Introduction
Severe genital bleeding is one of the most common
problems affecting women; it can affect a woman's physical, social,
and emotional quality of life. There are a variety of causes of
abnormal uterine bleeding, such as myomas, dysfunctional bleeding,
endometrial polyps, endometrial malignant diseases, uterine
arteriovenous malformations (AVMs), retained products of
conception, and gestational trophoblastic disease.
Kanaoka at Iseikai Hospital (Osaka, Japan) first
developed microwave endometrial ablation (MEA; frequency, 2.45 GHz)
for functional menorrhagia (1). The
uterine cavity is irradiated with microwaves at 2.45 GHz to ablate
the endometrium with a thin curved applicator, including the basal
layer, thus decreasing the menstrual amount. The MEA technique was
introduced to the Shimane University Hospital in August 2007. MEA
was approved as an advanced medical treatment by the Ministry of
Health, Labour and Welfare of Japan and our facility started to
provide MEA treatment in June 2009. In April 2012, MEA was approved
for national health insurance coverage as code K863-3:
hysteroscope-assisted endometrial ablation.
Approximately 40,000 women in Japan are known to
undergo hysterectomies annually. Twenty-five percent of these women
may be able to avoid hysterectomy by undergoing MEA treatment
(2). After endometrial ablation was
introduced in the UK, the number of hysterectomies decreased by one
third (3). Our previous report showed
that MEA is effective in controlling menorrhagia and
life-threatening uterine hemorrhage (4).
One hundred and sixty-nine patients at our hospital
underwent MEA treatment in the past 8 years. We have previously
evaluated the efficacy and safety of MEA, compared with
conventional hysterectomy (2,5), and found that it is effective, safe, and
cost-effective in patients with excessive menstruation (2,5). MEA is
considered a standard treatment for patients with excessive
menstruation resistant to conservative therapy. However, the
long-term outcomes of this treatment modality are still
unknown.
The primary aim of this study was to describe the
long-term outcomes of MEA in our institution. The secondary aim was
to identify the predictors of menorrhagia recurrence or re-surgery
after MEA.
Materials and methods
Patient selection and monitoring
This was a retrospective cohort study conducted from
August 2007 to August 2015 at Shimane University Hospital in
Shimane, Japan. Women who underwent MEA were identified and
enrolled in the study. All patients gave written informed consent
for the procedure and for study participation after proper
explanation of the risks and benefits of the procedure. The
inclusion criteria were severe menorrhagia, premenopausal status,
no desire for childbearing, and a desire to preserve the uterus, as
well as availability for at least 6 months of follow-up after MEA.
Patients diagnosed with endometrial carcinoma after surgery were
excluded from the study, as were those who did not undergo magnetic
resonance imaging (MRI) evaluation before surgery. Data regarding
patient characteristics, preoperative clinical findings, and
procedure complications were extracted from the medical records.
Before MEA was covered by health insurance in Japan, MEA was
approved by the Ethics Committee of Shimane University's Medical
Department; the same committee approved this study.
Patients were asked to schedule an initial
postoperative follow-up visit 1 month after MEA, followed by an
additional visit 6 months after the procedure. At these visits, a
visual analog scale (VAS) with a maximum score of 10 was used to
grade menstrual blood loss, menstrual cramping (dysmenorrhea), and
patient satisfaction. The recurrence of menorrhagia after MEA was
defined as the need for new pharmacotherapeutic interventions or
occurrence of new symptoms such as extraordinary urinary frequency,
accompanied by uterine enlargement. The time to recurrence was
defined as the time in months from the procedure until the new
treatment or symptoms began. Re-surgery was defined as worsening
menorrhagia with a concomitant symptom-related total hysterectomy.
We have previously reported the short-term efficacy of MEA in 76 of
169 patients who were included in this study (2).
Ablation procedure and follow-up
To perform the MEA, the lower abdominal region,
vulva, femoral region, and vaginal cavity were sterilized with
iodine, and ablation was performed using a Microtaze (Alfresa
Pharma, Osaka, Japan) according to procedure guidelines, with the
microwave output at 70 W for 50 sec. Transabdominal or rectal
ultrasonography was used for intra-procedure monitoring (6). The follow-up period ranged from 10 to 96
months, with a median of 35.5 months.
Statistical analyses
Univariate analysis was performed using binomial
logistic regression for ordered categorical variables. Patient
clinicopathological characteristics included in the model were age
at diagnosis, uterine cavity length (<10 vs. ≥10 cm), diagnosis
(myoma/adenomyosis vs. others), myoma type (submucosal vs.
intramural), largest myoma size (<5 vs. ≥5 cm), number of myomas
on preoperative MRI (<4 vs. ≥4), and preoperative hemoglobin
concentration (<9 vs. ≥9 mg/dl). The endpoints of the analysis
were recurrence of menorrhagia and re-surgery. These data were
plotted as Kaplan-Meier curves, and statistical significance was
determined using the log-rank test. Variables that were initially
entered into the model included those indicated to be significant
(P<0.30) in the univariate analysis. These variables were
hypothesized to potentially affect recurrence or re-surgery and
were entered into the multivariate analysis.
