Introduction
Antitumor immune responses represent extremely
complex biological processes involving a variety of immune cells,
proteins and signaling molecules (1,2). The
mechanisms underlying antitumor immune responses have been
extensively investigated through in vitro and in vivo
experiments as well as through clinical studies (3,4). It has
been reported that co-stimulatory molecules contribute to T-cell
activation and improve antitumor immunity, thereby playing an
important role in tumor diagnosis and treatment (5–7). For
example, B7-H3 (also known as CD276), a newly identified member of
the B7 family of co-stimulatory molecules, is a type I
transmembrane protein mainly expressed in activated T cells, B
cells, monocytes and dendritic cells (8). In addition to the B7-H3 membrane
protein, a serum soluble B7-H3 (sB7-H3) has also been identified.
This sB7-H3 is involved in the regulation of immune responses and
autoimmune diseases, but its role in the regulation of T-cell
responses remains controversial (9).
Our previous study revealed that the cancer tissue from
hepatocellular carcinoma (HCC) patients exhibited significantly
increased levels of B7-H3 (10).
B7-H3 has also been reported to be involved in the development of a
variety of other solid tumor tissue cancer types, including
osteosarcoma, pancreatic cancer, glioma, bladder cancer and
non-small cell lung cancer (11–15), as
well as in the development of inflammatory diseases (16).
It has been well established that tumor development
is closely associated with inflammatory reactions. Inflammatory
cytokines, including interleukin (IL)-17, IL-8 and IL-6, not only
induce inflammatory reactions by activating lymphocytes, but also
promote tumor cell proliferation and regulate tumor angiogenesis
and development (17). IL-17, a
recently discovered inflammatory cytokine, can induce the
expression of other inflammatory cytokines [including IL-6 and
tumor necrosis factor-α (TNF-α)] and chemotactic factors (18,19). In
this way, IL-17 promotes neutrophil migration to sites of
inflammation and mediates inflammatory responses to enhance the
antitumor effects of the body (20).
IL-8 is a chemotactic inflammatory factor that plays an important
role in maintaining cancer cell self-renewal and resistance to
chemotherapy drugs through its regulation of cancer stem cells
(21–23). IL-6, as a multifunctional cytokine
derived from activated lymphocytes, monocytes, macrophages, bone
marrow cells and certain tumor cells, can induce the synthesis of
infection- or injury-induced acute inflammatory proteins resulting
from acute phase responses.
HCC is the most common type of primary liver cancer
globally (24), and accurate clinical
diagnosis and screening for HCC are critical for improving the
survival rate and the quality of life of HCC patients. Serum
markers, including α-fetoprotein (AFP), carcinoembryonic antigen
(CEA) and carbohydrate antigen 19–9 (CA19-9), are widely used in
the diagnosis of HCC. However, their specificity and sensitivity
are far from meeting the standards required for clinical
evaluation, with the result being that there remains a high rate of
misdiagnosis. Thus, a search for serum inflammatory factors that
may be more specific and sensitive for HCC diagnosis has become the
focus of numerous investigations. At present, the potential
association between the co-stimulatory molecule sB7-H3 and the
cytokines IL-17, IL-8 and IL-6 remains unknown, as does the value
of combining sB7-H3 with IL-17, IL-8 and IL-6 for use in diagnosing
HCC. Therefore, the purpose of the present study was to investigate
the association between serum sB7-H3 levels and levels of IL-17,
IL-8 and IL-6 in HCC patients. The results of the study demonstrate
the potential value of combining sB7-H3 with IL-17, IL-8 and IL-6
for the diagnosis of HCC.
Patients and methods
Patients
A total of 63 hospitalized HCC patients (49 males
and 14 females; age range, 34–74; mean ± standard deviation age,
56±10.9), whose diagnosis was confirmed with pathological tests,
were selected from the Yuhuangding Hospital (Yantai, China) and the
Binzhou Medical College Affiliated Hospital (Yantai, China), and
were assessed between January and November in 2008. Another 50
healthy subjects (32 males and 18 females; age range, 26–75; mean ±
standard deviation age, 52±11.5 years), also from the Yuhuangding
Hospital and the Binzhou Medical College Affiliated Hospital, were
assessed over the same time period and served as controls. The
control subjects had no autoimmune diseases, no history of
allergies and no recent infections, and were otherwise healthy,
collected consecutively between January and November 2008. The sex
and ages of the control group matched those of the HCC group, with
no statistically significant differences being identified between
the two groups. Relative clinical information (age, sex, clinical
staging, distant metastasis and nodal metastasis) according to the
7th edition tumor-node-metastasis (TNM) classification of the
American Joint Committee on Cancer Staging system (25) was provided by the Yuhuangding Hospital
and the Binzhou Medical College Affiliated Hospital with informed
consent from the patients. This project was approved by the Ethics
Committee at Yuhuangding Hospital and the Binzhou Medical
University Affiliated Hospital (Yantai, China) and written informed
consent was obtained from each patient for specimen collection and
participation in the study.
