Introduction
Breast cancer is the most common type of cancer
among women, accounting for 29% of all newly diagnosed cases of
cancer and 15% of all cancer-associated mortalities in 2014 in the
United States (1). Genetic analysis
indicates that breast cancer is a disease of phenotypic
heterogeneity, which includes various molecular subtypes associated
with clinical prognoses. Triple-negative breast cancers (TNBCs),
which are characterized by lacking expression of estrogen receptor
(ER), progesterone receptor (PR) and human epidermal growth factor
receptor2 (HER2), comprise 15–20% of breast cancer cases and are
considered the most malignant subtype, with the highest risk of
metastasis (2). TNBCs more frequently
disseminate to the distant organs, including brain, lung and liver,
than to regional lymph nodes (2,3).
Metastasis is regarded as the key contributor to breast
cancer-associated mortality. In general, the 5-year survival rates
for patients with localized and regional breast cancer are 98.6 and
84.9%, respectively. However, if remote metastasis occurs, the
5-year relative survival rate is only 25.9% (4). Tumor progression and metastasis are
complex processes that are influenced by a variety of extrinsic and
intrinsic factors (5,6). Although the potential mechanisms
underlying tumor metastasis remain incompletely defined, cell
migration has attracted extensive attention as itis recognized as
the first and fundamental step for the dissemination of a
malignancy (7).
Hypoxia is an important component of the
microenvironment of various types of solid tumor, including breast
cancer (8). In hypoxia, whereas some
tumor cells will undergo apoptosis, the majority of the tumor cells
will adapt to the hypoxic conditions by favoring metabolic pathways
that do not require oxygen, or by promoting angiogenesis and
mutation to increase oxygen supply (9,10). It has
been identified thathypoxia-induciblefactor1 (HIF-1) serves an
important role in the response to hypoxia. HIF-1 is a transcription
factor consisting of a constitutively expressed HIF-1β subunit and
an oxygen-sensitive HIF-1α subunit. The transcriptional activity of
HIF-1 depends on the availability of HIF-1α protein, which is
accumulated under hypoxic conditions, and quickly degraded under
normoxic conditions. HIF-1 activates the transcription of numerous
genes involved in cancer progression.
A pool of studies have demonstrated that hypoxia
promotes cell migration; this process is associated with increased
HIF-1α stability and activity, as well as the upregulation of
vimentin, a marker for mesenchymal cells (11). Vimentin is a member of the
intermediate filament family, the members of which constitute part
of the cytoskeleton (12,13). In embryogenesis, vimentin serves a
pivotal role in the differentiation of organs and tissues (13). In the development of tumors, vimentin
may alter cellular polarity, regulate cell contact formation and
transport signal proteins involved in cell mobility (6). However, the dynamics linking the changes
in HIF-1α and vimentin levels in hypoxic conditions have not been
fully investigated.
Hypoxia universally occurs in solid tumors; however,
the duration of hypoxia varies greatly between and within tumors.
Previous observations have revealed there are two major forms of
hypoxia in tumors: Continuous hypoxia (CH) and intermittent hypoxia
(IH). CH develops due to the imbalance between the rapid
proliferation of cells and inadequate tumor angiogenesis/oxygen
supply; this occurs because the blood supply is primarily located
in tumor stroma, and the maximum oxygen diffusion distance in
malignant tissues is 100–150 µm (14,15).
Alternatively, in the tumor microenvironment, the structural
abnormalities of tumor vasculature can produce unstable
hemodynamics and cause IH (16,17).
Histological analyses have shown that tumor vasculatures are
characterized by an uneven thickness of the vascular basement
membrane, a loose association or absence of vascular endothelial
cells, a lack of vessel contractility, compression by tumor cells,
vessel formation that is tortuous and dilated, and numerous dead
ends. The duration of IH may range from min to days in various
tumors, depending on the level of maturation and the structural
complexity of the tumor vessel networks (18). Previous studies have focused on acute
or chronic CH and the results are controversial (19,20).
Although IH models have been tested in ovarian, lung and gastric
cancer cells, the effects of IH on breast cancer cells remain
unclear and require further investigation (16,21,22).
In the present study, human breast cancer MDA-MB-231
cells were cultured in an IH, CH or normoxic environment. The
effect of the conditions on the migration and proliferation of
MDA-MB-231 cells and the mechanisms involved were investigated. The
data demonstrated that multiple cycles of hypoxia and reoxygenation
induced a more invasive phenotype in breast cancer cells, mediated
by HIF-1α activation and associated vimentin upregulation.
Materials and methods
Cell culture and hypoxia
treatment
Human breast adenocarcinoma MDA-MB-231cells (a TNBC
cell line) were obtained from the Cell Bank of the Chinese Academy
of Sciences (Shanghai, China), and were cultured in Gibco
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; MRC Biological Technology Co., Ltd., Jiangsu China), 100 U/ml
penicillin, and 100 µg/ml streptomycin. The MDA-MB-231 cells were
cultured under normoxic, CH or IH conditions. For normoxic
cultures, the cells were grown in a humidified incubator (37°C, 21%
O2, 74% N2, 5% CO2). For CH
cultures, the cells were grown in a sealed hypoxic incubator for 48
h (37°C, 1% O2, 94% N2, 5% CO2).
