Introduction
Gastric cancer (GC) is one of the leading causes of
cancer-associated mortality worldwide, and the incidence rate of
this disease is high, particularly in Eastern Asia (1). According to a study in 2012, there were
an estimated 951,600 cases of newly diagnosed GC and 723,100
GC-associated mortalities (1), thus,
the application of effective treatment for GC is urgent. Surgical
resection with lymph node dissection is the cornerstone for
treatment of GC, particularly for those in the early stage;
however, the majority of patients are diagnosed at an advanced
stage in Eastern Asia, and even a number of those receiving radical
surgery have local and systemic recurrence (2). Several clinical trials have been
performed to compare between surgery with adjuvant chemotherapy
following curative D2 gastrectomy or neoadjuvant chemotherapy prior
to D2 or more extended surgery, and surgery alone, including the
ACTS-GC, CLASSIC, NSAS-GC, JCOG 0501 and PRODIGY studies (2–5). The
results of those trials demonstrated that adjuvant chemotherapy
with surgery may be more beneficial to patients compared with
surgery alone, and the phase III trials of neoadjuvant chemotherapy
(JCOG 0501 and PRODIGY) are still going on.
Metronomic chemotherapy, in last decade, has been
gradually recognized and became an alternative to conventionally
scheduled chemotherapy. The notion of ‘high time for low dose’ has
replaced ‘the higher the dose, the better’ with the purpose of
administering systemic therapy incessantly with minimal side
effects (6). Metronomic chemotherapy
not only disrupts the process of cell division, which inhibits the
proliferation of cancer cell, but also eliminates endothelial cells
involved in angiogenesis, termed an anti-angiogenetic effect
(7). In our previous studies,
capecitabine and 5-fluorouracil (5-Fu) was able to express a marked
anti-angiogenetic effect when administered at defined doses and
schedules in mice xenografts of gastrointestinal cancer cell lines
(8,9).
Besides tapering the tumor growth, the anti-angiogenetic activity
of metronomic chemotherapy was able to overcome drug resistance
(10). It is known that inherent and
acquired resistance are one of the major hinders for chemotherapy
(11). In recent years, studies on
chemotherapy resistance have focused on the tumor microenvironment.
Cancer-associated fibroblasts (CAFs), the dominant component of the
tumor microenvironment, have been confirmed to modulate
chemoresistance by secreting cytokines, including stromal
cell-derived factor-1α, interleukin (IL)-6 and IL-17A (12–14). The
present study aimed to evaluate whether capecitabine or 5-Fu
chemotherapy with the metronomic pattern may cause significant
chemoresistance compared with the traditional pattern, and whether
CAFs are involved in the drug resistance.
Materials and methods
Cell lines and culture
Human GC cell line, SGC-7901, was obtained from
Shanghai Institute of Digestive Surgery (Shanghai, China). These
cells were maintained in Dulbecco's modified Eagle's medium
(BasalMedia; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (AusgeneX Pty Ltd., Gold
Coast, Australia) at 37°C with 5% CO2 and saturated
humidity.
Establishment of GC xenografts and
tissue collection
Male Balb/c nude mice (n=25), 4–6 weeks of age, with
body weight of 15–20 g, were provided by the Research Center of
Experimental Medicine, Shanghai Jiaotong University School of
Medicine Affiliated Ruijin Hospital (Shanghai, China). Mice
received humane care, and the study protocol was approved by the
Animal Care and Use Committee and conducted in accordance with the
Guide for the Care and Use Laboratory Animals of Ruijin Hospital,
Shanghai Jiaotong University School of Medicine. Prior to
performing the experiment, animals were placed in separate cages
for 1 week to adapt to the new environment, which was under
specific pathogen-free (SPF) conditions. The temperature was
maintained at between 22 and 25°C, with between 40 and 70% relative
humidity, a 12-h light/12-h dark cycle and a light intensity of
between 15 and 20 lux. Water in drinking bottles and pelleted food
(Xietong-organism, Nanjing, China) were provided ad libitum.
The SGC-7901 cell suspension was adjusted to a cell density of
1×107/ml, and the nude mice were subcutaneously
inoculated with a 100 µl suspension. Administration of the therapy
was initiated when the subcutaneous nodules were ~2 mm in diameter.
The nude mice were randomly divided into the following groups: i)
Control group, intraperitoneally injected with normal saline; ii)
5-Fu conventional dose group [5-Fu maximum tolerated dose (MTD)
group], intraperitoneally injected with 50 mg/kg, twice per week
for 2 weeks, with a 1 week discontinuation for 6 weeks; iii) 5-Fu
metronomic group [5-Fu low-dose metronomic (LDM) group],
intraperitoneally injected with 15 mg/kg, twice a week for 6 weeks;
iv) capecitabine (Roche Diagnostics, Shanghai, China) conventional
dose (capecitabine MTD group), intragastric 500 mg/kg, twice per
week for 2 weeks, with a 1 week discontinuation for 6 weeks; and v)
capecitabine metronomic group (capecitabine LDM group),
intragastric administration at 200 mg/kg, twice a week for 6 weeks.