Sample collection
Fasting blood samples (3 ml) were drawn from the HCC
patients and controls in the morning. Serum was immediately
separated by centrifugation at 754 × g, 4°C for 5 min and stored at
−80°C prior to being assayed for serum levels of sB7-H3, IL-17,
IL-8 and IL-6.
sB7-H3, IL-17, IL-8 and IL-6
measurements
An sB7-H3 ELISA kit was developed by the
Biotechnology Research Institute of Suzhou University (Suzhou,
China) and was kindly gifted by Suzhou University for use in
analyzing serum sB7-H3 levels in HCC patients and controls
(26). IL-17, IL-8 and IL-6 ELISA
kits (IL-17, cat. no. D1700; IL-8, cat. no. D8000C; IL-6, cat. no.
D6050; R&D Systems, Inc., Minneapolis, MN, USA) were used for
assaying serum IL-17, IL-8 and IL-6 levels according to the
manufacturer's protocols.
Statistical analysis
Data were analyzed using SPSS15.0 software. Numeric
data are expressed as the mean ± standard error of the mean (SEM).
Comparisons between the two groups were performed using unpaired
Student's t-tests. The accuracy of the test in distinguishing HCC
patients from healthy patients was evaluated using receiver
operating characteristic (ROC) curve analysis, with the area under
curve (AUC) being used to measure predictive accuracy. The ROC was
drawn using Medcalc software (version 15.0; Medcalc Software,
Ostend, Belgium). The maximal Youden index (sensitivity +
specificity-1) was used to calculate the cutoff point. Logistic
regression analysis was used to analyze whether the biomarker
levels predicted an HCC diagnosis. P<0.05 was required for
results to be considered statistically significant.
Results
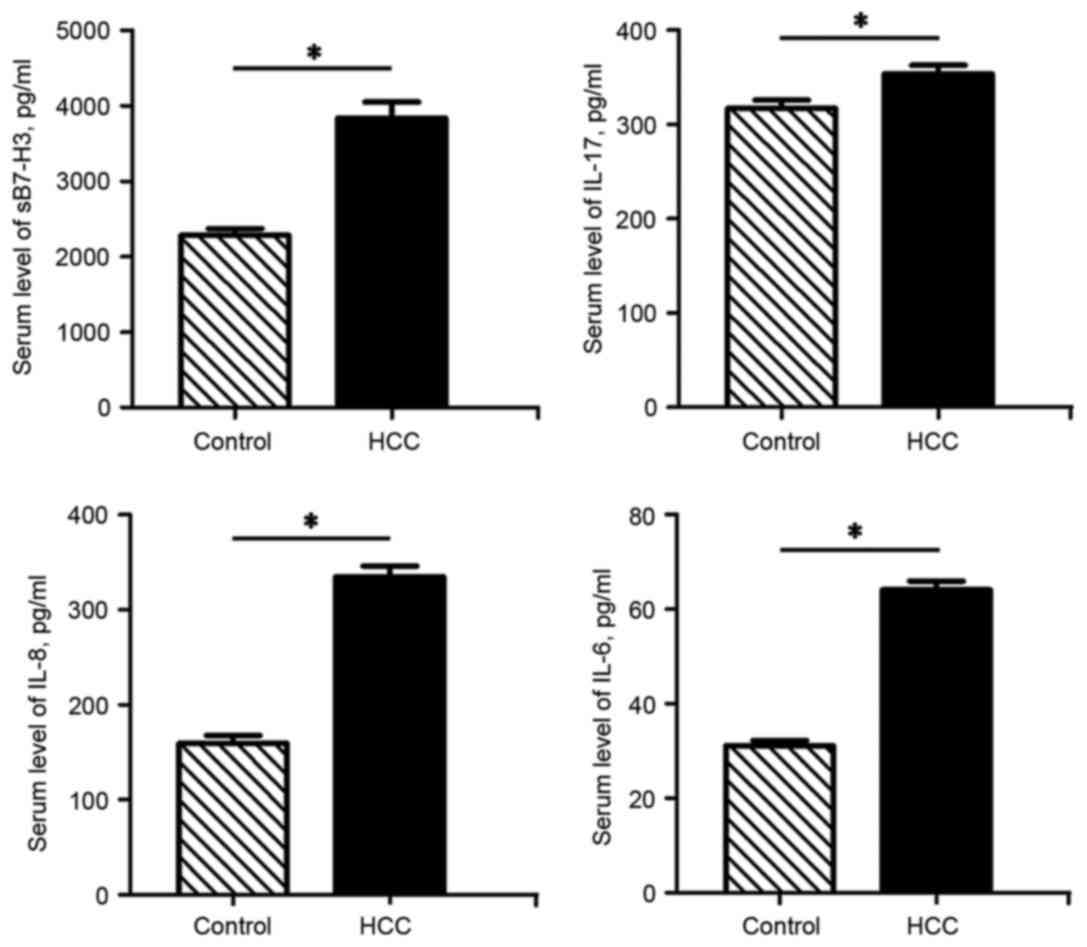
HCC patients exhibit elevated serum
levels of sB7-H3, IL-17, IL-8 and IL-6
In line with our previous findings, which revealed
increased B7-H3 levels in tumor tissues (10), the ELISA assay results from the
present study demonstrated that the serum sB7-H3 levels of the HCC
patients were significantly higher than those of the control
subjects (P<0.05). In addition, the serum IL-17, IL-8 and IL-6
levels in the HCC patients were also significantly increased
compared with those of the control group (P<0.05) (Fig. 1).
