Introduction
Esophageal carcinoma is the sixth most common cause
of cancer-associated mortality worldwide, with a 5-year survival
rate of 15–25% in 2000 (1). The
incidence and mortality of esophageal cancer are the fifth and
fourth in China, respectively; it is particularly prevalent in
rural areas (2). Esophageal squamous
cell carcinoma (ESCC) is more prevalent than esophageal
adenocarcinoma (EAC); it is the predominant form of esophageal
cancer worldwide and in Asia, with >100 cases per 100,000
population annually in 2000 (3).
Although epidemiological studies indicate that tobacco smoking and
alcohol consumption are the primary behavioral risk factors for
ESCC, not all tobacco and alcohol users ultimately develop ESCC
(4). Genetic risk factors, including
single nucleotide polymorphisms, may also affect the susceptibility
to ESCC (4,5). The methods by which esophageal cancer
typically progresses include direct infiltration, lymphatic
metastasis and hematogenous dissemination. Current treatment
modalities include surgery, radiotherapy, chemotherapy or
combinations of these treatments; however, the 5-year survival rate
of parents with ESCC remains unsatisfactory (3). Identifying novel valid tumor esophageal
cancer biomarkers, that may potentially be used to achieve early
diagnosis, identify therapeutic targets, evaluate the effects of
therapy and predict prognosis, is an emerging field (3). The human immune system aids the
resistance and elimination of tumors and can influence esophageal
carcinogenesis, with the most pivotal antitumor immune response
mediated by T lymphocytes (6,7). Therefore, polymorphisms in immune
response-associated genes and corresponding proteins that regulate
the activation and proliferation of T lymphocytes may contribute to
ESCC pathogenesis (8).
Similar to other members of the T-cell
immunoglobulin and mucin domain-containing protein (TIM) family,
TIM-3 has a structural organization that includes an N-terminal
immunoglobulin V (IgV) domain, amucin domain, a transmembrane
domain and a cytoplasmic tail, which is selectively expressed by
interferon-γ (IFN-γ)-secreting CD4+ T helper 1 (Th1),
CD8+ T cytotoxic 1 (Tc1) T cells and on cells of the
innate immune system (9–12). TIM-3 binds to its ligand galectin-9,
which can induce apoptosis in Th1 cells to downregulate effector
Th1/Tc1 cell responses and suppress, as well as induce, peripheral
tolerance (13,14). Monoclonal antibodies can block the
immune checkpoint pathway, which acts on T-cell inhibitory or
immune checkpoint receptors; immunotherapy has demonstrated as an
effective strategy for the treatment of cancer (15). TIM-3 is expressed by the majority of
suppressed or dysfunctional T cell populations, including
CD8+ T cells and Tregs, which exert key roles in
immunosuppression in tumor tissue based on preclinical trials
(16–18). Evidence indicates that TIM-3
represents a critical immune checkpoint in various types of
malignancy (16–19). Compared with the cytotoxic T
lymphocyte antigen or programmed cell death-1, which are expressed
on all T cells, TIM-3 is selectively expressed on IFN-γ-producing T
cells and is likely to be expressed primarily by intratumoral T
cells in patients with cancer (20–22).
Therefore, targeting TIM-3 could reduce the risk of adverse
autoimmune-like toxicity (22). In
addition, it has been demonstrated that the knockdown of TIM-3 in
tumor cells can weaken their proliferative and invasive ability
(23). Evidence indicates that TIM-3
may have a direct role in the occurrence and progression of tumors
as it has been observed on tumor cells; it may accelerate
oncogenesis, proliferation and invasion of the tumor cells directly
or act via immune inhibition (23,24).
The inhibitory capacity of TIM-3 was previously
assessed by identifying the effect of the blockade of the
interaction of TIM-3 with one or more of its ligands (25). Of these ligands, TIM-3 deficiency
decreased the galectin-9-mediated apoptosis of Th1 cells by ~40%
(26), which indicates that
galectin-9 has multiple target molecules in addition to TIM-3
(14). Further evidence revealed that
galectin-9 acts independently of TIM-3 in influencing T cell
function (27). Conversely, another
study refuted this, reporting thatgalectin-9 and TIM-3 may both
serve roles in regulating T cell responses (28). Galectin-9 is widely expressed on the
surface of cells and in the cytoplasm, and may interact with TIM-3
by binding to ligosaccharides present on the TIM-3 IgV domain.
IFN-γ can promote the expression of galectin-9. Previous research
demonstrated that the rate of cell death in TIM-3+ Th1
cells decreased and caused a consequent decline in IFN-γ production
when galectin-9 was bound by TIM-3. Thus, the TIM-3-galectin-9
pathway has an inhibitory effect on T cells (14,29).
Blockade of the TIM-3-galectin-9 signal pathway may
additionally interact with the external mechanism of interference
of T cells in immunosuppression. However, it has yet to be
identified whether TIM-3 and galectin-9 are expressed in ESCC
tumors and their roles in ESCC. The present study aimed to
investigate the association between the expression level of TIM-3
and galectin-9 with a range of clinicopathological characteristics
and patient survival time in ESCC patients, in order to investigate
the potential prognostic significance of TIM-3 and galectin-9 in
ECSS in Chinese patients.