Following drug administration, a Vernier caliper was used to
measure the length (L) and short track (W) of the tumor mass every
7 days in order to calculate the volume (V) according to the
following formula: V=(W + L)/(2 × W × L × 0.5236) (9). Tumor size did not exceed 20 mm in any
direction.
Immunohistochemical staining
(IHC)
The tumor was fixed with 10% formaldehyde for 24 h
at room temperature. Following hematoxylin-eosin staining (30 min;
room temperature) for tumor confirmation, immunohistochemical
staining was performed on 4-µm sections following the EnVision
two-step procedure of DakoREAL™ EnVision™ Detection system (Dako;
Agilent Technologies GmbH, Waldbronn, Germany). The slides were
incubated at 4°C overnight with primary antibodies for GSTP
(dilution, 1:100; catalog no. GT202729; Dako; Agilent Technologies
GmbH), MDR1 (dilution, 1:50; catalog no. BM0508; Wuhan Boster
Biological Technology, Ltd. Wuhan, China), α-smooth muscle actin
(α-SMA; dilution, 1:50; catalog no. GM085129; Dako; Agilent
Technologies GmbH), CD34 (dilution, 1:200; catalog no. SC-9095;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and vascular
endothelial growth factor (VEGF; dilution, 1:50; catalog no.
M727329; Dako; Agilent Technologies GmbH). The horseradish
peroxidase-labeled antibody to rabbit and mouse immunoglobulin were
used as secondary antibodies (used as supplied; catalog no. K5007;
Dako; Agilent Technologies GmbH) incubated at 37°C for 30 min. The
slides were visualized by diaminobenzidine under a light microscope
(BX51, Olympus Corporation, Tokyo, Japan; ×200 magnification). The
staining result criteria were as follows: A tumor with
brownish-yellow granules was positive for antibody staining.
Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA)
was used to measure the mean density of positive staining, which
was the equivalent to the total integrated optical
density/area.
Statistical analysis
SPSS software (version 13.0; SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. Data are presented as
the mean ± standard deviation, and differences between the groups