Association between sB7-H3, IL-17,
IL-8 and IL-6 levels in HCC patients and their clinicopathological
characteristics
Although our previous findings (10) revealed an association between tumor
tissue B7-H3 levels and clinicopathological characteristics, the
present study identified no significant association between sB7-H3
levels and certain clinicopathological characteristics, including
age, sex and lymph node metastasis. However, sB7-H3 levels were
found to differ significantly among the clinical stages examined,
and were associated with distant metastasis. Specifically, sB7-H3
levels for stage III patients were significantly higher than those
of the overall means at stages I and II (4,353.536±556.423 vs.
3,588.798±690.508 pg/ml; P<0.01), and patients with distant
metastasis had significantly increased sB7-H3 levels compared with
those without (4,205.617±704.864 vs. 3,719.306±726.908 pg/ml;
P<0.05). In the present study, the association between serum
levels of IL-17, IL-8 or IL-6 and clinicopathological
characteristics, including age, sex, pathological stage and lymph
node metastasis, was also analyzed. No significant differences for
age and sex were identified among IL-17, IL-8 and IL-6 levels.
Furthermore, while HCC patients with lymph node metastasis
exhibited lower IL-17 and IL-6 and higher IL-8 levels compared with
those without lymph node metastasis, these differences were not
statistically significant (Table
I).
 | Table I.Association between serum inflammatory
factors in hepatocellular carcinoma patients and their
clinicopathological characteristics. |
Table I.
Association between serum inflammatory
factors in hepatocellular carcinoma patients and their
clinicopathological characteristics.
| Group | n | sB7-H3, pg/ml | IL-17, pg/ml | IL-8, pg/ml | IL-6, pg/ml |
|---|
| Age, years |
|
|
|
|
|
|
<60 | 22 |
3,873.369±622.368 | 349.934±76.505 | 337.347±94.018 | 64.073±14.229 |
| ≥60 | 41 |
3,850.689±829.661 | 360.087±63.329 | 334.007±83.785 | 63.375±15.632 |
| Sex |
|
|
|
|
|
| Male | 49 |
3,940.958±891.735 | 354.797±67.336 | 319.177±81.509 | 63.839±14.332 |
|
Female | 14 |
3,650.267±819.650 | 350.698±86.772 | 385.828±97.451 | 64.535±16.088 |
| Clinical staging |
|
|
|
|
|
| I–II | 39 |
3,588.798±690.508 | 340.565±83.387 | 326.112±87.642 | 65.556±13.411 |
| III | 24 |
4,353.536±556.423a | 361.381±63.419 | 345.979±91.673 | 61.243±16.111 |
| Distant
metastasis |
|
|
|
|
|
| Yes | 14 |
4,205.617±704.864b | 360.297±69.329 | 361.410±98.941 | 57.015±16.041 |
| No | 49 |
3,719.306±726.908 | 333.003±76.051 | 325.027±84.597 | 66.542±13.371 |
| Nodal
metastasis |
|
|
|
|
|
|
Yes | 10 |
4,138.331±677.314 | 320.313±31.428 | 377.399±25.301 | 61.851±15.282 |
| No | 53 |
3,812.446±658.582 | 359.252±19.091 | 326.625±12.413 | 64.981±10.311 |
Associations among sB7-H3, IL-17, IL-8
and IL-6 levels
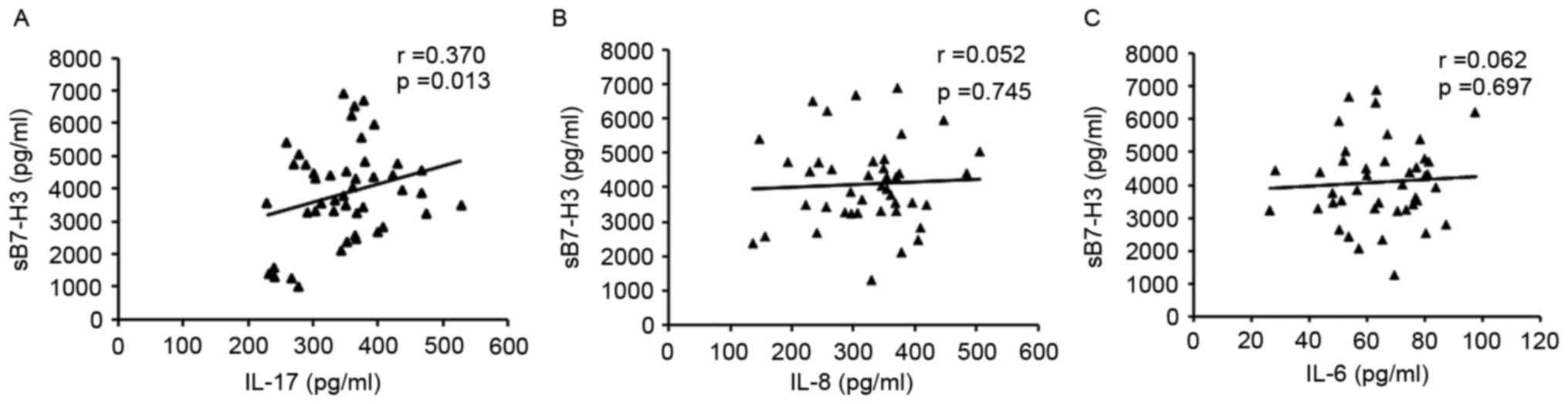
Analysis of the associations between serum sB7-H3
and IL-17, IL-8 or IL-6 in HCC patients revealed that serum sB7-H3
levels were positively associated with IL-17 levels, but that no
statistically significant associations were identified between IL-8
or IL-6 and sB7-H3 levels (Fig. 2).