Materials and methods
Clinical samples
Postoperative pathological specimens from 45
patients diagnosed with ESCC who received surgery at the Affiliated
Tumor Hospital of Zhengzhou University (Zhengzhou, China) between
January 2008 and December 2009 were included the present study. All
clinicopathological data was gathered by reviewing medical records
and conducting telephone interviews. No patients received
radiotherapy or chemotherapy prior to curative resection, from
which samples were obtained. Following surgery, patients received
postoperative adjuvant therapies and follow-ups regularly in line
with National Comprehensive Cancer Network (NCCN) guidelines
(30). Patient age ranged from 46–72
years (median, 58 years). The basic information of the 45 ESCC
patients is presented in Table I.
Tumor pathological specimens were used in the immunohistochemical
(IHC) assays. All patients agreed to inclusion in the present
study, providing written informed consent. The present study was
also approved by the ethics committee of the Affiliated Tumor
Hospital of Zhengzhou University.
 | Table I.Patient characteristics, and their
association with TIM-3 or galectin-9 expression. |
Table I.
Patient characteristics, and their
association with TIM-3 or galectin-9 expression.
|
|
| TIM-3, n |
|
| Galectin-9, n |
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Clinical
features | Patients, n
(%) | HEG | LEG | χ2 | P-value | HEG | LEG | χ2 | P-value |
|---|
| Total | 45 | 10 | 35 |
|
| 7 | 38 |
|
|
| Sex |
|
|
| 0.495 | 0.482 |
|
| 0.000 | 0.984 |
|
Female | 13 (28.89) | 2 | 11 |
|
| 2 | 11 |
|
|
|
Male | 32 (71.11) | 8 | 24 |
|
| 5 | 27 |
|
|
| Age, years |
|
|
| 0.319 | 0.572 |
|
| 2.899 | 0.089 |
|
<60 | 26 (57.78) | 5 | 21 |
|
| 2 | 24 |
|
|
|
≥60 | 19 (42.22) | 5 | 14 |
|
| 5 | 14 |
|
|
| Tumor size, cm |
|
|
| 3.401 | 0.065 |
|
| 2.445 | 0.118 |
|
<4 | 20 (44.44) | 7 | 13 |
|
| 5 | 15 |
|
|
| ≥4 | 25 (55.56) | 3 | 22 |
|
| 2 | 23 |
|
|
| Lymph node
metastasis |
|
|
| 0.294 | 0.588 |
|
| 0.534 | 0.465 |
|
Yes | 14 (31.11) | 4 | 10 |
|
| 3 | 11 |
|
|
| No | 31 (68.89) | 6 | 25 |
|
| 4 | 27 |
|
|
| Depth of
invasion |
|
|
| 1.086 | 0.297 |
|
| 0.846 | 0.358 |
|
TI+T2 | 20 (44.44) | 3 | 17 |
|
| 2 | 18 |
|
|
| T3 | 25 (55.56) | 7 | 18 |
|
| 5 | 20 |
|
|
| TNM stage |
|
|
| 1.684 | 0.194 |
|
| 1.522 | 0.217 |
| II | 34 (75.56) | 6 | 28 |
|
| 4 | 30 |
|
|
|
III | 11 (24.44) | 4 | 7 |
|
| 3 | 8 |
|
|
| Tumor location |
|
|
|
|
|
|
|
|
|
| Upper
thoracic | 11 (24.44) | 0 | 11 | 4.211 | 0.122 | 0 | 11 | 2.749 | 0.112 |
| Middle
thoracic | 30 (66.67) | 9 | 21 |
|
| 6 | 24 |
|
|
| Lower
thoracic | 4 (8.89) | 1 | 3 |
|
| 1 | 3 |
|
|
| Pathological
type |
|
|
| 0.817 | 0.366 |
|
| 1.322 | 0.250 |
|
Ulcerated | 28 (62.22) | 5 | 23 |
|
| 3 | 25 |
|
|
|
Non-ulcerated | 17 (37.78) | 5 | 12 |
|
| 4 | 13 |
|
|
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
Poor | 14 (31.11) | 3 | 11 | 0.030 | 0.985 | 1 | 13 | 1.097 | 0.578 |
|
Middle | 26 (57.78) | 6 | 20 |
|
| 5 | 21 |
|
|
|
Well | 5 (11.11) | 1 | 4 |
|
| 1 | 4 |
|
|
| Smoking
history |
|
|
| 1.435 | 0.231 |
|
| 0.048 | 0.826 |
| No | 21 (46.67) | 3 | 18 |
|
| 3 | 18 |
|
|
|
Yes | 24 (53.33) | 7 | 17 |
|
| 4 | 20 |
|
|
| Family history |
|
|
| 1.029 | 0.310 |
|
| 0.085 | 0.771 |
| No | 40 (88.89) | 8 | 32 |
|
| 6 | 34 |
|
|
|
Yes | 5 (11.11) | 2 | 3 |
|
| 1 | 4 |
|
|
Follow-up
To determine the condition of each patient, medical
records were reviewed or telephone interviews conducted every 3
months for the first year, every 6 months for the second year and
annually thereafter. The primary end point was patient mortality,
from which overall survival (OS) time was calculated, defined as
the time from the date of resection until death; the secondary
endpoint was the last follow-up.
IHC staining
Formalin-fixed, paraffin-embedded tissue samples
were cut into sections of 4-µm thickness, heated at 60°C overnight
and then deparaffinized and rehydrated using a graded alcohol
series: xylene I-xylene II-absolute ethanol I-absolute ethanol
II-95% ethanol-90% ethanol-80% ethanol-70% ethanol. Sections were
then boiled for 30 sec in 10 mmol/l sodium citrate buffer (pH 6) in
a high-pressure boiler. Following the blocking of the endogenous
peroxidase activity with 3% hydrogen peroxide for 10 min at room
temperature, slides were washed with 0.01 mol/lPBS for 3 min.