were compared using one-way analysis of variance with the Tukey's
multiple comparison post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of chemoresistance markers
is increased following chemotherapy
Our previous studies have confirmed that metronomic
5-Fu-based chemotherapy may perform an anti-angiogenetic role,
which is associated with the antitumor effects of metronomic
chemotherapy in vivo and in vitro. Compared with the
conventional dose traditional chemotherapy, the antitumor effect of
low dose metronomic chemotherapy is not inferior to the former
(8,9).
In addition to the anti-angiogenetic effect, it was speculated that
there are other factors that enhance the antitumor effect of
low-dose groups. Thus, the expression of chemoresistance markers,
including GSTP and MDR1, was examined in the two groups. The
expression of GSTP and MDR1 in GC was determined by IHC. MDR1
expression was present as brown-yellow particles in the plasma
membrane and cytoplasm, and GSTP was observed in the nucleus and
cytoplasm. IHC revealed that GSTP and MDR1 expression were
significantly higher in the 5-Fu-based MTD groups compared with
those of the LDM, and control group (Figs. 1 and 2).
In terms of GSTP, the mean density of staining positive in the 5-Fu
MTD, LDM and control groups was 0.120±0.01, 0.076±0.001 and
0.06±0.001, respectively, and the 5-Fu LDM group was significantly
lower than the 5-Fu MTD dose group (P<0.001) and slightly higher
compared with the control group (P<0.05; Fig. 1B). The mean density of staining
positive in capecitabine MTD, capecitabine LDM and the control
groups was 0.134±0.01, 0.109±0.002 and 0.06±0.001, respectively.
The mean density in the capecitabine LDM group was significantly
lower compared with the capecitabine MTD group (P<0.01) and
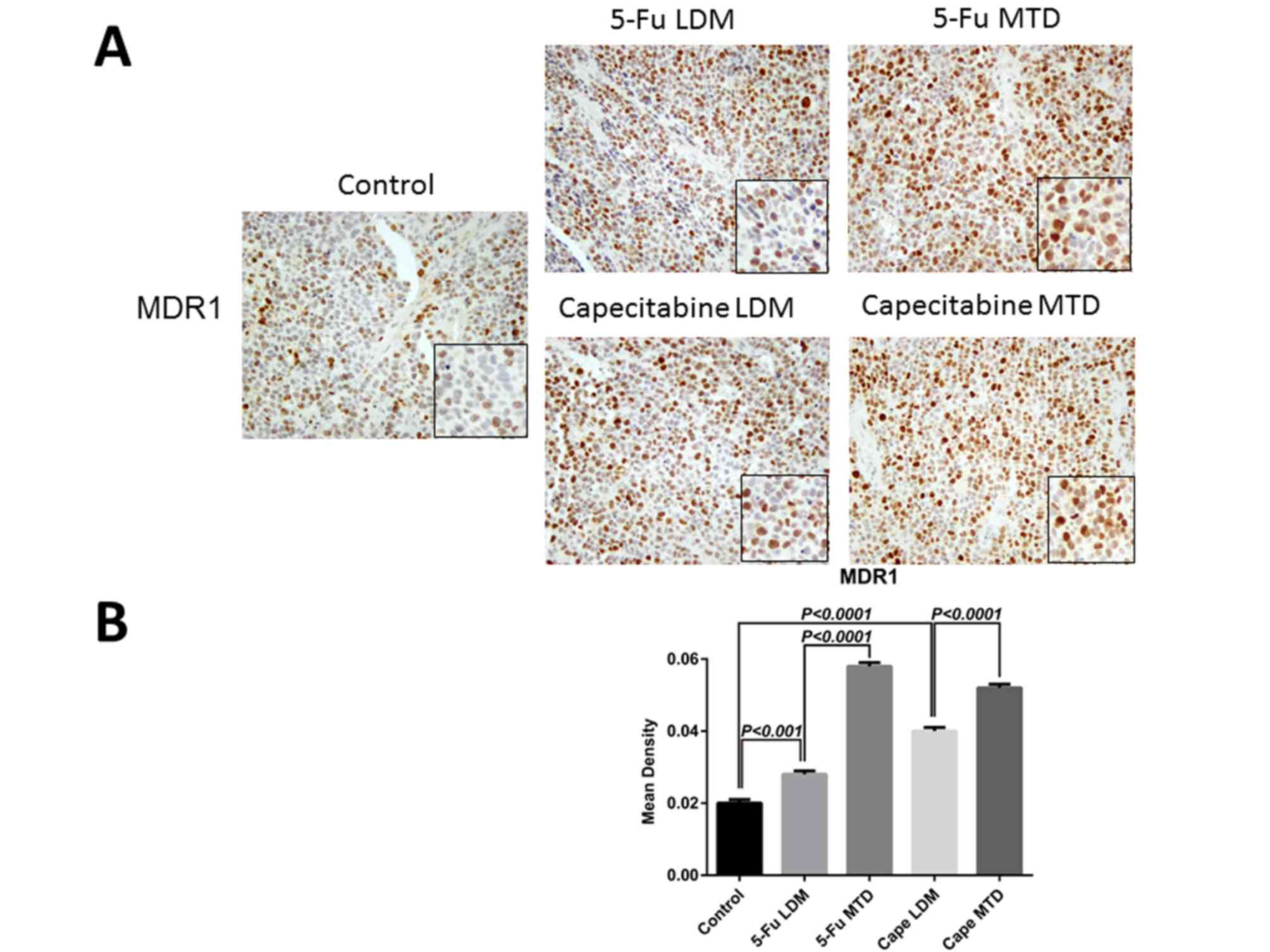
higher than the control group (P<0.001; Fig. 1B). In terms of MDR1, the mean density
of positive staining in the 5-Fu MTD, 5-Fu LDM and the control
groups was 0.058±0.001, 0.028±0.001 and 0.02±0.001, respectively.
The 5-Fu LDM group was lower than the 5-Fu MTD group (P<0.0001),