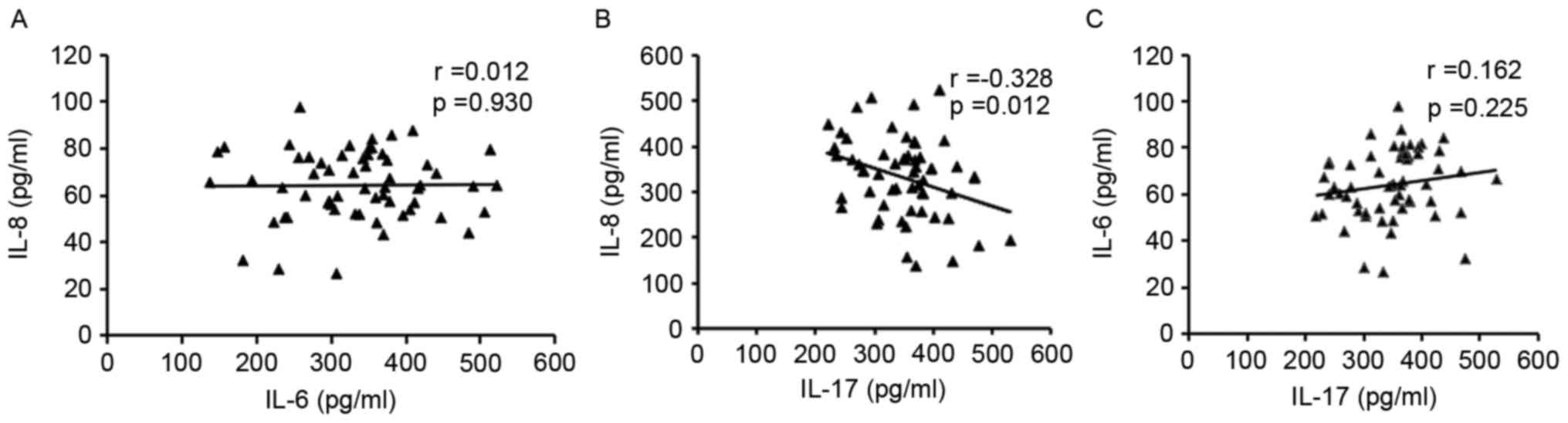
Analysis of the associations among these inflammatory cytokines
revealed that while IL-17 was negatively associated with IL-8,
there was no association with IL-6. Furthermore, no statistically
significant association was exhibited between IL-8 and IL-6 levels
(Fig. 3).