Following incubation with the primary human galectin-9 polyclonal
antibody (dilution, 1:250; ab69630; Abcam, Cambridge UK) and
Tim-3-phycoerythrin (dilution, 1:200; 565560; BD Biosciences,
Franklin Lakes, NJ, USA), respectively, for 1 h at room
temperature, biotinylated goat anti-rabbit immunoglobulin (Ig)G
(1:200; cat. no. NB7183; Novus Biologicals, LLC, Littleton, CO,
USA) was added for 30 min at 37°C. Then the slides were washed with
PBS, and counterstained with a diaminobenzidine peroxidase
substrate kit (Gene Tech Biotechnology Co., Ltd., Shanghai, China).
Hematoxylin and eosin staining was used to evaluate
histopathological expression for 1 min at room temperature.
Evaluation of IHC results
The expression of TIM-3 and galectin-9 were
evaluated with an optical microscope by two independent
pathologists who were unfamiliar with the clinical data of patients
and images were captured. To calculate the expression intensity of
TIM-3 and galectin-9, representative areas of the stained regions
were selected under low-power microscopy (magnification, ×100), and
high-power microscopy (magnification, ×400) was used to count TIM-3
and galectin-9 positive cells in 5 fields of view using a Leica DMI
4000B inverted research microscope (Leica Microsystems GmbH,
Wetzlar, Germany). As negative controls, the tumor slices were
treated with distilled water instead of primary antibody and
horseradish-conjugated goat anti-rabbit IgG (1:200; NB7183; Novus
Biologicals, LLC) was used instead of the secondary antibody for 30
min at 37°C; all negative controls showed negligible background
staining. The percentage of TIM-3-positive and
galectin-9-positivecells in ESCC samples were counted and the
immunostaining intensity quantified. According to the