but higher compared with control group (P<0.001; Fig. 2B). The mean density of positive
staining in the capecitabine MTD, capecitabine LDM and the control
groups was 0.052±0.001, 0.040±0.001, and 0.02±0.001, respectively.
The mean density in the capecitabine LDM group was significantly
lower than the capecitabine MTD group (P<0.0001) and higher
compared with the control group (P<0.0001; Fig. 2B). Taken together, these observations
advocated that the MTD group of capecitabine and 5-Fu may increase
the risk of drug resistance when compared with the LDM groups.
Tumor response to treatment is
associated with increased frequency of CAFs
Previous studies have demonstrated that stromal
compartments are changed by cytotoxic therapies (14,15),
indicating microenvironment-associated drug resistance. Therefore,
the stromal response in the MTD and LDM groups treated with 5-Fu or
capecitabine was examined. CAFs were investigated on the basis of
their proposed roles in supporting drug-resistance (16). As the characteristics of CAFs are
rather distinctive in different tumor types and stages, without
homogeneity, in order to compare the CAF evolution in GC following
different patterns of chemotherapies, matched samples from
identical xenografts of the control, MTD, and LDM groups were
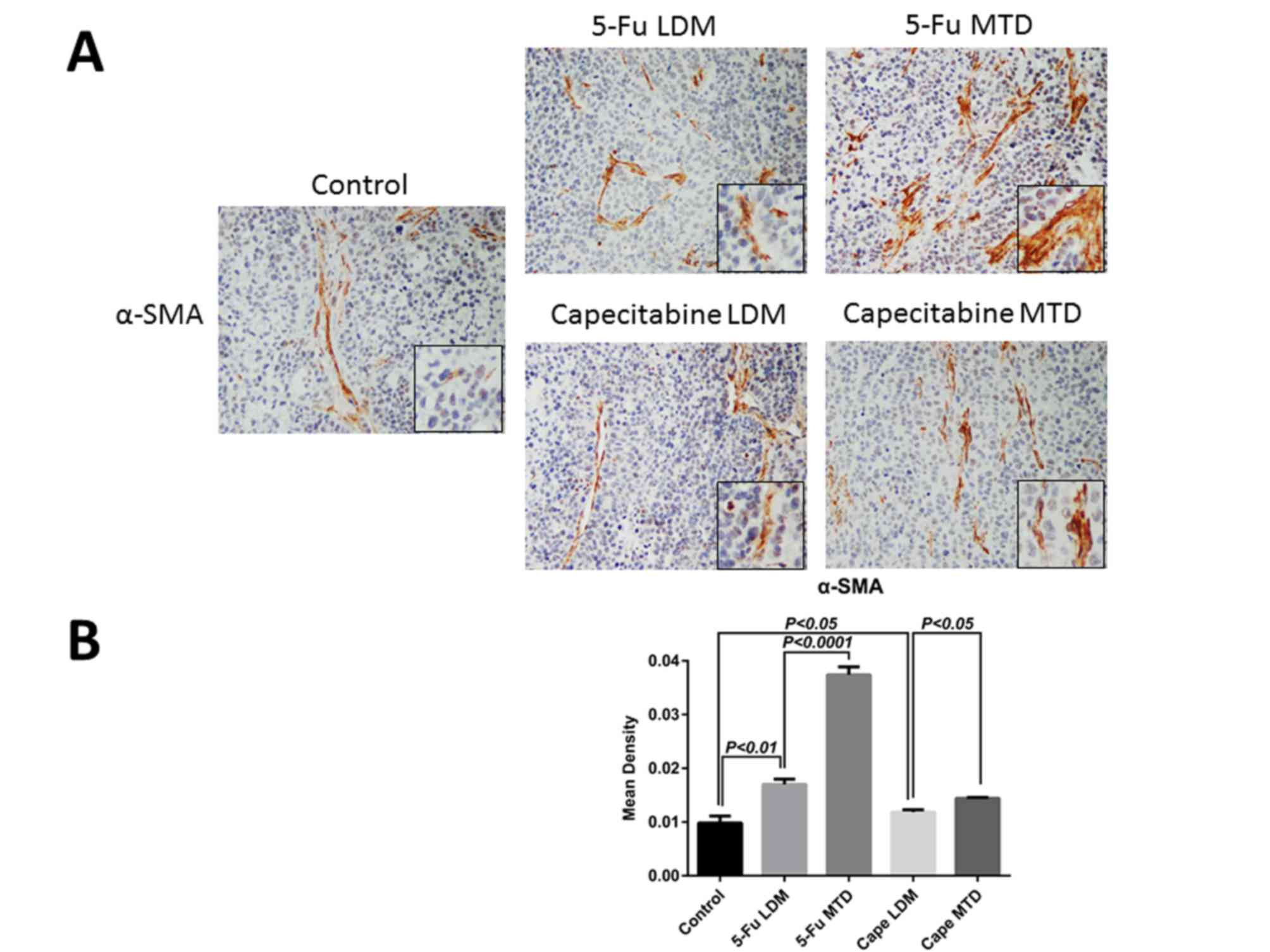
stained for the CAF marker α-SMA to reflect the difference
(Fig. 3A). The mean density of α-SMA
in the 5-Fu MTD, 5-Fu LDM, capecitabine MTD, capecitabine LDM and
control groups was 0.0374±0.0015, 0.017±0.001, 0.0144±0.0002,
0.0122±0.0002 and 0.0098±0.0013, respectively. IHC demonstrated
that α-SMA increased following chemotherapy, and the expression in
the 5-Fu MTD group was significantly higher compared with that of
the 5-Fu LDM group (P<0.0001; Fig.
3B). In addition, the expression of α-SMA in the capecitabine
MTD group was significantly higher compared with that of the
capecitabine LDM group (P<0.05; Fig.
3B). These results indicated that CAFs are enriched during
post-therapy tumor growth, particularly following conventional dose
traditional chemotherapy.