Multivariate prognostic analysis was performed using
a Cox proportional hazards model, and data from patients lost to
follow-up were censored. Statistical analyses were conducted using
the Statistical Package for the Social Sciences for Windows
software, Version 19.0 (IBM Corp., Armonk, NY, USA). All reported
P-values were two-sided, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Of 169 patients enrolled in this study, 5 were
excluded from analysis due to a final diagnosis of endometrial
carcinoma, and 4 were excluded due to the lack of MRI evaluation
before the MEA procedure. Data from 160 patients were analyzed in
this report. Patient characteristics are presented in Table I. Patients ranged in age from 34 to 56
years, with a median age of 47 years. All patients had a chief
complaint of excessive menstruation. Uterine myomas were present in
100 patients (63%), adenomyosis in 20 patients (13%), functional
excessive menstruation in 26 patients (16%), endometrial polyps in
12 patients (7.5%), and simple endometrial hyperplasia in 2
patients (1.3%). In women with a combination of uterine myomas and
adenomyosis, we judged which was dominant in each patient using MRI
evaluation and classified the patient in the appropriate group. The
preoperative hemoglobin concentration ranged from 2.7 to 14.3
mg/dl; the mean was 9.6 mg/dl. The uterine cavity length, as
determined on performing MRI, ranged from 7 to 14.5 cm, with a mean
of 9 cm.
 | Table I.Patient baseline characteristics
(n=160). |
Table I.
Patient baseline characteristics
(n=160).
| Characteristic | Median (range),
number (%) |
|---|
| Age (years) | 47 (34–56) |
| Uterine cavity length
(cm) | 9
(7–14.5) |
| Diagnosis |
|
|
Myomas | 100 (63%) |
|
Adenomyosis | 20
(13%) |
|
Functional excessive
menstruation | 26
(16%) |
|
Endometrial polyps | 12
(7.5%) |
| Simple
endometrial hyperplasia | 2
(1.3%) |
Univariate and multivariate analyses
of prognostic factors in the full cohort
In the entire cohort, the rate of menorrhagia
recurrence was 18.1% (29/160) and the rate of re-surgery was 9.3%
(15/160).
A univariate analysis was performed using age
(<40 or ≥40 years), diagnosis (uterine myoma, adenomyosis,
functional bleeding, endometrial polyps, or others), and uterine
cavity length (≥10 or <10 cm) as possible variables. Diagnosis
was categorized into 2 groups: i) myoma/adenomyosis; and ii)
others, including functional bleeding and endometrial polyps. This
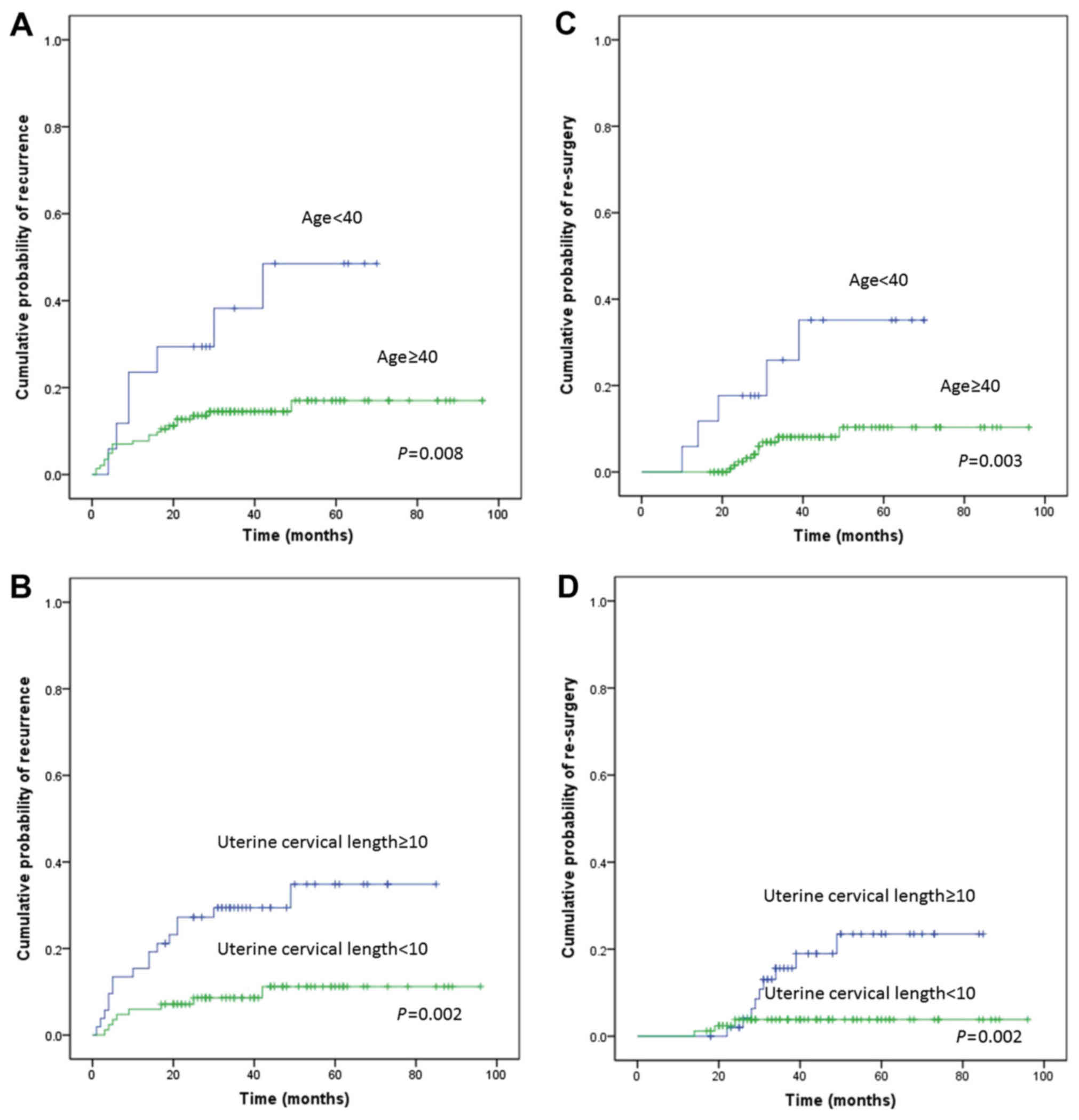
analysis identified age (P=0.008; Fig.