semi-quantitative HSCORE system (score=stain intensity × mean
percentage of stained cells), the stain intensity was ranked into 4
grades: No staining=−, weak staining=+1, moderate staining=+2 and
strong staining=+3.0 were pointed as negative (−), 1 as weakly
positive (+), 2 as moderately positive (+ +), ≥3 as strong positive
(+ + +). Those≥3 points were considered to be positive, the rest
were considered to demonstrate negative expression. The percentage
of stained cells was identified concurrently. If the final results
from the two pathologists were not consistent, specimens were
reviewed together until a consensus was agreed upon.
Statistical analysis
SPSS 19.0 statistical software (IBM Corp., Armonk,
NY, USA) was used to analyze all statistical data. χ2
test and Spearman's rank correlation coefficient were used to
analyze differences in classified variables between groups and the
correlation between the expression level of TIM-3 and galectin-9,
respectively. The OS rate was assessed using the Kaplan-Meier
method; survival differences were evaluated using the log-rank
test. Univariate and multivariate analyses of prognostic factors
for survival were performed using Cox's proportional hazards model.
P<0.05 was considered to indicate a statistically significant
difference.
Results
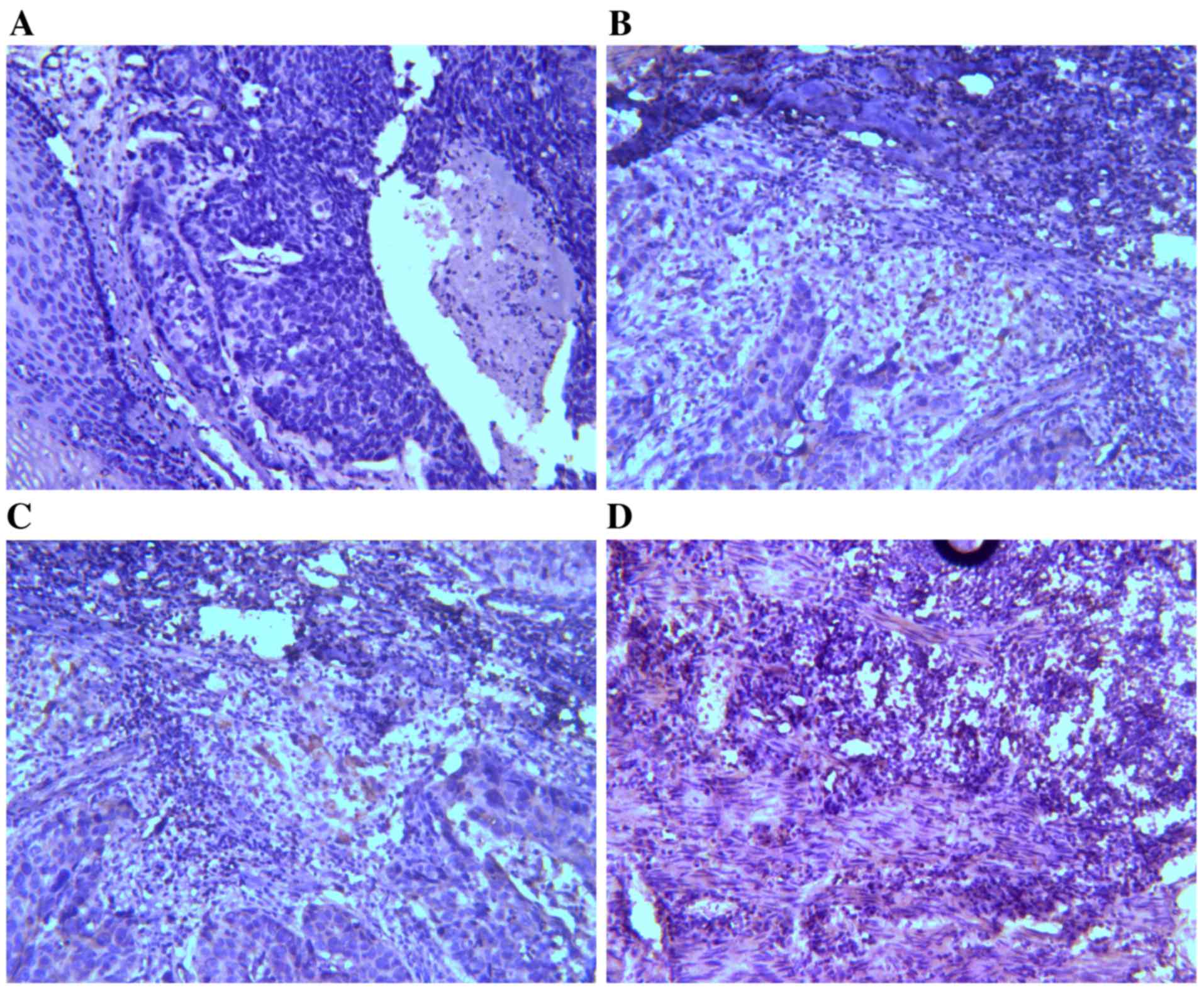
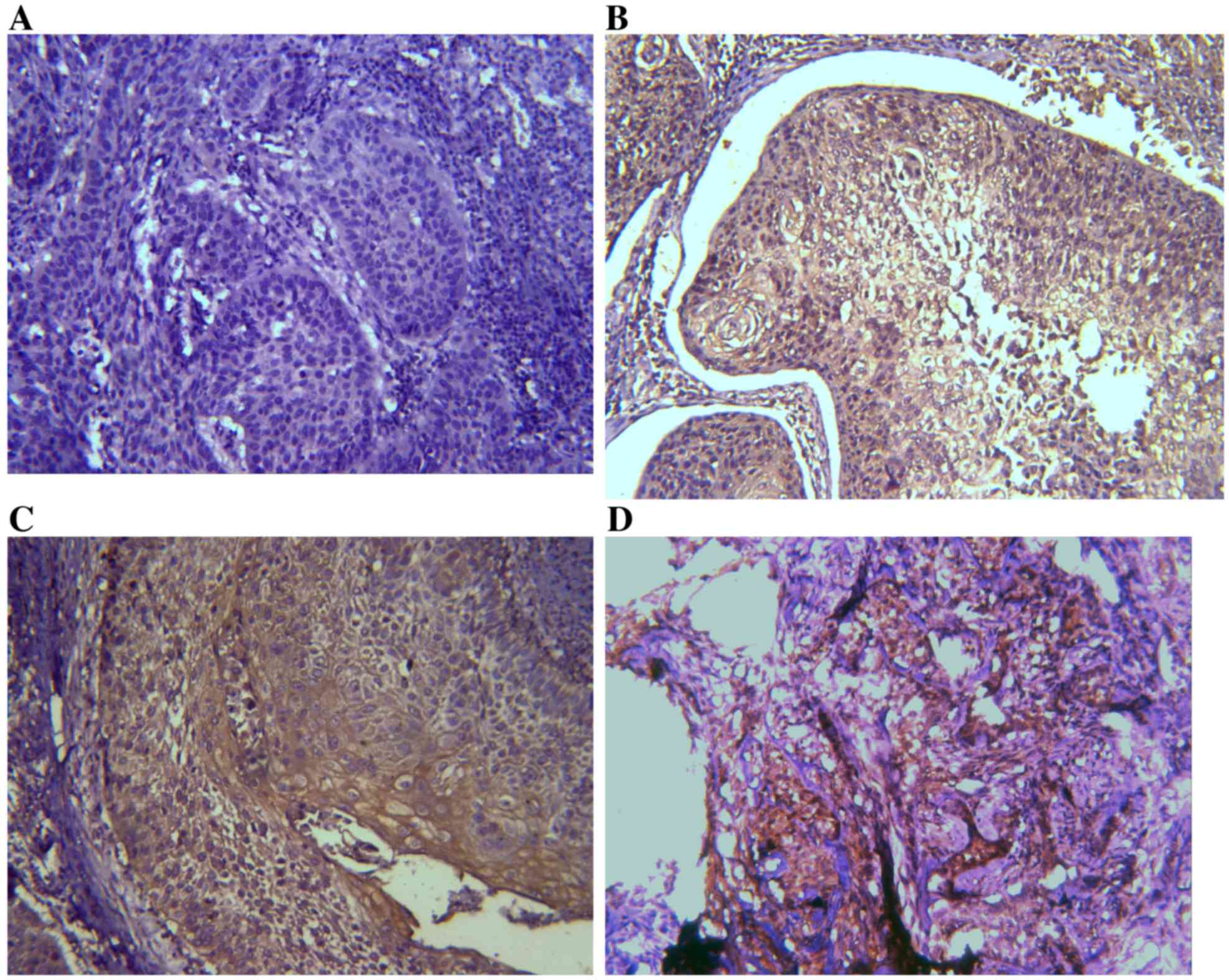
IHC results of TIM-3 and galectin-9
expression in ESCC tissues
Expression levels of TIM-3 and galectin-9 were
investigated by IHC analysis. TIM-3 and galectin-9 are expressed in
a variety of cells in the immune system and have also been
identified in certain types of tumor (24). It was assessed whether TIM-3 and
galectin-9 are also expressed in ESCC tissues (Figs. 1 and 2).