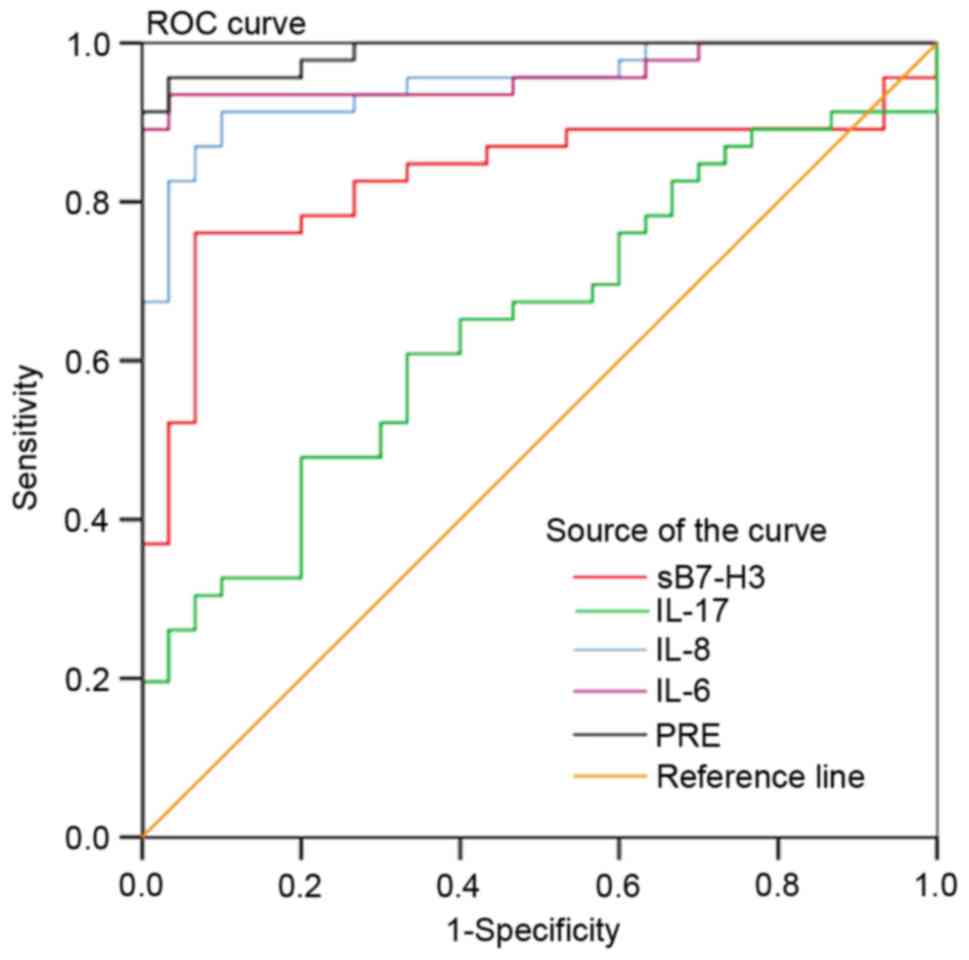
Analysis of accuracy in using sB7-H3,
IL-17, IL-8 or IL-6 to diagnose HCC
Serum sB7-H3, IL-17, IL-8 and IL-6 levels of the
healthy control subjects provided the reference levels for this
study, with which an ROC curve was generated to evaluate the
accuracy of using these biomarkers in HCC diagnosis. Results of
this analysis revealed that AUC values for the diagnosis of HCC
were 83.2% for sB7-H3 (P=0.0001), 65.7% for IL-17 (P=0.0066), 95.3%
for IL-8 (P=0.0001) and 97.0% for IL-6 (P=0.0001) (Fig. 4; Table
II). These findings suggest that each of these biomarkers may
serve as valid and reliable markers for the clinical diagnosis of
HCC.
 | Table II.Serum sB7-H3, IL-17, IL-8 and IL-6 in
diagnosing hepatocellular carcinoma, and their sensitivity,
specificity and Youden index. |
Table II.
Serum sB7-H3, IL-17, IL-8 and IL-6 in
diagnosing hepatocellular carcinoma, and their sensitivity,
specificity and Youden index.
| Biomarker | Sensitivity, % | Specificity, % | AUC, % | 95% CI | P-value | Std | Youden index |
|---|
| sB7-H3 | 76.09 | 90.20 | 83.2 | 0.742–0.900 | 0.0001 | 0.0424 | 0.6629 |
| IL-17 | 51.60 | 80.00 | 65.7 | 0.552–0.752 | 0.0066 | 0.0579 | 0.3160 |
| IL-8 | 91.70 | 91.40 | 95.3 | 0.889–0.986 | 0.0001 | 0.0208 | 0.8310 |
| IL-6 | 95.00 | 96.85 | 97.0 | 0.913–0.994 | 0.0001 | 0.0164 | 0.9185 |
| PRE | 96.67 | 97.14 | 99.2 | 0.947–1.000 | 0.0001 | 0.0084 | 0.9381 |
Application of a logistic regression
model with sB7-H3, IL-17, IL-8 and IL-6 in HCC diagnosis
A stepwise logistic regression model was used to
evaluate the accuracy of using sB7-H3, IL-17, IL-8 and IL-6 in HCC
diagnosis. The significance level for entry into the model was
0.10, and the significance level for remaining in the model was
0.15. The regression equation was as follows: P = 1/[1 +
e−(−11.23 +
0.001x1−0.018x2 +
0.035x3 +
0.191x4)], where x1
represents sB7-H3, x2 represents IL-17, x3
represents IL-8 and x4 represents IL-6. P was the
predicted probability for the logistic model with a range of 0–1,
and e was the natural logarithm (e≈2.718). These results
demonstrated that patients with high levels of these parameters
were at greater risk of HCC, with increases of 1.032 for IL-8 and
1.151 for IL-6 being observed (Table
III). Using the predicted probability (PRE) as a test variable
obtained by the combination of sB7-H3, IL-17, IL-8 and IL-6 in the
regression model, the value for AUC was 99.2%, which was
significantly greater than that observed when these inflammatory
factors were used as individual variables: sB7-H3 (AUC, 83.2%),
IL-17 (AUC, 65.7%), IL-8 (AUC, 95.3%) and IL-6 (AUC, 97.0%). The
sensitivity of the model using all four biomarkers was 96.67%,
which was significantly greater than the sensitivities of each
inflammatory marker when used as a single predictor. Furthermore,
the specificity of the model using all four biomarkers was 97.14%,
which was significantly greater than the individual specificities
of each when used as single predictors (Table II; Fig.