Effect of different chemotherapy
patterns on the expression of CD34 and VEGF
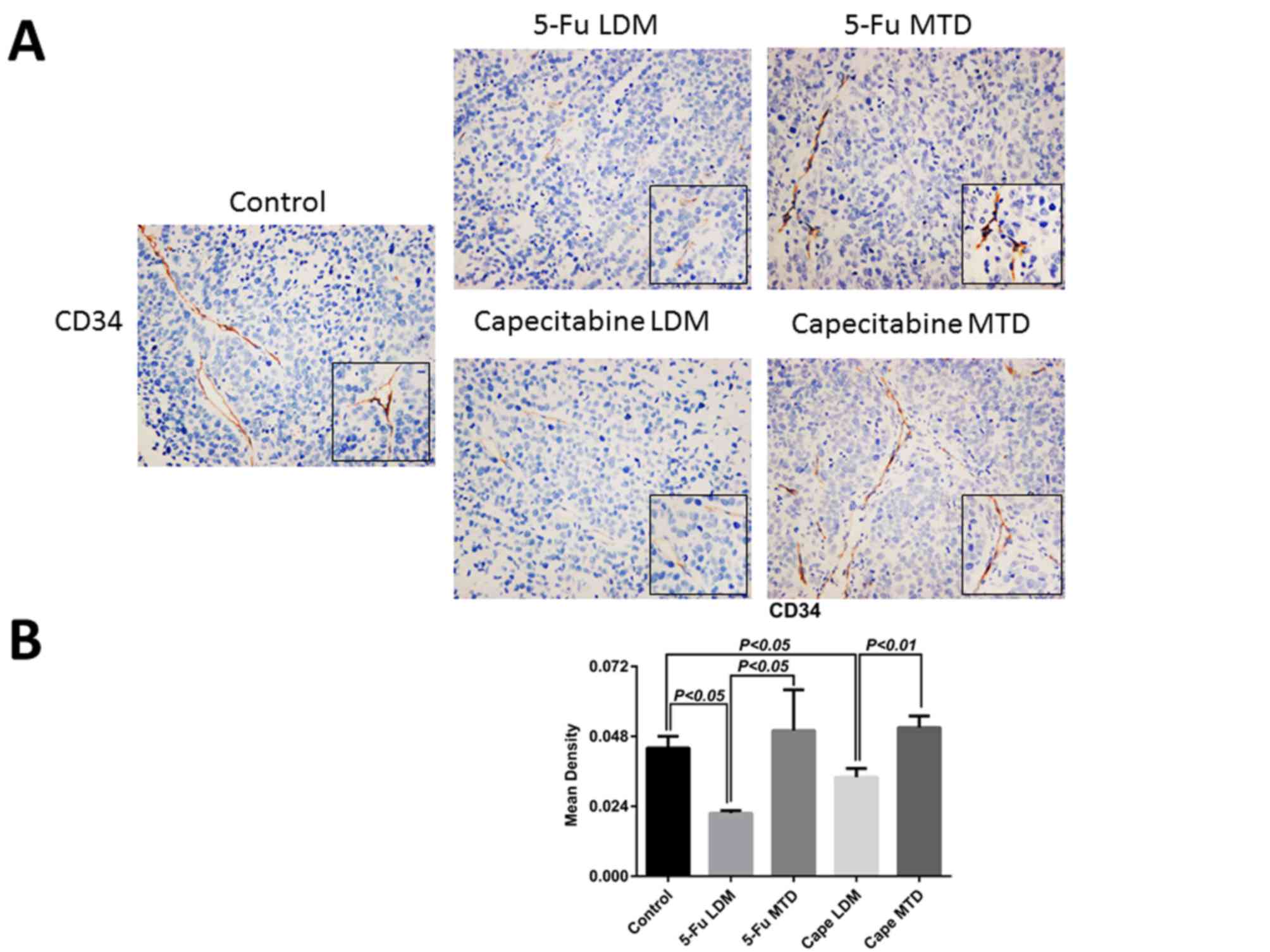
Matched samples from identical xenografts of the
control, MTD and LDM groups were stained for CD34, and VEGF
(Figs. 4 and 5). The IHC results revealed that the mean
density of CD34 in the control group was 0.044±0.004, while the
mean densities of CD34 in 5-Fu conventional dose and metronomic
groups were 0.050±0.014, and 0.0216±0.0009, respectively. The mean
densities of CD34 in the capecitabine conventional dose and
metronomic groups were 0.051±0.004, and 0.034±0.003, respectively.
This indicates that 5-Fu and capecitabine conventional dose
traditional chemotherapy have no significant effect on the
microvascular density (MVD) in GC xenografts, but 5-Fu and
capecitabine low dose metronomic chemotherapy significantly
decreased the MVD (P<0.05 and P<0.01, respectively; Fig. 4B). These results correspond with those
of our previous study revealing that it is 5-FU and capecitabine
metronomic chemotherapy rather than 5-FU and capecitabine
traditional chemotherapy that decrease the MVD in the GC xenografts
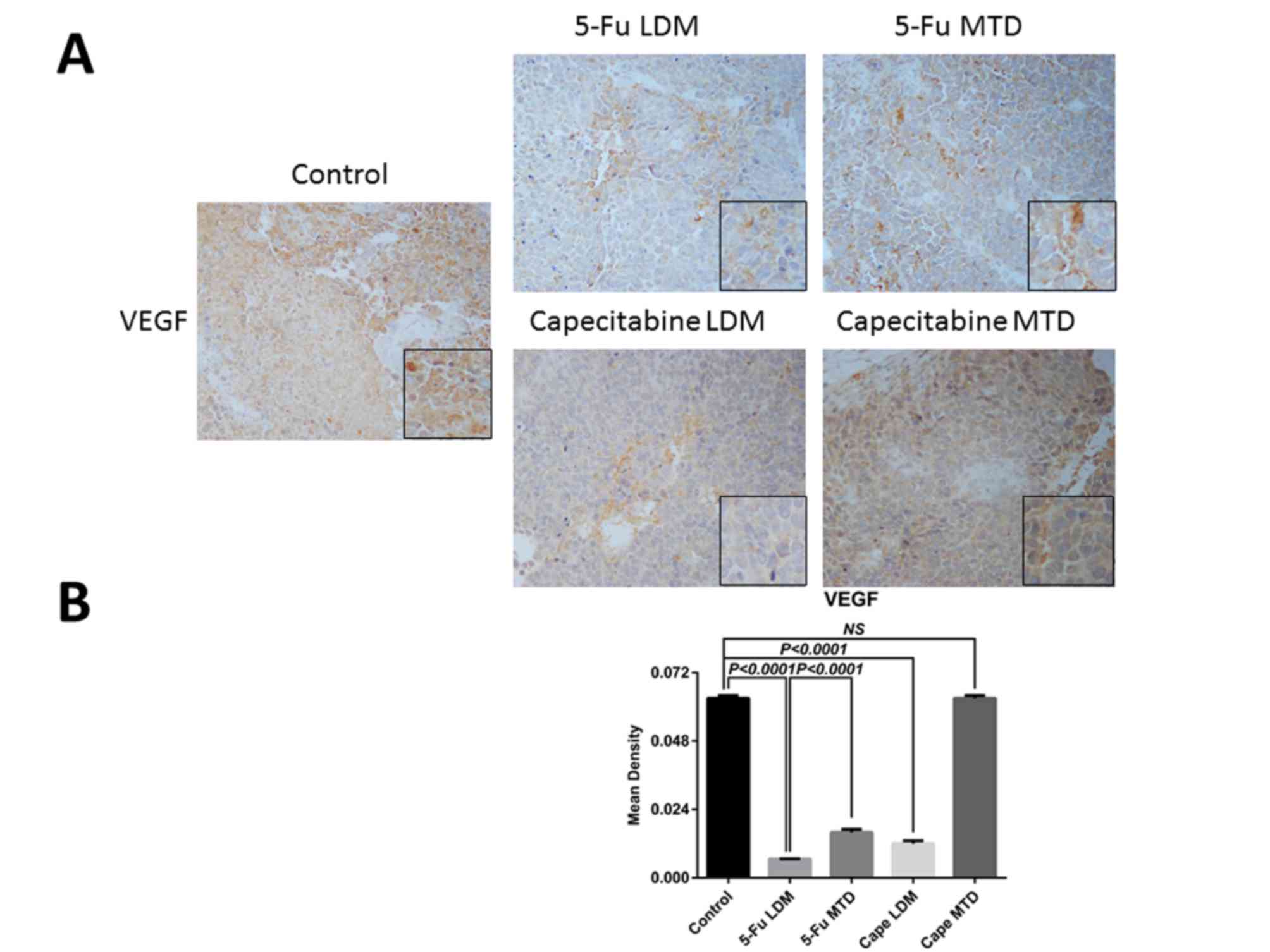
(9). The mean densities of VEGF in
the 5-Fu MTD, 5-Fu LDM, capecitabine MTD, capecitabine LDM and
control groups were 0.016±0.001, 0.0066±0.0001, 0.063±0.001,
0.012±0.001, and 0.063±0.001, respectively (Fig. 5). The results of IHC demonstrated
that, although the level of VEGF in the capecitabine MTD group was
similar to those of the control group (P>0.05), the VEGF
expression was significantly decreased following 5-Fu and
capecitabine LDM (both P<0.0001; Fig.
5B).
Discussion
5-Fu-based chemotherapy served as the first-line
treatment of GC (17). Capecitabine
is a precursor of 5-Fu and exhibits antitumor effects via
conversion by the thymidine phosphorylase enzyme in cancer cells
(8). To be an oral cytotoxic agent,
capecitabine has significant merits compared with intravenous drugs
with regards to being an appropriate choice for metronomic
chemotherapy (9). The characteristics
of better efficacy, low toxicity and good compliance, which have
been confirmed, made metronomic chemotherapy a novel trend in tumor
chemotherapy (8,9).