On the basis of the positive expression intensity of cancer cells,
the protein levels of TIM-3 and galectin-9 were quantified. Of the
45 cases, TIM-3 protein levels were of +3 staining intensity (TIM-3
high-expression group; TIM-3-HEG) in 10 cases (22.22%) and 35 cases
(77.78%) were of lower staining intensity (TIM-3 low-expression
group; TIM-3-LEG), of which 11 cases were +2, 23 cases were +1 and
1 case was 0. Concerning galectin-9 expression levels, 7 cases
(15.56%) were of +3 staining intensity (galectin-9-HEG) and 38
cases (84.44%) were of lower staining intensity (galectin-9-LEG),
including 14 cases that were +2, 13 cases that were +1 and 11 cases
that were 0.
Association between TIM-3 and
galectin-9 expression, and clinicopathological factors
The association of TIM-3 or galectin-9 expression
with a variety of associated clinicopathological factors
influencing the prognosis in ESCC was investigated. The outcomes
are included in Table I. There were
no significant statistical differences in age between the HEGs and
LEGs for TIM-3 and galectin-9. Additionally, other clinical
pathological characteristics, including tumor differentiation,
Tumor-Node-Metastasis (TNM) stage (the seventh edition of AJCCTNM
staging of esophageal cancer) (31),
lymph node metastasis status and tumor size, were also not
significantly associated with TIM-3 and galectin-9.
Rank correlation between TIM-3 and
galectin-9 expression
Of the 45 ESCC specimens, 3 were TIM-3-HEG and
galectin-9-HEG, 7 were TIM-3-HEG and galectin-9-LEG, 4 were
TIM-3-LEG and galectin-9-HEG and 31 were TIM-3-LEG and
galectin-9-LEG. Through a Spearman's rank correlation test, it was
determined that expression of the two proteins exhibited a
significant negative correlation (R=−0.71, P<0.001). The results
of this analysis are included in Table
II.
 | Table II.Correlation between the expression of
TIM-3 and galectin-9 protein in esophageal squamous cell
carcinoma. |
Table II.
Correlation between the expression of
TIM-3 and galectin-9 protein in esophageal squamous cell
carcinoma.
|
| Expression of
galectin-9 |
|
|
|---|
|
|
|
|
|
|---|
| Expression of
TIM-3 | HEG | LEG | R-value | P-value |
|---|
| HEG | 3 | 7 | −0.71 | <0.001 |
| LEG | 4 | 31 |
|
|
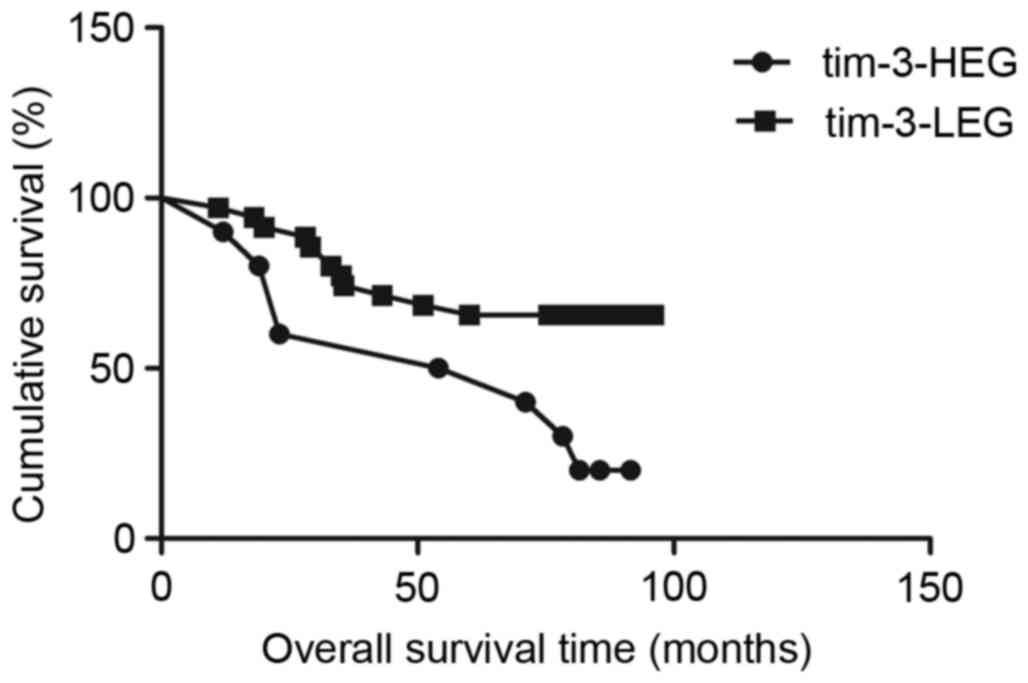
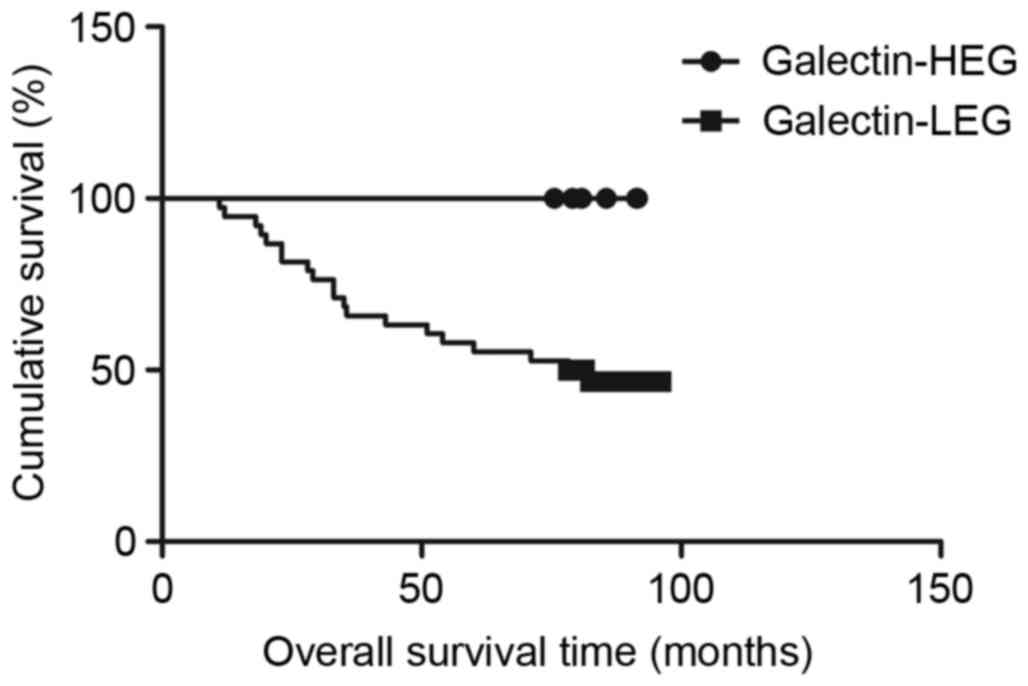
Association between TIM-3, galectin-9
and disease-specific survival time
Survival analysis with the Kaplan-Meier method
revealed that patients in the TIM-3-HEG attained a significantly
shorter disease-specific survival time than those in the TIM-3-LEG
(χ2=6.049, P=0.0139; Fig.
3); however, patients in the galectin-9-HEG exhibited a
significantly higher disease-specific survival time relative to
those in the galectin-9-LEG (χ2=4.915, P=0.0266;
Fig. 4). To assess the effect of
clinical pathological characteristics on the survival time for
patients with ESCC, a univariate analysis was conducted. As
included in Table III, TNM stage
was a significant risk factor for patients with ESCC (P<0.001).