4). Additionally, there was a clear cutoff value for the PRE
(0.5745) in the regression model obtained by the combination of
sB7-H3, IL-17, IL-8 and IL-6.
 | Table III.Stepwise logistic regression model
for diagnosing hepatocellular carcinoma (n=113). |
Table III.
Stepwise logistic regression model
for diagnosing hepatocellular carcinoma (n=113).
| Biomarker | β | P-value | OR value | 95% CI |
|---|
| IL-8 | 0.032 | 0.009 | 1.032 | 1.008–1.057 |
| IL-6 | 0.141 | 0.004 | 1.151 | 1.046–1.266 |
| Constant | −12.9 | <0.001 |
|
|
Discussion
HCC is one of the most common malignant tumors, and
is known to have a poor prognosis due to its invasive and
metastatic properties. At present, AFP and CEA are two of the most
common serum tumor markers used to diagnose liver cancer. However,
neither of these are highly specific markers for liver cancer
(27), and therefore, the attention
of numerous researchers has turned toward finding a more specific
diagnostic marker for liver cancer. Previously, we reported that
B7-H3 levels were increased in the tumor tissue of HCC patients
(10). In the present study, this
investigation was extended to include serum levels of sB7-H3 and
the cytokines IL-17, IL-8 and IL-6 in HCC patients, and the
potential of combining these factors for use as diagnostic
biomarkers of HCC was evaluated. The results revealed that serum
levels of sB7-H3 in HCC patients were significantly increased
compared with those of the healthy control group. In support of
these findings are data on the serum levels of sB7-H3 in patients
with non-small cell lung cancer, which were also significantly
increased when compared with those of healthy controls (26). Taken together, these findings suggest
that sB7-H3 could serve as a serum diagnostic biomarker for
HCC.
The present study also observed that serum IL-17,
IL-8 and IL-6 levels were increased in HCC patients compared with
those in the healthy control group, but the design of this study
did not provide an opportunity to determine the underlying
mechanisms for such increases. However, it has been reported that
increases in the number of Th17 cells in HCC patients, which occur
concurrently with increases in serum IL-17 levels, are associated
with decreases in overall survival and disease-free survival time
(28,29). Additionally, Park et al
(30) reported that anticancer drugs
can induce IL-8 secretion and the expression of IL-8 receptors on
liver cells. Furthermore, serum IL-8 levels were positively
correlated with tumor size, whereas silencing of the IL-8 gene
within hepatocytes significantly decreased tumor size. It has also
been reported that IL-8 is involved in tumor angiogenesis, growth
and metastasis (31), and can
therefore serve as an important chemokine for blood vessel
formation in HCC (32). In order to
clarify the clinical effects and associations of IL-6, IL-27, TNF-α
and vascular endothelial growth factor with signal transducer and
activator of transcription proteins under different clinical and
pathological stages of HCC, Kao et al (33) demonstrated that overexpression of IL-6
was closely associated with maintaining liver function and 6-month
mortality rate in HCC while the elevated IL-27, TNF-α, or VEGF
presented no significant correlation with 6-month mortality rate in
HCC. In addition, determinations of preoperative IL-6 serum levels
can serve as a potential biomarker for the early prediction of
hepatitis B virus-related HCC relapse (34). Results from the present study revealed
that serum levels of soluble IL-17 in HCC patients were
significantly increased and positively associated with sB7-H3
levels, but that they were negatively associated with IL-8 levels.
The level of IL-6 was also increased in the HCC patients,
indicating that IL-6 participates in the development of HCC.
Notably, no association was observed between serum levels of sB7-H3
and IL-8. Together, these data suggest that the immune regulation
of sB7-H3 and cytokines in HCC involves a complex process. However,
details regarding the mechanisms involved require further
investigations.
Given our previous findings that B7-H3 was highly
expressed in tumor tissue (10), and
the results of the present study demonstrating increased serum
levels of sB7-H3 and a positive correlation between serum sB7-H3
and IL-17 levels in HCC patients, efforts were directed toward
assessing the accuracy of using sB7-H3, IL-17, IL-8 and IL-6 in HCC
diagnosis using ROC analysis. The potential value of using sB7-H3,
IL-17, IL-8 and IL-6 in HCC diagnosis was also evaluated using
logistic regression analysis. Results from the ROC analyses
indicated that levels of sB7-H3, IL-17, IL-8 and IL-6 all differed
significantly between the HCC patients and the control group.
Further logistic regression analysis revealed that combining the
responses obtained from all four of these biomarkers significantly
enhanced the potential for predicting HCC. An optimal cutoff value
of 0.5745 for PRE was obtained by combining the four biomarkers in
the regression model. The specificity and AUC achieved maximal
values of 97.14 and 99.2%, respectively, for this combination of
biomarkers, which were higher in comparison with the specificity
and AUC observed when the four biomarkers were used individually.