In the present study, 5-Fu-based metronomic
chemotherapy significantly reduced the expression level of GSTP and
MDR1 compared with those of conventional dose chemotherapy. A
previous study has demonstrated that multidrug resistance (MDR) is
the main cause for the failure of chemotherapy, particularly in GC,
and the occurrence of MDR proceeds through an increased expression
level of P-glycoprotein and a decreased level of topoisomerase II
(18). MDR1 protein, also termed
P-glycoprotein, may reduce the intracellular concentration of
chemotherapeutic drugs via inducing the efflux of anticancer drug,
and GSTP may protect cells against toxic electrophiles and
oxidative stress products, both of which are classical MDR pathways
leading to drug resistance (19).
Additionally, GSTP-positive GCs are resistant to 5-Fu (20), which corresponds to the present study
findings whereby 5-Fu-based conventional chemotherapy group
acquired drug-resistance.
To the best of our knowledge, no studies have
previously compared the drug resistance abilities between different
chemotherapeutic routes. It was found that conventional
chemotherapy with 5-Fu or capecitabine may increase the risk of
drug resistance compared with a metronomic approach. The increased
expression of CAFs in the tissue of patients receiving conventional
therapy suggests the possibility of drug resistance (16). Thus, it was speculated that CAFs may
rebuild the vessel in the microenvironment via the production of
VEGF, leading to drug resistance following chemotherapy. The
results in mice have essential clinical implications, which may aid
in explaining why a number of patients receiving metronomic or
maintenance chemotherapy continue to have stable disease exceeding
the expected duration for cancer cells to acquire chemoresistance.
Studies associated with metronomic chemotherapy mainly focus on
anti-angiogenic functions without consideration of the interaction
with the microenvironment; however, the change in the tumor
microenvironment caused by metronomic chemotherapy may affect the
chemoresistance (7–9). The study of metronomic chemotherapy to
reduce drug resistance may be of use to improve patient outcomes in
clinical practice.