To study the independent factors affecting the prognosis among all
the clinicopathological characteristics, Cox's proportional
regression analysis was used (Table
IV), which revealed that TIM-3 and galectin-9 expression were
not independent factors affecting patient prognosis (TIM-3
expression hazard ratio, 1.102; 95% confidence interval,
0.292–4.157; P=0.886 and galectin-9 expression hazard ratio, 0.905;
95% confidence interval, 0.189–4.322; P=0.900).
 | Table III.Univariate analysis of the
association of prognosis among clinicopatholocal parameters, TIM-3,
galectin-9 in patients with esophageal squamous cell carcinoma. |
Table III.
Univariate analysis of the
association of prognosis among clinicopatholocal parameters, TIM-3,
galectin-9 in patients with esophageal squamous cell carcinoma.
| Parameters | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Sex (female vs.
male) | 0.837 | 0.303–2.306 | 0.730 |
| Age (≤60 vs. >60
years) | 1.423 | 0.517–3.917 | 0.495 |
| Tumor size (≥5 vs.
<5 cm) | 0.360 | 0.116–1.121 | 0.078 |
| Lymph node
metastasis (yes vs. no) | 0.458 | 0.170–1.234 | 0.123 |
| Depth of invasion
(TI/2 vs. T3) | 0.354 | 0.114–1.100 | 0.734 |
| TNM stage (II vs.
III) | 0.179 | 0.066–0.485 | 0.001 |
| Tumor
location(upper/lower vs. middle) | 0.048 |
0.000–23,863.841 | 0.649 |
| Pathological type
(ulcerated vs. non-ulcerated) | 1.347 | 0.468–3.882 | 0.581 |
| Differentiation
(poor vs. moderate/well) | 1.629 | 0.591–4.488 | 0.345 |
| Smoking history
(yes vs. no) | 1.158 | 0.434–3.088 | 0.769 |
| Family history (yes
vs. no) | 25.568 | 0.57–11453.023 | 0.298 |
| TIM-3 expression
(HEG vs. LEG) | 2.517 | 0.913–6.937 | 0.074 |
| Galectin-9
expression (HEG vs. LEG) | 1.816 | 0.585–5.639 | 0.302 |
 | Table IV.Multivariate analysis of the
association of prognosis with clinicopathological parameters, and
TIM-3 and galectin-9 expression, in patients with esophageal
squamous cell carcinoma. |
Table IV.
Multivariate analysis of the
association of prognosis with clinicopathological parameters, and
TIM-3 and galectin-9 expression, in patients with esophageal
squamous cell carcinoma.
| Parameters | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Tumor size (≥5 vs.
<5 cm) | 0.592 | 0.169–2.067 | 0.411 |
| TNM stage (II vs.
III) | 0.148 | 0.022–1.007 | 0.051 |
| Lymph node
metastasis (yes vs. no) | 1.625 | 0.257–10.284 | 0.051 |
| Depth of invasion
(TI/2 vs. T3) | 0.803 | 0.201–3.206 | 0.756 |
| Pathological type
(ulcerated vs. non-ulcerated) | 1.670 | 0.522–5.345 | 0.387 |
| Differentiation
(poor vs. moderate/well) | 1.094 | 0.307–3.900 | 0.890 |
| TIM-3 expression
(HEG vs. LEG) | 1.102 | 0.292–4.157 | 0.886 |
| Galectin-9
expression (HEG vs. LEG) | 0.905 | 0.189–4.322 | 0.900 |
Discussion
The results of the present study revealed that the
expression levels of TIM-3 and galectin-9 ranged from low to high
among different specimens of ESCC tumors. The disease-specific
survival rate differed significantly between TIM-3-LEG and HEG, and
similarly between galectin-9-LEG and HEG.