These findings suggest that this model has potential applications
for the clinical diagnosis of HCC.
In the present study, combinations of serum sB7-H3,
IL-17, IL-8 and IL-6 yielded a high AUC and were revealed to be
highly specific and sensitive in diagnosing HCC. To the best of our
knowledge, there exists no report proposing the combined use of
sB7-H3 and cytokines for the diagnosis of HCC. However, one
limitation of this study was the small sample size, which may have
caused multicollinearity in the logistic regression model.
Additionally, no comparison was made between the proposed model and
the commonly used clinical serological markers, AFP and CEA, with
regards to their relative efficacies in diagnosing HCC. Therefore,
a future study would incorporate larger samples and a comparison
between the proposed model and AFP and CEA, in order to assess the
relative effectiveness of this model.
To conclude, an accurate clinical diagnosis and
screening tool for HCC is vital for improving the survival rate and
quality of life of HCC patients. The present study investigated the
association among sB7-H3 and cytokine levels of IL-17, IL-8 and
IL-6 in HCC patients. Based on these findings, the combination of
serum B7-H3, IL-17, IL-8 and IL-6 may provide a novel and effective
protocol for use in HCC screening and diagnosis.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Shandong Province (grant no. ZR2010HL048) and
by the Medical and Health Development Projects of Shandong Province
(grant no. 2013WS0132).
References
|
1
|
Zhao X and Subramanian S: Oncogenic
pathways that affect antitumor immune response and immune
checkpoint blockade therapy. Pharmacol Ther. Jul 15–2017.(Epub
ahead of print). View Article : Google Scholar
|
|
2
|
Marinelli L, Tenore GC and Novellino E:
Probiotic species in the modulation of the anticancer immune
response. Semin Cancer Biol. 46:182–190. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang A, Peng S, Farmer E, Zeng Q, Cheng
MA, Pang X, Wu TC and Hung CF: Enhancing antitumor immunogenicity
of HPV16-E7 DNA vaccine by fusing DNA encoding E7-antigenic peptide
to DNA encoding capsid protein L1 of Bovine papillomavirus. Cell
Biosci. 7:462017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wein L, Savas P, Luen SJ, Virassamy B,
Salgado R and Loi S: Clinical validity and utility of
tumor-infiltrating lymphocytes in routine clinical practice for
breast cancer patients: Current and future directions. Front Oncol.
7:1562017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lan KH, Liu YC, Shih YS, Tsaid CL, Yen SH
and Lan KL: A DNA vaccine against cytotoxic T-lymphocyte associated
antigen-4 (CTLA-4) prevents tumor growth. Biochem Biophys Res
Commun. 440:222–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao Q, Fu A, Yang S, He X, Wang X, Zhang
X, Zhou J, Luan X, Yu W and Xue J: Leukocyte-associated
immunoglobulin-like receptor-1 expressed in epithelial ovarian
cancer cells and involved in cell proliferation and invasion.
Biochem Biophys Res Commun. 458:399–404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hellmann MD, Friedman CF and Wolchok JD:
Combinatorial cancer immunotherapies. Adv Immunol. 130:251–277.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steinberger P, Majdic O, Derdak SV,
Pfistershammer K, Kirchberger S, Klauser C, Zlabinger G, Pickl WF,
Stöckl J and Knapp W: Molecular characterization of human
4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J
Immunol. 172:2352–2359. 2014. View Article : Google Scholar
|
|
9
|
Yan R, Yang S, Gu A, Zhan F, He C, Qin C,
Zhang X and Feng P: Murine b7-h3 is a co-stimulatory molecule for T
cell activation. Monoclon Antib Immunodiagn Immunother. 32:395–398.
2013.PubMed/NCBI
|
|
10
|
Wang F, Wang G, Liu T, Yu G, Zhang G and
Luan X: B7-H3 was highly expressed in human primary hepatocellular
carcinoma and promoted tumor progression. Cancer invest.
32:262–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao JL, Chen FL, Zhou Q, Pan W, Wang XH,
Xu J, Zhang SX, Ni LI and Yang HL: B7-H3 protein expression in a
murine model of osteosarcoma. Oncol Lett. 12:383–386.
2016.PubMed/NCBI
|
|
12
|
Xu H, Chen X, Tao M, Chen K, Chen C, Xu G,
Li W, Yuan S and Mao Y: B7-H3 and B7-H4 are independent predictors
of a poor prognosis in patients with pancreatic cancer. Oncol Lett.
11:1841–1846. 2016.PubMed/NCBI
|
|
13
|
Wang Z, Yang J, Zhu Y, Zhu Y, Zhang B and
Zhou Y: Differential expression of 2IgB7-H3 and 4IgB7-H3 in cancer
cell lines and glioma tissues. Oncol Lett. 10:2204–2208.
2015.PubMed/NCBI
|
|
14
|
Wu D, Zhang Z, Pan H, Fan Y, Qu P and Zhou
J: Upregulation of the B7/CD28 family member B7-H3 in bladder
cancer. Oncol Lett. 9:1420–1424. 2015.PubMed/NCBI
|
|
15
|
Sun J, Mao Y, Zhang YQ, Guo YD, Mu CY, Fu
FQ and Zhang XG: Clinical significance of the induction of
macrophage differentiation by the costimulatory molecule B7-H3 in
human non-small cell lung cancer. Oncol Lett. 6:1253–1260.