1A), uterine cavity length (P=0.002; Fig. 1B), and diagnosis (P=0.007) as
potential predictors of recurrence. A multivariate analysis
confirmed that age <40 [hazard ratio (HR), 3.992; 95% confidence
interval (CI): 1.560–10.217; P=0.004] and uterine cavity length ≥10
(HR, 4.035; 95% CI: 1.706–9.541; P=0.001) were independent risk
factors for recurrence (Table II).
Univariate and multivariate analyses were also performed on
re-surgery. As shown in Table III,
the univariate analysis revealed that age (P=0.003; Fig. 1C), uterine cavity length (P=0.015;
Fig. 1D), and diagnosis of
myoma/adenomyosis (P=0.132) were potential predictors of
re-surgery. The multivariate analysis showed that age (HR, 5.618,
95% CI: 1.678–18.806; P=0.005) and uterine cavity length (HR,
5.041; 95% CI: 1.353–18.779; P=0.016) were independent risk factors
for re-surgery (Table III).
 | Table II.Univariate and multivariate analyses
of prognostic factors for menorrhagia recurrence in the entire
cohort (rate of menorrhagia recurrence: 18.1%, 29/160). |
Table II.
Univariate and multivariate analyses
of prognostic factors for menorrhagia recurrence in the entire
cohort (rate of menorrhagia recurrence: 18.1%, 29/160).
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | Patients | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age at surgery,
years |
|
|
|
|
|
|
|
|
<40 | 17 | 3.019 | 1.282–7.106 | 0.008 | 3.992 | 1.560–10.217 | 0.004 |
| ≥40 | 143 | Ref |
|
| Ref |
|
|
| Uterine cavity
length, cm |
|
|
|
|
|
|
|
| ≥10 | 62 | 3.524 | 1.508–8.237 | 0.002 | 4.035 | 1.706–9.541 | 0.001 |
|
<10 | 98 | Ref |
|
| Ref |
|
|
| Diagnosis |
|
|
|
|
|
|
|
|
Myoma/adenomyosis | 120 | 9.464 | 1.286–69.670 | 0.007 |
|
|
|
|
Others | 40 | Ref |
|
|
|
|
|
 | Table III.Univariate and multivariate analyses
of prognostic factors for re-surgery in the entire cohort of
patients (rate of re-surgery: 9.3%, 15/160). |
Table III.
Univariate and multivariate analyses
of prognostic factors for re-surgery in the entire cohort of
patients (rate of re-surgery: 9.3%, 15/160).
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | Patients | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age at surgery,
years |
|
|
|
|
|
|
|
|
<40 | 17 | 4.477 | 1.530–13.102 | 0.003 | 5.618 | 1.678–18.806 | 0.005 |
| ≥40 | 143 | Ref |
|
| Ref |
|
|
| Uterine cavity
length, cm |
|
|
|
|
|
|
|
| ≥10 | 62 | 4.401 | 1.191–16.267 | 0.015 | 5.041 | 1.353–18.779 | 0.016 |
|
<10 | 98 | Ref |
|
| Ref |
|
|
| Diagnosis |
|
|
|
|
|
|
|
|
Myoma/Adenomyosis | 120 | 4.189 | 0.550–31.895 | 0.132 |
|
|
|
|
Others | 40 | Ref |
|
|
|
|
|
Univariate and multivariate analyses
of prognostic factors in patients with uterine myomas
We next performed a subgroup analysis of patients
with uterine myomas. In patients with uterine myomas, the rate of
menorrhagia recurrence was 19.0% (19/100) and the rate of
re-surgery was 13.0% (13/100).
We first set various cut-off values and performed
statistical analyses. We finally decided to use optimal cut-off
values and set the age to 48 years, cut-off value of largest myoma
size to 5 cm, and cut-off value of number of myomas to four, which
gave the significant differences statistically.