Although there no association was observed between
TIM-3 or galectin-9 expression and clinicopathological parameters
associated with the prognosis of patients with ESCC, including the
TNM stage, lymph node metastasis, tumor size, depth of invasion,
pathological type, differentiation and patient age, the
disease-specific survival time differed significantly depending on
the expression levels of TIM-3 and galectin-9. The results of the
present study are in accord with previous similar studies in breast
cancer and gastric cancer (24,32). A
study by Cao et al (23)
revealed that the migratory and invasive ability of HeLa cells was
markedly reduced by the knockdown of TIM-3 expression,
demonstrating that TIM-3 expression may be associated with tumor
metastasis. TIM-3 has also been demonstrated to exert a direct role
in promoting the occurrence and development of certain types of
tumor, including cervical, gastric and colon cancer (23,24,33). The
results of the present study were not consistent with the previous
reports. A role for TIM-3 in ESCC was not explicitly identified,
which may be associated with the small sample of TIM-3-HEG
tumors.
All patients obtained further treatment following
surgery, as per NCCN guidelines. Therefore, TIM-3 and galectin-9
could represent novel molecular markers to forecast the response of
patients to ESCC treatments. In recent years, studies have aimed to
identify potential ESCC risk factors and strategies to interfere
with them, and at the time of writing, few markers have been
identified that maybe used as predictors of ESCC patient prognosis,
including interleukin 6 (34), early
mitotic inhibitor-1 (35) and X-ray
repair cross complementing 5 (36).
However, the pathogenesis of ESCC cannot be adequately predicted
using the current criteria for risk factors (37,38). It is
therefore necessary to search for more accurate and specific
indexes to evaluate the occurrence, development or prognosis of
ESCC.
Previous studies have identified that TIM-3 and
galectin-9 expression levels may be associated with patient
prognosis in other types of human cancer (23,24,32,33),
but the association between them and the prognosis of ESCC is not
clear. Therefore, the purpose of the present study was to assess
whether TIM-3 and galectin-9 were suitable as prognostic molecular
markers for ESCC. In this experiment, it was concluded that
TIM-3-HEG andgalectin-9-LEG were associated with shorter
disease-specific survival times for Chinese patients with ESCC.
TIM-3-HEG is a poor prognostic factor for ESCC, which is consistent
with the review by Anderson (22).
To conclude, the present study identified that the
expression levels of TIM-3 and galectin-9 varied in ESCC, and that
the high expression of TIM-3 or the low expression of galectin-9
may indicate a poor prognosis for patients with ESCC. The data of
the present study indicated that TIM-3 and galectin-9 may be
suitable for development as novel biomarkers in ESCC.
Acknowledgements
The authors would like to thank Dr Quanli Gao
(Department of Biological Immunotherapy, Henan Cancer Hospital,
Zhengzhou, China), for his professional guidance.
References
|
1
|
Domper Arnal MJ, Ferrández Arenas Á and
Lanas Arbeloa Á: Esophageal cancer: Risk factors, screening and
endoscopic treatment in Western and Eastern countries. World J
Gastroenterol. 21:7933–7943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Napier KJ, Scheerer M and Misra S:
Esophageal cancer: A review of epidemiology, pathogenesis, staging
workup and treatment modalities. World J Gastrointest Oncol.
6:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen W, Zheng R, Zhang S, Zhao P, Li G, Wu
L and He J: The incidences and mortalities of major cancers in
China, 2009. Chin J Cancer. 32:106–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xie J, Wang J, Cheng Sh, Zheng L, Ji F,
Yang L, Zhang Y and Ji H: Expression of immune checkpoints in T
cells of esophageal cancer patients. Oncotarget. 27:63669–63678.
2016. View Article : Google Scholar
|
|
7
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qiu H, Zheng L, Tang W, Yin P, Cheng F and
Wang L: Programmed death-1 (PD-1) polymorphisms in Chinese patients
with esophageal cancer. Clin Biochem. 47:612–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monney L, Sabatos CA, Gaglia JL, Ryu A,
Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel
RA, et al: Th1-specific cell surface protein Tim-3 regulates
macrophage activation and severity of an autoimmune disease.
Nature. 415:536–541. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao E, Zang X, Ramagopal UA, Mukhopadhaya
A, Fedorov A, Fedorov E, Zencheck WD, Lary JW, Cole JL, Deng H, et
al: T cell immunoglobulin mucin-3 crystal structure reveals a
galectin-9-independent ligand-binding surface. Immunity.
26:311–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santiago C, Ballesteros A, Martínez-Muñoz
L, Mellado M, Kaplan GG, Freeman GJ and Casasnovas JM: Structures
of T cell immunoglobulin mucin protein 4 show a metal-Ion-dependent
ligand binding site where phosphatidylserine binds. Immunity.
27:941–951. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Santiago C, Ballesteros A, Tami C,
Martínez-Muñoz L, Kaplan GG and Casasnovas JM: Structures of T Cell
immunoglobulin mucin receptors 1 and 2 reveal mechanisms for
regulation of immune responses by the TIM receptor family.