2013.PubMed/NCBI
|
|
16
|
Steinberger P: B7-H3 ameliorates GVHD.
Blood. 125:3219–3221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rusolo F, Pucci B, Colonna G, Capone F,
Guerriero E, Milone MR, Nazzaro M, Volpe MG, Di Bernardo G,
Castello G and Costantini S: Evaluation of selenite effects on
selenoproteins and cytokinome in human hepatoma cell lines.
Molecules. 18:2549–2562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jovanovic DV, Di Battista JA,
Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F and
Pelletier JP: IL-17 stimulates the production and expression of
proinflammatory cytokines, IL-beta and TNF-alpha, by human
macrophages. J Immunol. 160:3513–3521. 1998.PubMed/NCBI
|
|
19
|
Zrioual S, Toh ML, Tournadre A, Zhou Y,
Cazalis MA, Pachot A, Miossec V and Miossec P: IL-17RA and IL-17RC
receptors are essential for IL-17A-induced ELR+ CXC
chemokine expression in synoviocytes and are overexpressed in
rheumatoid blood. J Immunol. 180:655–663. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang G, Zhou KF and Lu ZH: Interleukin-17
enhances the removal of respiratory syncytial virus in mice by
promoting neutrophil migration and reducing interferon-gamma
expression. Genet Mol Res. 15:2016.
|
|
21
|
Sanacora S, Urdinez J, Chang TP and
Vancurova I: Anticancer drug bortezomib increases interleukin-8
expression in human monocytes. Biochem Biophys Res Commun.
460:375–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Duan Y, Cheng X, Chen X, Xie W,
Long H, Lin Z and Zhu B: IL-17 is associated with poor prognosis
and promotes angiogenesis via stimulating VEGF production of cancer
cells in colorectal carcinoma. Biochem Biophys Res Commun.
407:348–354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiu BL, Li CH and Chang CC: Selective
modulation of MHC class II chaperons by a novel IFN-γ-inducible
class II transactivator variant in lung adenocarcinoma A549 cells.
Biochem Biophys Res Commun. 440:190–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang JH, Wei W, Guo ZX, Shi M and Guo RP:
Decreased Cezanne expression is associated with the progression and
poor prognosis in hepatocellular carcinoma. J Transl Med.
13:412015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B
and Zhang X: Diagnosis value of serum B7-H3 expression in non-small
cell lung cancer. Lung Cancer. 66:245–249. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ching CT, Van Hieu N, Cheng TY, Fu LS, Sun
TP, Liu MY, Huang SH and Yao YD: Liver cancer detection by a
simple, inexpensive and effective immunosensor with zinc oxide
nanoparticles. Sensors (Basel). 15:29408–29418. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW,
Cai XY, Zhou J, Cheng YF, Fan J and Qiu SJ: High expression of
IL-17 and IL-17RE associate with poor prognosis of hepatocellular
carcinoma. J Exp Clin Cancer Res. 32:32013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang Q, Luan W, Warren L, Fiel MI, Blank
S, Kadri H, Mandeli J and Hiotis SP: Prognostic role of immune
cells in hepatitis B-associated hepatocellular carcinoma following
surgical resection depends on their localization and tumor size. J
Immunother. 39:36–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SY, Han J, Kim JB, Yang MG, Kim YJ,
Lim HJ, An SY and Kim JH: Interleukin-8 is related to poor
chemotherapeutic response and tumourigenicity in hepatocellular
carcinoma. Eur J Cancer. 50:341–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang KH, Ma S, Lee TK, Chan YP, Kwan PS,
Tong CM, Ng IO, Man K, To KF, Lai PB, et al: CD133(+) liver
tumor-initiating cells promote tumor angiogenesis, growth, and
self-renewal through neurotensin/interleukin-8/CXCL1 signaling.
Hepatology. 55:807–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu B, Lin N, Zhang M, Zhu Y, Cheng H,
Chen S, Ling Y, Pan W and Xu R: Activated hepatic stellate cells
promote angiogenesis via interleukin-8 in hepatocellular carcinoma.
J Transl Med. 13:3652015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kao JT, Feng CL, Yu CJ, Tsai SM, Hsu PN,
Chen YL and Wu YY: IL-6, through p-STAT3 rather than p-STAT1,
activates hepatocarcinogenesis and affects survival of
hepatocellular carcinoma patients: A cohort study. BMC
Gastroenterol. 15:502015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sheng T, Wang B, Wang SY, Deng B, Qu L, Qi
XS, Wang XL, Deng GL and Sun X: The relationship between serum
interleukin-6 and the recurrence of hepatitis B virus related
hepatocellular carcinoma after curative resection. Medicine
(Baltimore). 94:e9412015. View Article : Google Scholar : PubMed/NCBI
|