A univariate analysis was performed using age
(<48 or ≥48), myoma type (submucosal or intramural), largest
myoma size (≥5 or <5 cm), number of myomas on preoperative MRI
(≥4 or <4), uterine cavity length (≥10 or <10), and
preoperative hemoglobin concentration (<9 or ≥9 mg/dl). This
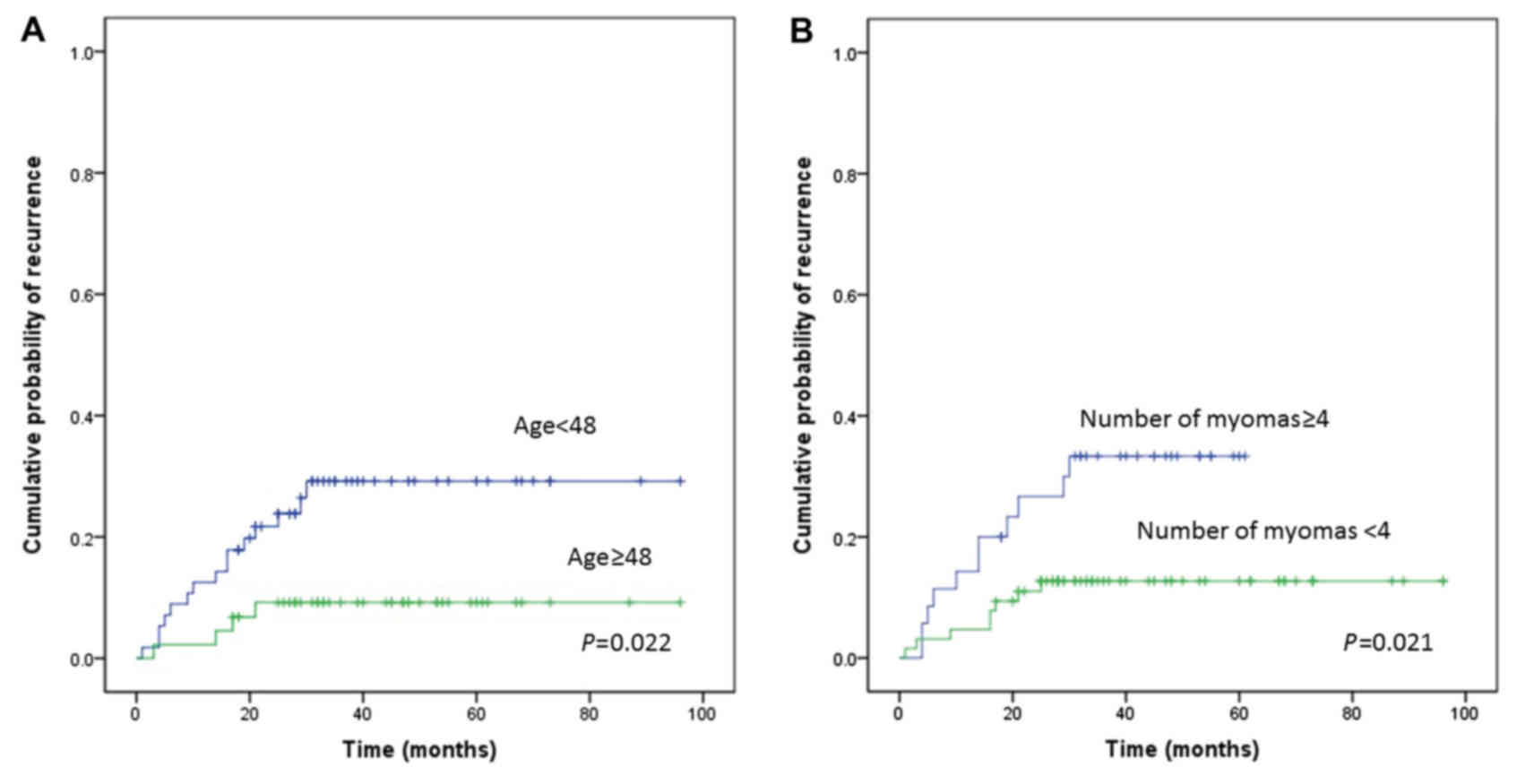
analysis identified age (P=0.022; Fig.
2A), largest myoma size (P=0.073), number of myomas (P=0.021;
Fig. 2B), and uterine cavity length
(P=0.061) as potential predictors of recurrence. A multivariate
analysis confirmed that age <48 years (HR, 3.465; 95% CI:
1.147–10.468; P=0.028) and number of myomas ≥4 (HR, 2.993; 95% CI:
1.200–7.466; P=0.019) were independent risk factors for recurrence
(Table IV). Univariate and
multivariate analyses were also performed on re-surgery. As shown
in Table V, the univariate analysis
revealed that age (P=0.012), largest myoma size (P=0.006), number
of myomas (P=0.013), uterine cavity length (P=0.023), and
preoperative hemoglobin concentration (P=0.037) were potential
predictors of re-surgery. The multivariate analysis showed that
largest myoma size ≥5 cm (HR, 5.567: 95% CI: 1.175–26.381; P=0.031)
and preoperative hemoglobin concentration <9 mg/dl (HR, 3.743;
95% CI: 1.145–12.232; P=0.029) were independent risk factors for
re-surgery (Table V).
 | Table IV.Univariate and multivariate analyses
of prognostic factors for recurrence in patients with uterine
myomas (rate of recurrence: 19.0%, 19/100). |
Table IV.