As demonstrated in a previous study (10), the anti-angiogenetic characteristic of
cyclophosphamide has been demonstrated through increasing the
apoptosis of tumor cells and maintaining the cytotoxic pressure on
the vascular endothelial cells within the tumor bed, overall
leading to no drug resistance being acquired. These results were
not achieved using the conventional schedule, whereby mice
harboring tumors developed acquired drug resistance (10). The anti-angiogenetic schedule used by
Browder et al (10), is
similar to the metronomic chemotherapy in current use, as is the
metronomic administration pattern of 5-Fu or capecitabine. All the
methods utilize the drugs in innovative ways which are able to have
an improved effect over the traditional pattern.
The significant increase in the amount of CAFs
following chemotherapy indicated that chemotherapy may induce the
remodeling of the tumor microenvironment as well, and CAFs may
offer microenvironmental cues instructing tumor drug resistance
(14,21). In vitro assays have also
demonstrated that CAFs induce resistance to chemotherapy via
secreting cytokines (16,22,23).
Besides chemotherapy agents, inhibiting CAFs may also enhance the
effects of bevacizumab (rhuMab VEGF, Avastin) even in
bevacizumab-resistant GC cells (24),
which is similar to the selective susceptibility of α-SMA-deficient
vessels to bevacizumab (25). CAFs
are the primarily source of VEGF, cancer epithelial cells are able
to produce VEGF and the level of VEGF is increased through the
cancer-stromal interaction (26).
VEGFs and their receptors have been revealed to modulate vascular
permeability activity, leading to enhanced interstitial fluid
pressure in the tumor stroma, which is associated with
chemotherapeutic resistance (25).
The participation of CAFs in tumor progression and
metastasis is well established, particularly in GC (27,28),
therefore, anti-CAF therapy may have a triple effect through its
anti-angiogenesis potency, antitumor qualities, and the ability to
increase chemotherapeutic drugs being absorbed by the tumor
(26). Similar to the oral
anti-fungal agent itraconazole, which is able to suppress the
angiogenetic factors secreted from CAFs, a synergic effect was
demonstrated with pemetrexed in a second-line therapy trial for
lung cancer (24,29).
In conclusion, the present study demonstrated that
low dose metronomic chemotherapy was able to significantly reduce
the risk of acquired chemoresistance compared with the normal dose
conventional chemotherapy, and the difference in the level of CAFs
following both chemotherapy patterns confirmed the diversity of
drug-resistance. Furthermore, the downregulation of VEGF expression
may not only reflect the anti-angiogenesis effect of metronomic
chemotherapy, but also corresponds with the reduced number of CAFs
that occur, which may contribute to the development of
chemoresistance. To better understand the crosstalk between
metronomic chemotherapy and the tumor microenvironment, including
CAFs, mechanisms that lead to chemoresistance and molecules
secreted by CAFs require further characterization.
Acknowledgements
The study was supported by the National Science
Foundation of China (grant nos. 81672327, 81372645 and 81502013),
The Program of Shanghai Academic/Technology Research Leader (grant
no. 17XD1402600), The Shanghai Municipal Education
Commission-Gaofeng Clinical Medicine Grant Support (grant no.
20161410), The FONG SHU FOOK TONG Foundation and National Key
Clinical Discipline (Oncology) and the innovation foundation of
translational medicine of Shanghai Jiao Tong University School of
Medicine (grant no. 15ZH3001), The Program for Outstanding Medical
Academic Leader and Shanghai Municipal Commission of Health and
Family Planning (grant no. 20154Y496) and SCORE Foundation (grant
no. Y-MX2015-078).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujitani K: Overview of adjuvant and
neoadjuvant therapy for resectable gastric cancer in the East. Dig
Surg. 30:119–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al: Adjuvant
capecitabine and oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled
trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakajima T, Kinoshita T, Nashimoto A,
Sairenji M, Yamaguchi T, Sakamoto J, Fujiya T, Inada T, Sasako M
and Ohashi Y: National Surgical Adjuvant Study of Gastric Cancer
Group: Randomized controlled trial of adjuvant uracil-tegafur
versus surgery alone for serosa-negative, locally advanced gastric
cancer. Br J Surg. 94:1468–1476. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banys-Paluchowski M, Schütz F, Ruckhäberle
E, Krawczyk N and Fehm T: Metronomic chemotherapy for metastatic
breast cancer - a systematic review of the literature. Geburtshilfe
Frauenheilkd. 76:525–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pasquier E, Kavallaris M and André N:
Metronomic chemotherapy: New rationale for new directions. Nat Rev
Clin Oncol. 7:455–465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi H, Jiang J, Ji J, Shi M, Cai Q, Chen
X, Yu Y, Liu B, Zhu Z and Zhang J: Anti-angiogenesis participates
in antitumor effects of metronomic capecitabine on colon cancer.