For IH cultures, the cells were subjected to a specified number of
hypoxia (37°C, 1% O2, 94% N2, 5%
CO2) and reoxygenation (37°C, 21% O2, 74%
N2, 5% CO2) cycles; each hypoxia and
reoxygenation cycle included 12 h of hypoxic incubation followed by
12 h of normoxic culture. When reoxygenated, the cell culture
medium was replaced and the dishes were placed into a normoxic
chamber.
Wound healing assay
To evaluate the mobility of MDA-MB-231 cells
indifferent oxygenation conditions, cells were cultured in 6-well
plates or 60-mm dishes with DMEM. For the IH group, the cells were
subjected to 10 cycles of 12 h hypoxic incubation (37°C, 1%
O2, 94% N2, 5% CO2) and 12 h
reoxygenation (37°C, 21% O2, 74% N2, 5%
CO2) cycles prior to testing and were continually
exposed to IH conditions for 48 h during testing. For the CH group,
the cells were cultured under hypoxia for 48 h (37°C, 1%
O2, 94% N2,5% CO2) during the
testing. For the normoxic group, the cells were exposed to normoxia
for 48 h (37°C, 21% O2, 74% N2, 5%
CO2) during the testing, without the 10 cycles of
hypoxia. When 90% confluence was reached, a ‘wound’ was created on
the cell monolayer with a 200-µl pipette tip, the plates were
washed three times with PBS and the medium was replaced with
FBS-free DMEM. Cell migration at 0 and 48 h was assessed and
photographed under an inverted fluorescence microscope (Leica
Microsystems GmbH, Wetzlar, Germany). The wound area was assessed
using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.,
Rockville, MD, USA). The relative mobility was calculated by the
following migration index: Relative mobility=(wound width at 0
h-wound width at 48 h)/wound width at 0 h. Wound width was
calculated wound area divided by image height.
Trans-well migration assay
A Boyden chamber system (Corning Life Sciences,
Lowell, MA, USA) was applied to assess the migration ability of
breast cancer cells. Cells were pre-treated with or without hypoxic
conditions, then trypsinized and resuspended in serum-free DMEM at
a density of 2×104/250 µl. The resuspended cells were
placed on the upper layer of a cell-permeable membrane, and 500 µl
complete medium was placed below the membrane. The plates were
incubated for 48 h in IH, CH and normoxic conditions, respectively.
The cells on the upper layer of the membrane were removed with a
cotton swab; the migrated cells on the lower membrane were fixed in
4% paraformaldehyde and stained with 0.1% crystal violet. The
migrated cells were photographed and counted using an inverted
microscope and random fields were scanned (5 fields/filter).
Subsequently, cells were incubated in 10% glacial acetic acid for
20 min, and the medium was collected in 96-well plates. The optical
density (OD) at 570 nm was measured by a microplate reader (Thermo
Fisher Scientific, Inc.), which represented the relative levels of
cell migration.
Cell proliferation assay
The proliferation of MDA-MB-231 cells in normoxia or
hypoxia was assessed with a Cell Counting Kit-8 (CCK-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan). For the IH group,
the cells were subjected to 10 cycles of hypoxia (37°C, 1%
O2, 94% N2, 5% CO2) and
reoxygenation (37°C, 21% O2, 74% N2, 5%
CO2) cycles prior to the start of the CCK-8 assay and
were continually cultured under IH conditions during the assay. For
the CH group, the cells were continuously exposed to hypoxic
conditions (37°C, 1% O2, 94% N2, 5%
CO2) during the CCK-8 assay. For the normoxic group, the
cells were exposed to normoxic conditions (37°C, 21% O2,
74% N2, 5% CO2) during the assay, without the
10 cycles of hypoxia. A total of 1.5×103 cells/well (or
3.0×103 cells/well when subsequent to transfection) were
seeded in 96-well culture plates and cultured for 4–6 h at 37°C
(21% O2, 74% N2, 5% CO2). When the
cells had adhered, CCK-8 reagents were added and incubated at 37°C
(21% O2, 74% N2, 5% CO2) for an
additional 2 h. The OD was measured at 450 nm on a microplate
reader to ensure an equal number of cells had been seeded, and this
represented the number of cells on the 0th day. Then, the cells
were cultured at 37°C under IH, CH and normoxic conditions for four
days with DMEM and the OD was subsequently determined on the 1st,
2nd, 3rd and 4th days of culture.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.), and
the first-strand cDNA was synthesized using a Prime Script First
Strand cDNA Synthesis Kit (Takara Biotechnology Co., Ltd., Dalian,
China) following the manufacturer's protocol. PCR reactions were
performed with SYBR® Premix Ex Taq™ II (Takara
Biotechnology Co., Ltd.) and Quant Studio Dx Real-Time PCR
Instrument (Applied Biosystems, Thermo Fisher Scientific, Inc.).