Univariate and multivariate analyses
of prognostic factors for recurrence in patients with uterine
myomas (rate of recurrence: 19.0%, 19/100).
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | Patients | Hazard ratio | 95% CI | P | Hazard ratio | 95% CI | P |
|---|
| Age at surgery,
years |
|
|
|
|
|
|
|
|
<48 | 56 | 3.358 | 1.113–10.132 | 0.022 | 3.465 | 1.147–10.468 | 0.028 |
|
≥48 | 44 | Ref |
|
| Ref |
|
|
| Myoma type |
|
|
|
|
|
|
|
|
Submucosal | 40 | 1.706 | 0.693–4.201 | 0.238 |
|
|
|
|
Intramural | 60 | Ref |
|
|
|
|
|
| Largest myoma size,
cm |
|
|
|
|
|
|
|
| ≥5 | 50 | 2.356 | 0.895–6.205 | 0.073 |
|
|
|
|
<5 | 50 | Ref |
|
|
|
|
|
| Number of myomas on
preoperative MRI |
|
|
|
|
|
|
|
| ≥4 | 35 | 2.781 | 1.117–2.781 | 0.021 | 2.993 | 1.200–7.466 | 0.019 |
|
<4 | 65 | Ref |
|
| Ref |
|
|
| Uterine cavity
length, cm |
|
|
|
|
|
|
|
|
≥10 | 42 | 2.371 | 0.933–6.026 | 0.061 |
|
|
|
|
<10 | 58 | Ref |
|
|
|
|
|
| Preoperative
hemoglobin concentration, mg/dl |
|
|
|
|
|
|
|
|
<9 | 29 | 2.371 | 0.632–1.608 | 0.313 |
|
|
|
| ≥9 | 71 | Ref |
|
|
|
|
|
 | Table V.Univariate and multivariate analyses
of prognostic factors for re-surgery in patients with uterine
myomas (rate of re-surgery: 13.0%, 13/100). |
Table V.
Univariate and multivariate analyses
of prognostic factors for re-surgery in patients with uterine
myomas (rate of re-surgery: 13.0%, 13/100).
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | Patients | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age at surgery,
years |
|
|
|
|
|
|
|
|
<48 | 56 | 5.522 | 1.222–24.955 | 0.012 | 3.863 | 0.831–17.968 | 0.085 |
|
≥48 | 44 | Ref |
|
| Ref |
|
|
| Myoma type |
|
|
|
|
|
|
|
|
Submucosal | 40 | 1.369 | 0.459–4.080 | 0.571 |
|
|
|
|
Intramural | 60 | Ref |
|
|
|
|
|
| Largest myoma size,
cm |
|
|
|
|
|
|
|
| ≥5 | 50 | 6.294 | 1.392–28.451 | 0.006 | 5.567 | 1.175–26.381 | 0.031 |
|
<5 | 50 | Rref |
|
| Ref |
|
|
| Number of myomas on
preoperative MRI |
|
|
|
|
|
|
|
| ≥4 | 35 | 3.993 | 1.228–12.986 | 0.013 | 3.094 | 0.934–10.245 | 0.065 |
|
<4 | 65 | Ref |
|
| Ref |
|
|
| Uterine cavity
length, cm |
|
|
|
|
|
|
|
|
≥10 | 42 | 4.010 | 1.102–14.589 | 0.023 |
|
|
|
|
<10 | 58 | Ref |
|
|
|
|
|
| Preoperative
hemoglobin concentration, mg/dl |
|
|
|
|
|
|
|
|
<9 | 29 | 3.038 | 1.015–9.091 | 0.037 | 3.743 | 1.145–12.232 | 0.029 |
| ≥9 | 71 | Ref |
|
| Ref |
|
|
Univariate and multivariate analyses
of prognostic factors in patients with adenomyosis
Finally, we performed a subgroup analysis of
patients with adenomyosis. In patients with adenomyosis, the rate
of menorrhagia recurrence was 35.0% (7/20).
We first set various cut-off values and performed
statistical analysis. We finally decided to use an optimal cut-off
value of the thickness of myometrium to 60 mm, which gave the
significant differences statistically.
