
5
|
Schlezinger JJ, Liu D, Farago M, Seldin
DC, Belguise K, Sonenshein GE and Sherr DH: A role for the aryl
hydrocarbon receptor in mammary gland tumorigenesis. Biol Chem.
387:1175–1187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fritz WA, Lin TM, Safe S, Moore RW and
Peterson RE: The selective aryl hydrocarbon receptor modulator
6-methyl-1,3,8-trichlorodibenzofuran inhibits prostate tumor
metastasis in TRAMP mice. Biochem Pharmacol. 77:1151–1160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin XF, Chen J, Mao W, Wang YH and Chen
MH: A selective aryl hydrocarbon receptor modulator
3,3′-diindolylmethane inhibits gastric cancer cell growth. J Exp
Clin Cancer Res. 31:462012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen J, Röcken C, Klein-Hitpass L, Götze
T, Leodolter A, Malfertheiner P and Ebert MP: Microarray analysis
of gene expression in metastatic gastric cancer cells after
incubation with the methylation inhibitor 5-aza-2′-deoxycytidine.
Clin Exp Metastasis. 21:389–397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng TL, Chen J, Mao W, Liu X, Tao Y, Chen
LZ and Chen MH: Potential therapeutic significance of increased
expression of aryl hydrocarbon receptor in human gastric cancer.
World J Gastroenterol. 15:1719–1729. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin XF, Chen J, Mao W, Wang YH and Chen
MH: A selective aryl hydrocarbon receptor modulator
3,3′-diindolylmethane inhibits gastric cancer cell growth. J Exp
Clin Cancer Res. 31:462012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang YC, Riby J, Chang GH, Peng BC,
Firestone G and Bjeldanes LF: Cytostatic and antiestrogenic effects
of 2-(indol-3-ylmethyl)-3,3′-diindolylmethane, a major in vivo
product of dietary indole-3-carbinol. Biochem Pharmacol.
58:825–834. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loganathan S, Kandala PK, Gupta P and
Srivastava SK: Inhibition of EGFR-AKT axis results in the
suppression of ovarian tumors in vitro and in preclinical mouse
model. PLoS One. 7:e435772012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen D, Banerjee S, Cui QC, Kong D, Sarkar
FH and Dou QP: Activation of AMP-activated protein kinase by
3,3′-diindolylmethane (DIM) is associated with human prostate
cancer cell death in vitro and in vivo. PLoS One. 7:e471862012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pappa G, Strathmann J, Löwinger M, Bartsch
H and Gerhäuser C: Quantitative combination effects between
sulforaphane and 3,3′-diindolylmethane on proliferation of human
colon cancer cells in vitro. Carcinogenesis. 28:1471–1477. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ali S, Banerjee S, Schaffert JM, El-Rayes
BF, Philip PA and Sarkar FH: Concurrent inhibition of NF-kappaB,
cyclooxygenase-2, and epidermal growth factor receptor leads to
greater anti-tumor activity in pancreatic cancer. J Cell Biochem.
110:171–181. 2010.PubMed/NCBI
|
|
16
|
Choi HJ, Lim DY and Park JH: Induction of
G1 and G2/M cell cycle arrests by the dietary compound
3,3′-diindolylmethane in HT-29 human colon cancer cells. BMC
Gastroenterol. 9:392009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tadi K, Chang Y, Ashok BT, Chen Y,
Moscatello A, Schaefer SD, Schantz SP, Policastro AJ, Geliebter J
and Tiwari RK: 3,3′-Diindolylmethane, a cruciferous vegetable
derived synthetic anti-proliferative compound in thyroid disease.
Biochem Biophys Res Commun. 337:1019–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang X, Firestone GL and Bjeldanes LF:
Inhibition of growth factor-induced Ras signaling in vascular
endothelial cells and angiogenesis by 3,3′-diindolylmethane.
Carcinogenesis. 27:541–550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim EJ, Shin M, Park H, Hong JE, Shin HK,
Kim J, Kwon DY and Park JH: Oral administration of
3,3′-diindolylmethane inhibits lung metastasis of 4T1 murine
mammary carcinoma cells in BALB/c mice. J Nutr. 139:2373–2379.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahmad A, Kong D, Wang Z, Sarkar SH,
Banerjee S and Sarkar FH: Down-regulation of uPA and uPAR by
3,3′-diindolylmethane contributes to the inhibition of cell growth
and migration of breast cancer cells. J Cell Biochem. 108:916–925.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Banerjee S, Wang Z, Kong D and Sarkar FH:
3,3′-Diindolylmethane enhances chemosensitivity of multiple
chemotherapeutic agents in pancreatic cancer. Cancer Res.
69:5592–5600. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ali S, Banerjee S, Ahmad A, El-Rayes BF,
Philip PA and Sarkar FH: Apoptosis-inducing effect of erlotinib is
potentiated by 3,3′-diindolylmethane in vitro and in vivo using an
orthotopic model of pancreatic cancer. Mol Cancer Ther.
7:1708–1719. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fares F, Azzam N, Appel B, Fares B and
Stein A: The potential efficacy of 3,3′-diindolylmethane in
prevention of prostate cancer development. Eur J Cancer Prev.
19:199–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong D, Li Y, Wang Z, Banerjee S and
Sarkar FH: Inhibition of angiogenesis and invasion by
3,3′-diindolylmethane is mediated by the nuclear factor-kappaB
downstream target genes MMP-9 and uPA that regulated
bioavailability of vascular endothelial growth factor in prostate
cancer. Cancer Res. 67:3310–3319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jin Y: 3,3′-Diindolylmethane inhibits
breast cancer cell growth via miR-21-mediated Cdc25A degradation.
Mol Cell Biochem. 358:345–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mimura J, Yamashita K, Nakamura K, Morita
M, Takagi TN, Nakao K, Ema M, Sogawa K, Yasuda M, Katsuki M and
Fujii-Kuriyama Y: Loss of teratogenic response to
2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah
(dioxin) receptor. Genes Cells. 2:645–654. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ichite N, Chougule MB, Jackson T, Fulzele
SV, Safe S and Singh M: Enhancement of docetaxel anticancer
activity by a novel diindolylmethane compound in human non-small
cell lung cancer. Clin Cancer Res. 15:543–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Chinni SR and Sarkar FH: Selective
growth regulatory and pro-apoptotic effects of DIM is mediated by
AKT and NF-kappaB pathways in prostate cancer cells. Front Biosci.
10:236–243. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, Zhang J, Shi W and Liu Y: Anticancer
effects of 3,3′-diindolylmethane are associated with G1 arrest and
mitochondria-dependent apoptosis in human nasopharyngeal carcinoma
cells. Oncol Lett. 5:655–662. 2013.PubMed/NCBI
|
|
30
|
Roh YS, Cho A, Islam MR, Cho SD, Kim J,
Kim JH, Lee JW, Lim CW and Kim B: 3,3′-Diindolylmethane induces
immunotoxicity via splenocyte apoptosis in neonatal mice. Toxicol
Lett. 206:218–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anderton MJ, Manson MM, Verschoyle R,
Gescher A, Steward WP, Williams ML and Mager DE: Physiological
modeling of formulated and crystalline 3,3′-diindolylmethane
pharmacokinetics following oral administration in mice. Drug Metab
Dispos. 32:632–638. 2004. View Article : Google Scholar : PubMed/NCBI
|