The reaction volume included 10 µl SYBR® Premix Ex Taq™
II, 2 µl cDNA, 7 µl H2O, 0.5 µM forward and reverse
primers and 1 µl template cDNA. The PCR conditions were as follows:
95°C for 30 sec, and 40 cycles of 95°C for 5 sec, 60°C for 1 min
and 72°C for 30 sec. The forward and reverse primers (synthesized
by Sangon Biotech Co., Ltd., Shanghai, China) are listed in
Table I. Target mRNA expression was
determined by normalizing to β-actin levels and was analyzed using
the 2−ΔΔCq method (21,23). The
number of experimental repeats was three.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer
sequence | Product size
(bp) |
|---|
| HIF-1α |
| 90 |
|
Forward |
5′-CTGCCACCACTGATGAATTA-3′ |
|
|
Reverse |
5′-GTATGTGGGTAGGAGATGGA-3′ |
|
| Vimentin |
| 106 |
|
Forward |
5′-CCTTGAACGCAAAGTGGAATC-3′ |
|
|
Reverse |
5′-GACATGCTGTTCCTGAATCTGAG-3′ |
|
| β-actin |
| 101 |
|
Forward |
5′-GATCATTGCTCCTCCTGAGC-3′ |
|
|
Reverse |
5′-ACTCCTGCTTGCTGATCCAC-3′ |
|
Western blotting
Total protein was extracted using radio immuno
precipitation assay buffer (Solarbio; Beijing Soledad Co., Ltd.,
Beijing, China) supplemented with 1 m Mphenyl methanesul fonyl
fluoride. Protein content was determined using BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Equal amounts of protein were separated
with 8% SDS-PAGE and transferred to poly vinylidene fluoride
membranes (pore size, 0.45-µm; EMD Millipore, Billerica, MA, USA).
The blots were blocked with Tris-buffered saline containing 0.1%
Tween-20 (TBST) and 5% skimmed milk for 1.5 h at room temperature.
Membranes were incubated with primary antibodies overnight at 4°C.
Primary antibodies included rabbit polyclonal anti-HIF-1α
(dilution, 1:2,000; cat. no. NB100-479; Novus Biologicals,
Littleton, CO, USA), rabbit polyclonal anti-vimentin (dilution,
1:1,000; cat. no. 3932s; Cell Signaling Technology, Inc., Danvers,
MA, USA), rabbit polyclonal anti-β-actin (dilution, 1:5,000; cat.
no. ab6046; Abcam, Cambridge, MA, USA) and mouse monoclonal
anti-GAPDH (dilution, 1:5,000; cat. no. ab8245; Abcam). All
membranes were washed three times with TBST and were further
incubated with a peroxidase-conjugated anti-rabbit secondary
antibody (dilution, 1:500; cat. no. DY3002; Shanghai Ex Cell
Biology, Inc., Shanghai, China) at room temperature for 1 h. Blots
were washed and detected using an enhanced chem ilumine scence
reagent (WBKLS0100; EMD Millipore) and visualized using a Bio
Spectrum Imaging System (version no., Alliance 4.7, UVItec,
Cambridge, UK).
siRNA transfection
MDA-MB-231 cells that had been grown in IH
conditions were seeded in a 6-well culture plate and incubated in
antibiotic-free DMEM supplemented with 10% FBS. The cells were
grown to subconfluence (60–70%) and transfected with HIF-1α siRNA
(cat. no. sc-35561; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) or control siRNA (cat. no. sc-13515; Santa Cruz Biotechnology,
Inc.) using siRNA transfection reagent (cat. no. sc-29528; Santa
Cruz Biotechnology, Inc.) according to the manufacturer's protocol.
Subsequent to transfection, the cells were harvested for western
blotting or migration assays.
Statistical analysis
All statistical analyses were performed using SPSS
17.0 software (SPSS Inc., Chicago, IL, USA). Data obtained from two
groups were analyzed by two-tailed Student's t-tests, and data from
three or more groups were analyzed using a one-way analysis of
variance with a post hoc Fisher's least significant difference
test. All values are expressed as the means ± standard deviation of
≥3 independent experiments. P<0.05 was considered to represent a
statistically significant difference.
Results
Effect of IH on the migration of
MDA-MB-231 cells
MDA-MB-231 cells were exposed to an IH environment
for 5, 10, 15 or 20 cycles prior to a wound healing assay. As shown
in Fig. 1A, MDA-MB-231 cells on each
side of the wound edge migrated into the wound area, and reached
confluence at 48 h for the 10-cycle IH group, whereas cells in
other groups did not reach confluence. The relative migration
abilities of cells in the 5-, 10-, 15- and 20-cycle groups were
38.6, 76.3, 41.5 and 33.5%, respectively (Fig. 1B), indicating that 10 cycles of IH
produced the greatest effect in promoting the migration of
MDA-MB-231 cells (P<0.01, 10-cycle compared with the 5-, 15- and
20-cycle). Based on this result, 10 cycles was selected to
represent IH in subsequent experiments.