A univariate analysis was performed using age
(<48 or ≥48 years), thickness of myometrium (≥60 or <60 mm),
uterine cavity length (≥10 or <10 cm), and preoperative
hemoglobin concentration (<9 or ≥9 mg/dl). This analysis
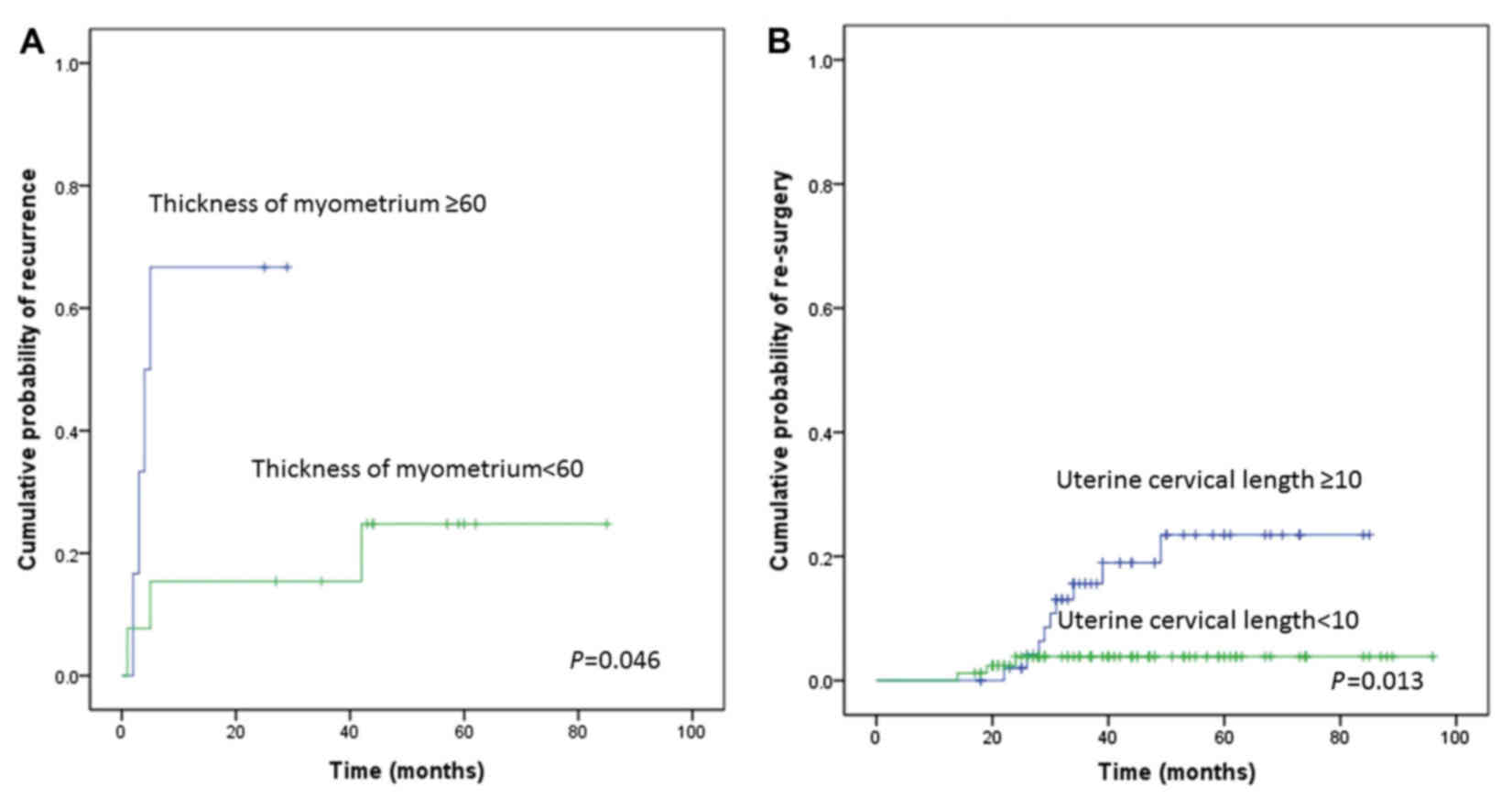
identified thickness of myometrium (P=0.046; Fig. 3A) and uterine cavity length (P=0.013;
Fig. 3B) as potential predictors of
recurrence. A multivariate analysis confirmed that uterine cavity
length ≥10 cm (HR=15.467; 95% CI=1.792–133.519; P=0.013) was an
independent risk factor for recurrence (Table VI). An analysis of the predictors of
re-surgery could not be performed in this subgroup due to the small
sample size of patients who underwent re-surgery.
 | Table VI.Univariate and multivariate analyses
of prognostic factors for recurrence in patients with uterine
adenomyosis (rate of recurrence: 35.0%, 7/20). |
Table VI.
Univariate and multivariate analyses
of prognostic factors for recurrence in patients with uterine
adenomyosis (rate of recurrence: 35.0%, 7/20).