Cancer Lett. 349:128–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan F, Shi H, Ji J, Cai Q, Chen X, Yu Y,
Liu B, Zhu Z and Zhang J: Capecitabine metronomic chemotherapy
inhibits the proliferation of gastric cancer cells through
anti-angiogenesis. Oncol Rep. 33:1753–1762. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Browder T, Butterfield CE, Kräling BM, Shi
B, Marshall B, O'Reilly MS and Folkman J: Antiangiogenic scheduling
of chemotherapy improves efficacy against experimental
drug-resistant cancer. Cancer Res. 60:1878–1886. 2000.PubMed/NCBI
|
|
11
|
Wang W, McLeod HL, Cassidy J and
Collie-Duguid ES: Mechanisms of acquired chemoresistance to
5-fluorouracil and tomudex: Thymidylate synthase dependent and
independent networks. Cancer Chemother Pharmacol. 59:839–845. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Guan J, Long X, Wang Y and Xiang X:
mir-1-mediated paracrine effect of cancer-associated fibroblasts on
lung cancer cell proliferation and chemoresistance. Oncol Rep.
35:3523–3531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shintani Y, Fujiwara A, Kimura T, Kawamura
T, Funaki S, Minami M and Okumura M: IL-6 secreted from cancer
associated fibroblasts mediates chemoresistance in NSCLC by
increasing epithelial-mesenchymal transition signaling. J Thorac
Oncol. 11:1482–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lotti F, Jarrar AM, Pai RK, Hitomi M,
Lathia J, Mace A, Gantt GA Jr, Sukhdeo K, DeVecchio J, Vasanji A,
et al: Chemotherapy activates cancer-associated fibroblasts to
maintain colorectal cancer-initiating cells by IL-17A. J Exp Med.
210:2851–2872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanaka K, Miyata H, Sugimura K, Fukuda S,
Kanemura T, Yamashita K, Miyazaki Y, Takahashi T, Kurokawa Y,
Yamasaki M, et al: miR-27 is associated with chemoresistance in
esophageal cancer through transformation of normal fibroblasts to
cancer-associated fibroblasts. Carcinogenesis. 36:894–903. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amornsupak K, Insawang T, Thuwajit P,
O-Charoenrat P, Eccles SA and Thuwajit C: Cancer-associated
fibroblasts induce high mobility group box 1 and contribute to
resistance to doxorubicin in breast cancer cells. BMC Cancer.
14:9552014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen L, Shan YS, Hu HM, Price TJ, Sirohi
B, Yeh KH, Yang YH, Sano T, Yang HK, Zhang X, et al: Management of
gastric cancer in Asia: Resource-stratified guidelines. Lancet
Oncol. 14:e535–e547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao W, Chen R, Zhao M, Li L, Fan L and
Che XM: High glucose promotes gastric cancer chemoresistance in
vivo and in vitro. Mol Med Rep. 12:843–850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Longley DB and Johnston PG: Molecular
mechanisms of drug resistance. J Pathol. 205:275–292. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Geng M, Wang L, Chen X, Cao R and Li P:
The association between chemosensitivity and Pgp, GST-π and Topo II
expression in gastric cancer. Diagn Pathol. 8:1982013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verset L, Tommelein J, Lopez Moles X,
Decaestecker C, Boterberg T, De Vlieghere E, Salmon I, Mareel M,
Bracke M, De Wever O and Demetter P: Impact of neoadjuvant therapy
on cancer-associated fibroblasts in rectal cancer. Radiother Oncol.
116:449–454. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan H, Guo BY and Zhang S:
Cancer-associated fibroblasts attenuate Cisplatin-induced apoptosis
in ovarian cancer cells by promoting STAT3 signaling. Biochem
Biophys Res Commun. 470:947–954. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Steinbichler TB, Metzler V, Pritz C,
Riechelmann H and Dudas J: Tumor-associated fibroblast-conditioned
medium induces CDDP resistance in HNSCC cells. Oncotarget.
7:2508–2518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hara M, Nagasaki T, Shiga K and Takeyama
H: Suppression of cancer-associated fibroblasts and endothelial
cells by itraconazole in bevacizumab-resistant gastrointestinal
cancer. Anticancer Res. 36:169–177. 2016.PubMed/NCBI
|
|
25
|
Salnikov AV, Heldin NE, Stuhr LB, Wiig H,
Gerber H, Reed RK and Rubin K: Inhibition of carcinoma cell-derived
VEGF reduces inflammatory characteristics in xenograft carcinoma.
Int J Cancer. 119:2795–2802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J and Liu J: Tumor stroma as targets
for cancer therapy. Pharmacol Ther. 137:200–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Franco OE, Shaw AK, Strand DW and Hayward
SW: Cancer associated fibroblasts in cancer pathogenesis. Semin
Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan Y, Wang LF and Wang RF: Role of
cancer-associated fibroblasts in invasion and metastasis of gastric
cancer. World J Gastroenterol. 21:9717–9726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rudin CM, Brahmer JR, Juergens RA, Hann
CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P and Liu JO:
Phase 2 study of pemetrexed and itraconazole as second-line therapy
for metastatic nonsquamous non-small-cell lung cancer. J Thorac
Oncol. 8:619–623. 2013. View Article : Google Scholar : PubMed/NCBI
|