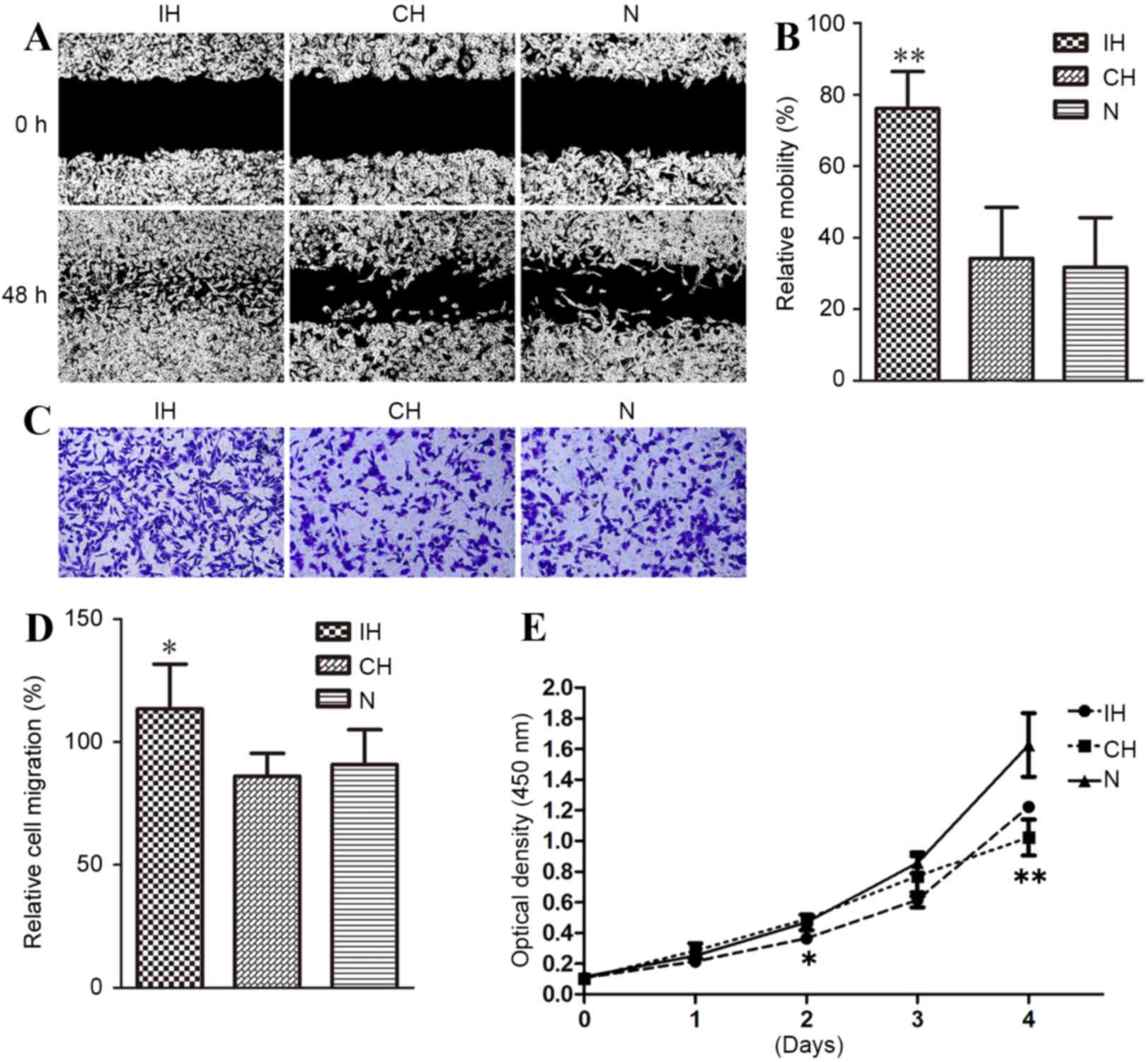
Effects of CH and IH on MDA-MB-231
cell migration and proliferation
The effects of IH or CH on MDA-MB-231 cell migration
and proliferation were compared. As shown in Fig. 2A, the IH group exhibited an
accelerated rate of migration compared with CH and normoxic groups,
whereas the CH and normoxic groups showed similar rates of cell
migration, with relative mobility 76.1% for IH, 34.1% for CH and
31.7% for normoxic cells (P<0.01; Fig.
2B). A trans-well migration assay further verified the a for
ementioned results. Direct cell number counting (Fig. 2C) and relative OD (Fig. 2D) measurements revealed that the
number of cells migrating to the lower chamber in the IH group was
significantly greater compared with that of the CH and normoxic
groups, with ODs of 1.135100 for IH, 0.861450 for CH and 0.908983
for normoxic cells (P<0.05; Fig. 2C
and D).
To determine whether IH-induced migration of
MDA-MB-231 cells was due to accelerated cell proliferation, a CCK-8
assay was performed on MDA-MB-231 cells cultured in IH, CH or
normoxic conditions. As demonstrated in Fig. 2E, the cells cultured under IH
conditions revealed decreased proliferation compared with the
normoxic cells (P<0.05), although such inhibition was not
obvious until after the 3rd day of CH exposure. This indicated that
IH promoted MDA-MB-231 cell migration by a mechanism other than
increased cellular proliferation.
Effects of IH on HIF-1 andvimentin
expression
As HIF-1α and vimentin are two important molecules
implicated in cancer migration (6,12), it was
next examined whether IH promoted MDA-MB231 cell migration through
affecting these two molecules. As shown by the western blot
analysis in Fig. 3A, HIF-1α protein
was accumulated in MDA-MB231 cells in the IH group during hypoxia,
and was degraded during reoxygenation, suggesting
post-translational HIF-1α regulation; that is, in normoxic
conditions, HIF-1α may be hydroxylated by prolyl hydroxylase and
degraded by the proteasome, whereas hypoxia inhibits the activity
of prolyl hydroxylase and, therefore, HIF-1α degradation.