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | Patients | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
|---|
| Age at surgery,
years |
|
|
|
|
|
|
|
|
<48 | 13 | 1.940 | 0.431–8.734 | 0.388 |
|
|
|
|
≥48 | 7 | Ref |
|
|
|
|
|
| Thickness of
myometrium, mm |
|
|
|
|
|
|
|
|
<60 | 6 | 5.704 | 1.030–31.605 | 0.046 |
|
|
|
|
≥60 | 13 | Ref |
|
|
|
|
|
| Uterine cavity
length, cm |
|
|
|
|
|
|
|
|
≥10 | 6 | 15.467 | 1.792–133.519 | 0.013 | 15.467 | 1.792–133.519 | 0.013 |
|
<10 | 12 | Ref |
|
| Ref |
|
|
| Preoperative
hemoglobin concentration, mg/dl |
|
|
|
|
|
|
|
|
<9 | 12 | 0.868 | 0.193–3.891 | 0.868 |
|
|
|
| ≥9 | 8 | Ref |
|
|
|
|
|
Discussion
The use of MEA was approved as an advanced medical
treatment by the Ministry of Health, Labour and Welfare of Japan in
December 2008. The pharmaceutical company, Alfressa Pharma,
reported that over 500 patients underwent MEA treatment in Japan as
of April 2012. Reports on the experiences and outcomes from several
facilities have been published (2,5,7). MEA has generally been associated with a
good safety profile (8–10). We have previously reported comparison
of MEA to conventional surgical treatments, including total
abdominal hysterectomy and laparoscopically assisted vaginal
hysterectomy (5). MEA was associated
with significantly less blood loss, as well as shorter surgery time
and hospitalization period (2,5). However,
there are no reports addressing the long-term outcomes of MEA
treatment. To our knowledge, this is the first study to evaluate
the long-term outcomes of patients after MEA (frequency, 2.45 GHz)
and identify the prognostic factors for recurrence and re-surgery
in patients with menorrhagia who underwent MEA.
We have encountered several patients with myomas or
adenomyosis that are resistant to MEA treatment. Myoma growth is
thought to depend on ovarian hormones. As such, myomas are common
among women before menopause and typically reduce or become
asymptomatic after menopause (11–13), which
occurs at a mean age of 51 years (14). Peddada et al (15) prospectively examined myoma growth
rates using MRI. The median growth rate was 9% during 1 year. White
women older than 45 years had the slowest growth among the age
groups, with a 2% growth rate. The relative odds of rapid growth
(>20% increase in volume per 6 months) in white women less than
35 years was 17 times higher than that of white women older than 45
years (15). Several studies in Japan
showed similar growth rates of myomas in Japanese women (16–20).
Therefore, we postulated that women younger than 48 years of age at
MEA treatment might be at higher risk for recurrence or re-surgery.
As expected, we found significantly higher rates of recurrence of
menorrhagia and re-surgery in women younger than 48 years, compared
with those 48 years and older. These findings suggest that the MEA
treatment method may be less effective for younger women with
myomas-namely, for women with a longer period of time until the
onset of menopause. Furthermore, we found high rates of recurrence
or re-surgery in women with myomas that are 4 or more in number or
5 cm or greater in maximum size, in those with a large uterus
(uterine cavity length ≥10 cm), and in those with a low
preoperative hemoglobin level (<9 mg/dl). These clinical factors
are all considered to reflect aggressive characteristics of myomas,
thus it is not surprising that they are associated with an
increased risk for recurrence and re-surgery.
We have also encountered several patients with
adenomyosis resistant to MEA treatment (2). In that study, we found that MEA tended
to be less effective in this patient population, than in women with
myomas (2). We also found that
multiple rounds of MEA treatment may more successfully control
menorrhagia from adenomyosis (21).
We postulated that the thickened myometrium in women with
adenomyosis caused the resistance to MEA treatment and was
associated with recurrence or re-surgery. The results of the
present study support this hypothesis, as there was a higher rate
of recurrence of menorrhagia among women with adenomyosis in which
the myometrium is 60 mm or thicker, compared with those in which it
is thinner than 60 mm. Furthermore, there was a high rate of
recurrence in all women with an enlarged uterus (uterine cavity
length ≥10 cm). These findings suggest that the MEA treatment
method may be less effective for adenomyosis associated with a
thickened myometrium or an enlarged uterus.
MEA treatment showed extremely high efficacy for
functional bleeding and endometrial polyps. The difference in
efficacy for these conditions may be related to the depth of
penetration of the microwaves. In the MEA procedure, only the
endometrium can be ablated, therefore the myomas themselves or the
adenomyosis itself cannot truly be ablated. However, although
functional bleeding is not associated solely with the endometrium,
ablation of the endometrium is nonetheless a good way to control
menorrhagia. Similarly, when an endometrial polyp is small, it can
be ablated easily using MEA.
The strength of our study is that it was conducted
in a single facility in which a strict and well-defined MEA
management policy was in place throughout the study period.
However, there were some limitations in this study. First, our
study is retrospective in design. Second, our data may have been
affected by a small sample size effect because of errors made in
assumptions while calculating the sample size, especially for
adenomyosis. Similarly, we were not able to analyze the re-surgery
rate in the adenomyosis group due to the small sample size.
Finally, the discrepancy of prognostic factors between recurrence
risk and re-surgery risk may be due to the differences between the
number of re-surgery cases and the number of recurrence cases.
In conclusion, MEA is thought to be a highly
efficacious method to control menorrhagia caused by functional
bleeding and endometrial polyps. However, our results indicate that
MEA may be less effective for patients younger than 48 years with
myomas, especially for those with 4 or more myomas or those with a
myoma 5 cm or larger in size. Our findings also suggest that MEA
may be less effective for women with adenomyosis and an enlarged
uterus. Assessment of these clinical parameters may be a method by
which to predict resistance to MEA among patients with menorrhagia
who are considering the procedure. Additional large-scale,
prospective, multicenter studies are needed to confirm these
results.