Immunity. 26:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu B, Yuan L, Gao Q, Yuan P, Zhao P, Yuan
H, Fan H, Li T, Qin P, Han L, et al: Circulating and
tumor-infiltrating Tim-3 in patients with colorectal cancer.
Oncotarget. 6:20592–20603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freeman GJ, Casasnovas JM, Umetsu DT and
DeKruyff RH: TIM genes: A family of cell surface phosphatidylserine
receptors that regulate innate and adaptive immunity. Immunol Rev.
235:172–189. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nicodemus CF: Antibody-based immunotherapy
of solid cancers: Progress and possibilities. Immunotherapy.
7:923–939. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Q, Munger ME, Veenstra RG, Weigel BJ,
Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK
and Blazar BR: Coexpression of Tim-3 and PD-1 identifies a CD8+
T-cell exhaustion phenotype in mice with disseminated acute
myelogenous leukemia. Blood. 117:4501–4510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fourcade J, Sun Z, Benallaoua M, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V and Zarour HM:
Upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+ T cell dysfunction in melanoma patients. J
Exp Med. 207:2175–2186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakuishi K, Ngiow SF, Sullivan JM, Teng
MW, Kuchroo VK, Smyth MJ and Anderson AC: TIM3+FOXP3+ regulatory T
cells are tissue-specific promoters of T-cell dysfunction in
cancer. Oncoimmunology. 2:e238492013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tarhini A: Immune-mediated adverse events
associated with ipilimumab ctla-4 blockade therapy: The underlying
mechanisms and clinical management. Scientifica (Cairo).
2013:8575192013.PubMed/NCBI
|
|
21
|
Hamid O, Robert C, Daud A, Hodi FS, Hwu
WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al:
Safety and tumor responses with lambrolizumab (anti-PD-1) in
melanoma. N Engl J Med. 369:134–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Anderson AC: Tim-3: An emerging target in
the cancer immunotherapy landscape. Cancer Immunol Res. 2:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao Y, Zhou X, Huang X, Li Q, Gao L, Jiang
L, Huang M and Zhou J: Tim-3 expression in cervical cancer promotes
tumor metastasis. PLoS One. 8:e538342013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang J, Jin MS, Kong F, Cao D, Ma HX, Jia
Z, Wang YP, Suo J and Cao X: Decreased galectin-9 and increased
Tim-3 expression are related to poor prognosis in gastric cancer.
PLoS One. 8:e817992013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferris RL, Lu B and Kane LP: Too much of a
good thing? Tim-3 and TCR signaling in T cell exhaustion. J
Immunol. 193:1525–1530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su EW, Bi S and Kane LP: Galectin-9
regulates T helper cell function independently of Tim-3.
Glycobiology. 21:1258–1265. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leitner J, Rieger A, Pickl WF, Zlabinger
G, Grabmeier-Pfistershammer K and Steinberger P: TIM-3 does not act
as a receptor for galectin-9. PLoS Pathog. 9:e10032532013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakuishi K, Jayaraman P, Behar SM,
Anderson AC and Kuchroo VK: Emerging Tim-3 functions in
antimicrobial and tumor immunity. Trends Immunol. 32:345–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
National Comprehensive Cancer Network:
(NCCN) Clinical Practice Guidelines in Oncology. Esophageal Cancer.
Version 1. 2009, https://www.nccn.org/professionals/physician_gls/f_guidelines.asp
|
|
31
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union Against Cancer (eds): Title
section/chapterTNM Classification of Malignant Tumours. 7th
edition. Wiley-Blackwell; Hoboken, NJ: pp. 4552009
|
|
32
|
Irie A, Yamauchi A, Kontani K, Kihara M,
Liu D, Shirato Y, Seki M, Nishi N, Nakamura T, Yokomise H and
Hirashima M: Galectin-9 as a prognostic factor with antimetastatic
potential in breast cancer. Clin Cancer Res. 11:2962–2968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou E, Huang Q, Wang J, Fang C, Yang L,
Zhu M, Chen J, Chen L and Dong M: Up-regulation of Tim-3 is
associated with poor prognosis of patients with colon cancer. Int J
Clin Exp Pathol. 8:8018–8027. 2015.PubMed/NCBI
|
|
34
|
Zhao ZF, Li JX, Ye R, Wu X, Gao LL and Niu
BL: Interleukin-6 as a potential molecular target in esophageal
squamous cell carcinoma. Oncol Lett. 11:925–932. 2016.PubMed/NCBI
|
|
35
|
Guan C, Zhang J, Zhang J, Shi H and Ni R:
Enhanced expression of early mitotic inhibitor-1 predicts a poor
prognosis in esophageal squamous cell carcinoma patients. Oncol
Lett. 12:114–120. 2016.PubMed/NCBI
|
|
36
|
Wang S, Wang Z, Yang YU, Shi MO and Sun Z:
Overexpression of Ku80 correlates with aggressive
clinicopathological features and adverse prognosis in esophageal
squamous cell carcinoma. Oncol Lett. 10:2705–2712. 2015.PubMed/NCBI
|
|
37
|
Feng Z, Xiang-Lei L, Hai-Tao W, Zuo-Pei W,
Bao-Li H, Hai-Feng Z, Xiao-Long W and Li L: Programmed cell death 1
expression in esophageal squamous cell carcinoma and association
with clinical characteristics. Indian J Cancer. 52 Suppl
3:E176–E178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jackie Oh S, Han S, Lee W and Lockhart AC:
Emerging immunotherapy for the treatment of esophageal cancer.
Expert Opin Investig Drugs. 25:667–677. 2016. View Article : Google Scholar : PubMed/NCBI
|