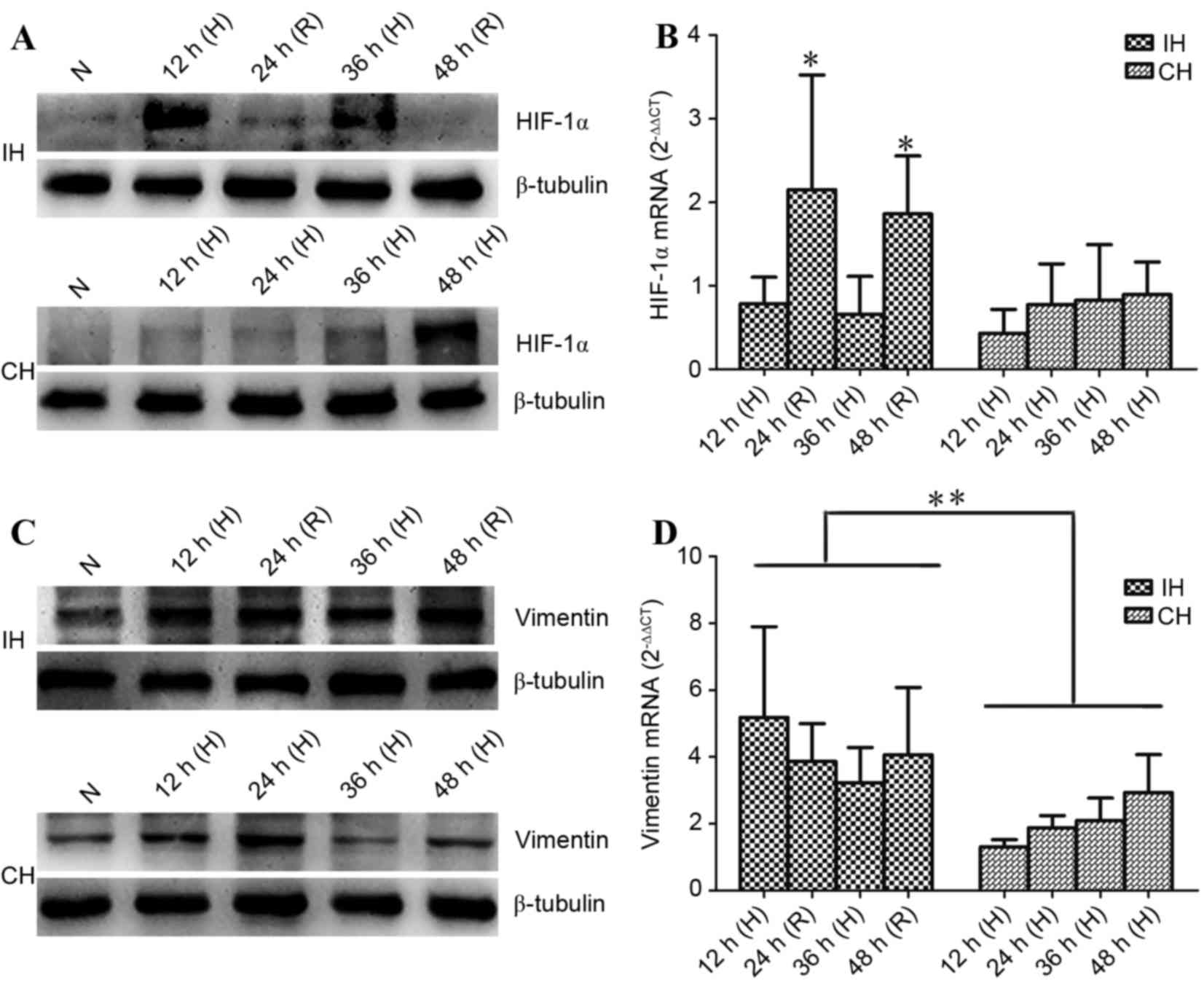 | Figure 3.Effects of IH and CH on HIF-1 and
vimentin expression. MDA-MB-231 cells were exposed to IH, CH and N
for the times indicated, and then total proteins and mRNA were
extracted. (A) In IH cells, HIF-1α protein was accumulated during H
phases and was degraded during R. However, in CH cells, it remained
low for the first 36 h of exposure and was enhanced at 48 h. (B)
HIF-1α mRNA expression was comparable between the IH (H stage) and
CH groups. However, it was significantly increased during R
compared with H. (C) Vimentin protein level remained steady high in
the IH group, while in CH cells, vimentin protein level appeared to
be transient. (D) Vimentin mRNA expression was significantly higher
in IH cells than in CH cells. Data are expressed as the mean ±
standard deviation of three repetitions. *P<0.05 vs. H stage;
**P<0.01 vs. IH group. IH, intermittent hypoxia; CH, continuous
hypoxia; HIF-1, hypoxia-induced factor 1; N, normoxia; H, hypoxia;
R, reoxygenation. |
For the cells exposed to CH, HIF-1α protein level
remained low during the first 36 h of exposure, and was
dramatically enhanced at 48 h. HIF-1α mRNA expression was assessed
by RT-qPCR and the results revealed that HIF-1α mRNA expression was
comparable between the IH (during hypoxia) and CH groups. However,
HIF-1α mRNA expression in IH cells was significantly increased
during reoxygenation when compared with during hypoxia (P<0.05;
Fig. 3B).