References
|
1
|
Kanaoka Y, Hirai K and Ishiko O: Microwave
endometrial ablation for an enlarged uterus. Arch Gynecol Obstet.
269:30–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakayama K, Ishibashi K, Ishikawa M,
Katagiri A, Katagiri H, Iida K, Nakayama N and Miyazaki K:
Microwave endometrial ablation at a frequency of 2.45 GHz for
menorrhagia: Analysis of treatment results at a single facility. J
Obstet Gynaecol Res. 40:224–229. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Overton C, Hargreaves J and Maresh M: A
national survey of the complications of endometrial destruction for
menstrual disorders: The MISTLETOE study. Minimally Invasive
surgical techniques-laser, EndoThermal or Endoresection. Br J
Obstet Gynaecol. 104:1351–1359. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nakayama K, Rahman MT, Rahman M, Ishikawa
M, Yeasmin S, Katagiri A, Iida K, Nakayama N, Aoki S and Miyazaki
K: Microwave endometrial ablation is a highly efficacious way to
emergently control life-threatening uterine hemorrhage. Arch
Gynecol Obstet. 283:1065–1068. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakayama K, Yeasmin S, Katagiri A, Rahman
MT, Rahman M, Ishikawa M, Iida K, Nakayama N, Aoki S and Miyazaki
K: A comparative study between microwave endometrial ablation and
conventional surgical procedures for treatment of menorrhagia. Clin
Exp Obstet Gynecol. 38:33–37. 2011.PubMed/NCBI
|
|
6
|
Kanaoka Y, Ishikawa N, Asakawa Y and
Nakayama K: Practice guideline of MEA. http://www.alfresa-pharma.co.jp/microtaze/MEAApril
1–2012
|
|
7
|
Akashi Y, Shimizu A and Mizuuchi M: A
survey of outcomes of microwave endometrial ablation for
menorrhagia in our hospital. J Obstet Gynecol (Tokyo). 76:229–233.
2009.(In Japanese).
|
|
8
|
Milligan MP and Etokowo GA: Microwave
endometrial ablation for menorrhagia. J Obstet Gynaecol.
19:496–499. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Downes E and O'Donovan P: Microwave
endometrial ablation in the management of menorrhagia: Current
status. Curr Opin Obstet Gynecol. 12:293–296. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cooper KG, Bain C, Lawrie L and Parkin DE:
A randomized comparison of microwave endometrial ablation with
transcervical resection of the endometrium; follow up at a minimum
of five years. BJOG. 112:470–475. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flake GP, Anderson J and Dixon D: Etiology
and pathogenesis of uterine leiomyomas: A review. Environ Health
Perspect. 111:1037–1054. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sato F, Mori M, Nishi M, Kudo R and Miyake
H: Familial aggregation of uterine myomas in Japanese women. J
Epidemiol. 12:249–253. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Faerstein E, Szklo M and Rosenshein N:
Risk factors for uterine leiomyoma: A practice-based case-control
study. I. African-American heritage, reproductive history, body
size, and smoking. Am J Epidemiol. 153:1–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shifren JL and Schiff I: MenopauseBerek
& Novak's gynecology. Berek JS, Rinehart RD and Hengst TC: 14th
edition. Lippincott Williams & Wilkins; Philadelphia, PA:
c2007
|
|
15
|
Peddada SD, Laughlin SK, Miner K, Guyon
JP, Haneke K, Vahdat HL, Semelka RC, Kowalik A, Armao D, Davis B
and Baird DD: Growth of uterine leiomyomata among premenopausal
black and white women. Proc Natl Acad Sci USA. 105:pp. 19887–19892.
2008, View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fedele L, Parazzini F, Luchini L,
Mezzopane R, Tozzi L and Villa L: Recurrence of fibroids after
myomectomy: A transvaginal ultrasonographic study. Hum Reprod.
10:1795–1796. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanafi M: Predictors of leiomyoma
recurrence after myomectomy. Obstet Gynecol. 105:877–881. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jacobson GF, Shaber RE, Armstrong MA and
Hung YY: Changes in rates of hysterectomy and uterine conserving
procedures for treatment of uterine leiomyoma. Am J Obstet Gynecol.
196:601.e1–6. 2007. View Article : Google Scholar
|
|
19
|
Nishiyama S, Saito M, Sato K, Kurishita M,
Itasaka T and Shioda K: High recurrence rate of uterine fibroids on
transvaginal ultrasound after abdominal myomectomy in Japanese
women. Gynecol Obstet Invest. 61:155–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoo EH, Lee PI, Huh CY, Kim DH, Lee BS,
Lee JK and Kim D: Predictors of leiomyoma recurrence after
laparoscopic myomectomy. J Minim Invasive Gynecol. 14:690–697.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura K, Nakayama K, Ishikawa M,
Katagiri H, Katagiri A, Ishibashi T, Sato E, Asakawa Y and Kyo S:
Efficacy of multiple microwave endometrial ablation technique for
menorrhagia resulting from adenomyosis. J Obstet Gynaecol Res.
41:1769–1172. 2015. View Article : Google Scholar : PubMed/NCBI
|