Vimentin upregulation is associated with cancer cell
migration (12,13). Western blotting data revealed that IH
increased vimentin protein levels during the hypoxia and
reoxygenation stages when compared with normoxic cells, indicating
that the vimentin protein level remained high in the IH group. In
CH cells, the increase in vimentin protein appeared to be
transient; it increased at 12 h, peaked at 24 h, and returned back
to the basal level (i.e. as in normoxic conditions) at 48 h
(Fig. 3C). Vimentin mRNA expression
was also examined. The data revealed that vimentin mRNA expression
was significantly higher in IH cells than in CH cells (P<0.01);
however, no difference in mRNA expression was observed in the
groups between different time points (Fig. 3D).
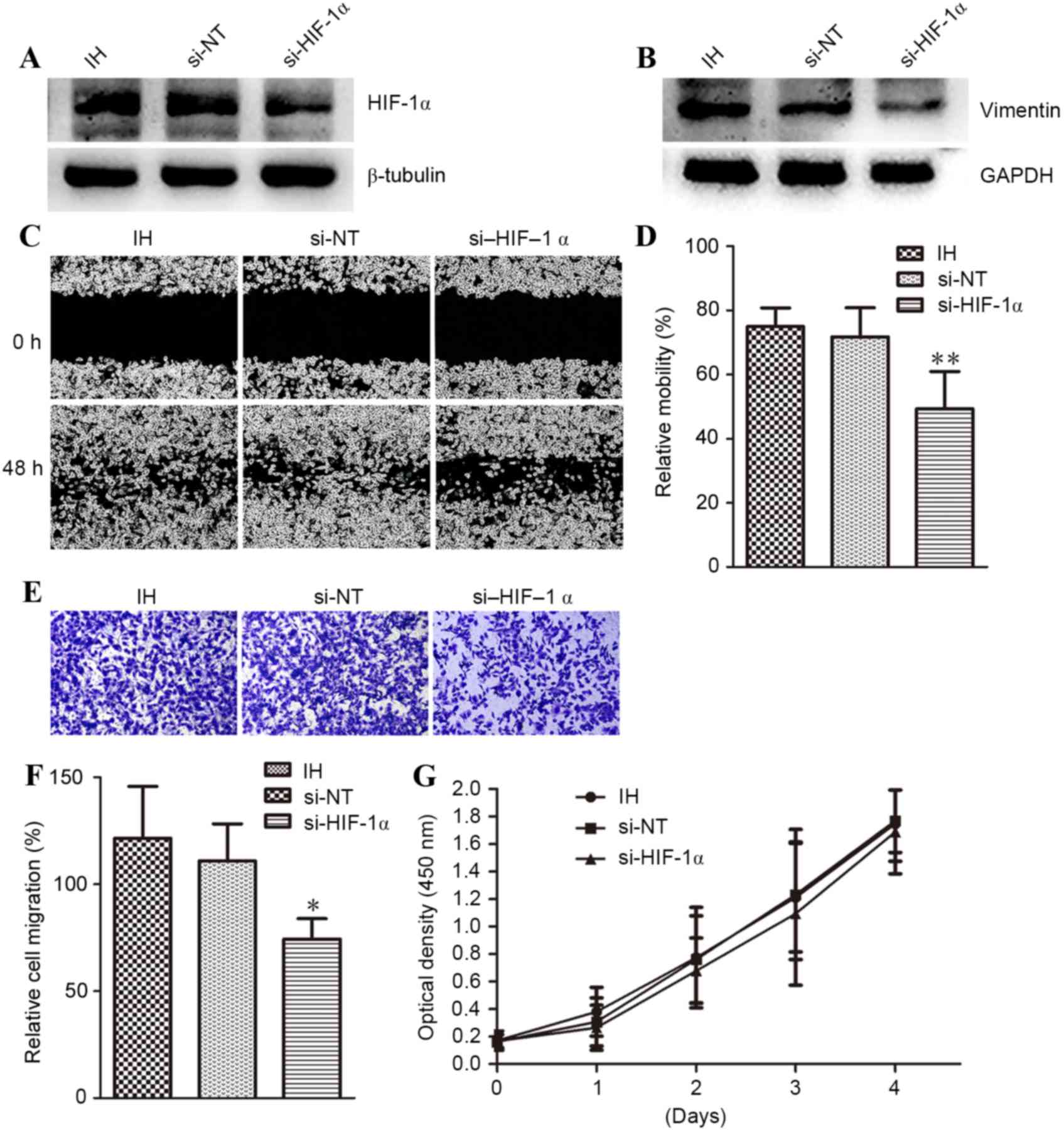
Knockdown of HIF-1α expression
abolishes hypoxia-induced vimentin expression and MDA-MB-231 cell
migration
It was evaluated whether HIF-1α-mediated IH-induced
MDA-MB-231 cell migration. The cells were cultured for 10 cycles of
IH, and then transfected with HIF-1α (si-HIF-1α) or control (si-NT)
siRNA. Western blot analysis verified the efficacy of si-HIF-1α;
si-HIF-1α successfully knocked down HIF-1α protein expression,
while si-NT had no effect (Fig. 4A).
In addition to knockdown of HIF-1α, vimentin expression and cell
migration (as assessed by wound healing or trans-well assay) were
also inhibited by HIF-1α siRNA, when compared with si-NT (Fig. 4B-F). The relative migration into the
wound are a was 75% for IH, 71.7% for si-NT and 49.2% for si-HIF-1α
(P<0.01). The relative OD values, reflecting the number of cells
that migrated to the lower chamber, were 1.214733 for IH, 1.109700
for si-NT, and 0.743567 for si-HIF-1α (P<0.05). Notably,
knockdown of HIF-1α with siRNA had no effect on the proliferation
of MDA-MB-231 cells (Fig. 4G). These
results indicated that HIF-1α mediated IH-induced cell
migration.
Discussion
IH, which is characterized by hypoxia and
reoxygenation, is a result of fluctuations in oxygen perfusion
caused by the inefficient structure of tumor microvasculature, and
has a significant effect on tumor biology (18). The present study demonstrated that IH
significantly increased the migration of MDA-MB-231 cells, and that
this effect was dependent on the number of cycles of
hypoxia-reoxygenation. Unexpectedly, IH significantly inhibited
cell proliferation, while CH did not if hypoxia persisted for >3
days. IH and CH induced HIF-1α protein accumulation and vimentin
upregulation, with the greatest effect observed for IH. Knockdown
of HIF-1α with siRNA eliminated IH-induced cell migration and
vimentin upregulation. This was consistent with a number of
previous studies, which reported that IH may positively modulate
stem cell transformation, therapy resistance and cell autophagy in
human cancer (17,24).
The effects of IH on cancer cells are diverse and
contradictory (25). Accumulating
evidence has demonstrated that multiple cycles of IH promote tumor
metastasis by selecting cells with the invasiveness phenotype
(17), which is consistent with the
results of the present study. However, high-frequency exposure to
IH or excessively long exposure to CH leads to the generation of
reactive oxygen species (ROS), which induce DNA strand breakage and
cellular injury (26,27). In the present study, a decreased rate
of migration was observed when IH cycles were increased to 15 and
20 cycles, indicating that there may be a ceiling effect for IH in
promoting tumor migration. The data of the present study indicates
that the CH and normoxic groups exhibit similar rates of cell
migration, which is inconsistent with previous reports (9). This inconsistency may arise from the
differences in cell type, hypoxia severity and hypoxia tolerance. A
fluctuation in oxygen concentration in IH could inflict genotoxic
stress upon cancer cells and inhibit proliferation, whereas CH
induced cell death in an oxygen concentration-dependent and
exposure time-dependent manner (28,29). In
the CH group, the proliferation-inhibition effect became apparent
on the 4th day, which may have arisen from an increased rate of
apoptosis in MDA-MB-231 cells, whereas IH may improve hypoxia
tolerance.
The data that cell migration decreased when HIF-1α
was knocked down with siRNA implicates HIF-1α as being important in
IH-induced metastasis. The regulation of HIF-1α predominantly
occurs at the post-transcriptional level (6). However, the data from the present study
revealed that HIF-1α mRNA expression is significantly increased in
IH cells during reoxygenation. A possible explanation for this
finding is that HIF-1α protein degradation may trigger a feedback
loop to promote expression of its mRNA. It is important to note
that IH and CH are two distinct stimuli that activate different
signal transduction pathways to affect HIF-1α protein level. For
example, protein kinase A is reported to be involved in the
regulation of HIF-1α phosphorylation in IH; however, it is not
involved in CH (30). Conversely,
inhibition of mitogen-activated protein kinase 1/3 or
phosphatidylinositol-4,5-bisphosphate 3-kinasehas no effect on
HIF-1α stabilization and transcriptional activity in IH; however,
if these kinases are inhibited in CH, gene regulation by
HIF-1issuppressed (31). HIF-1α
protein additionally appears to be more stable in IH than CH, as
HIF-1α protein is upregulated in two cycles of IH within 48 h,
whereas equal protein expression only a rises after 48 h in CH
cells, consistent with previous studies (18,21).
Unexpectedly, HIF-1α protein level remained low within the first 36
h of exposure to CH. There are two possible explanations for this
finding; either HIF-1α degradation by the proteasome continues to
occur in CH conditions as previously reported (21,32), or CH
may suppress HIF-1α synthesis at a transcriptional or translational
level. Further study is required to explore these
possibilities.
CH invoked a pronounced increase in HIF-1α protein
after 48 h and a marked decrease in cell viability thereafter,
which raises the possibility that a substantial increase in HIF-1α
protein level may represent a turning point for stressed cells
entering crisis. Typically, HIF-1α protein is degraded by the
proteasome within 5 min of reoxygenation (33). However, in the present study, even if
the HIF-1α protein was degraded completely, reoxygenation
stimulated HIF-1α signaling and increased the expression of target
genes such as vimentin. If HIF-1α is knocked down by siRNA,
vimentin protein expression and cell migration are suppressed. A
possible explanation for this observation is that a number of
HIF-1α target gene transcripts may remain untranslated during
hypoxic conditions to form HIF-1-mRNA complexes aggregated in
stress granules; and, upon reoxygenation, these stress granules are
depolymerized to allow the transcription of HIF-1α target genes
(34,35).
The role of vimentin in cell migration is
particularly complex and not yet fully understood. Vimentin has
been reported to regulate cytoskeletal organization and the loss of
cell polarization and adhesion (13).
Fas and integrins act as receptors for the extracellular matrix,
with vimentin-mediated signal transduction between cells and the
extracellular matrix, to regulate cytoskeletal rearrangement and
focal adhesion organization (36,37).
Accumulating studies have demonstrated that increasing HIF-1α
stability and activity lead to upregulation of vimentin expression,
and this process is closely associated with epithelial-mesenchymal
transition (7,11). In the present study, although IH and
CH induced vimentin expression, the IH group exhibited
significantly higher vimentin mRNA expression and more consistent
protein levels than the CH group. In CH-exposed cells, vimentin
protein elevation appears to be transient, as it increases for the
first 24 h, and then decreases with continuing hypoxia. An
important characteristic of vimentin is that it is prone to
protease cleavage. Calpains and caspases can be activated by
hypoxia, and they have been reported to cleave and digest vimentin
(12,38). It is thus speculated that long periods
of hypoxia may induce calpains and caspases to target vimentin.
Although sustained oxidative stress injury and toxic metabolites
generated from anaerobic glycolysis may obstruct vimentin
translation, future studies are required to elucidate how hypoxia
dynamically regulates vimentin expression in CH.
In conclusion, the results presented in the present
study clearly suggest that multiple cycles of hypoxia and
reoxygenation have a more pronounced effect on promoting an
invasive TNBC phenotype than CH, and that HIF-1α activation,
together with vimentin upregulation, may account for this
phenotypic change.
Acknowledgements
The authors would like to thank Professor Zesong Li
and his team for their technical support, and Dr Hanchao Gao for
his guidance in statistics. The present study was supported by
Science and Technology Plan Projects of Guangdong Province (grant
no. 2014A020212038), a Knowledge Innovation Basic Research Grant
from Shenzhen Science & Technology Commission (grant no.
JCYJ20150330102720122), a grant from the Natural Science Foundation
of Guangdong Province (grant no. 2016A030313029) and the
International Cooperation Foundation of Shenzhen (grant no.
GJHZ20160301163138685).
Glossary
Abbreviations
Abbreviations:
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
IH
|
intermittent hypoxia
|
|
CH
|
continuous hypoxia
|
|
TNBC
|
triple-negative breast cancer
|
|
ER
|
estrogen receptor
|
|
HER2
|
human epidermal growth factor
receptor-2
|
|
PR
|
progesterone receptor
|
|
DMEM
|
Dulbecco's modified Eagle medium
|
|
FBS
|
fetal bovine serum
|
|
TBST
|
Tris-buffered saline with Tween-20
|
|
siRNA
|
small interfering RNA
|
|
si-HIF-1α
|
HIF-1α siRNA
|
|
si-NT
|
non-targeting siRNA
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papa A, Caruso D, Tomao S, Rossi L,
Zaccarelli E and Tomao F: Triple-negative breast cancer:
Investigating potential molecular therapeutic target. Expert Opin
Ther Targets. 19:55–75. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Surazynski A, Miltyk W, Prokop I and Palka
J: The effect of estrogen on prolidase-dependent regulation of
HIF-1α expression in breast cancer cells. Mol Cell Biochem.
379:29–36. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Cancer Institute, . SEER fact
sheet for breast cancer. http://seer.cancer.gov/statfacts/html/breast.html.2005-2011
|
|
5
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsai YP and Wu KJ: Hypoxia-regulated
target genes implicated in tumor metastasis. J Biomed Sci.
19:1022012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu ZJ, Semenza GL and Zhang HF:
Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang
Univ Sci B. 16:32–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agani F and Jiang BH: Oxygen-independent
regulation of HIF-1: Novel involvement of PI3K/AKT/mTOR pathway in
cancer. Curr Cancer Drug Targets. 13:245–251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du J, Sun B, Zhao X, Gu Q, Dong X, Mo J,
Sun T, Wang J, Sun R and Liu Y: Hypoxia promotes vasculogenic
mimicry formation by inducing epithelial-mesenchymal transition in
ovarian carcinoma. Gynecol Oncol. 133:575–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He G, Jiang Y, Zhang B and Wu G: The
effect of HIF-1α on glucose metabolism, growth and apoptosis of
pancreatic cancerous cells. Asia Pac J Clin Nutr. 23:174–180.
2014.PubMed/NCBI
|
|
11
|
Lei J, Fan L, Wei G, Chen X, Duan W, Xu Q,
Sheng W, Wang K and Li X: Gli-1 is crucial for hypoxia-induced
epithelial-mesenchymal transition and invasion of breast cancer.
Tumour Biol. 36:3119–3126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dave JM and Bayless KJ: Vimentin as an
integral regulator of cell adhesion and endothelial sprouting.
Microcirculation. 21:333–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chernoivanenko IS and Minin AA and Minin
AA: Role of vimentin in cell migration. Ontogenez. 44:186–202.
2013.(In Russian). PubMed/NCBI
|
|
14
|
Brown JM: Tumor hypoxia, drug resistance,
and metastases. J Natl Cancer Inst. 82:338–339. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Helmlinger G, Yuan F, Dellian M and Jain
RK: Interstitial pH and pO2 gradients in solid tumors in vivo:
High-resolution measurements reveal a lack of correlation. Nat Med.
3:177–182. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Song X, Wang X, Wei L, Liu X, Yuan
S and Lv L: Effect of chronic intermittent hypoxia on biological
behavior and hypoxia-associated gene expression in lung cancer
cells. J Cell Biochem. 111:554–563. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Verduzco D, Lloyd M, Xu L, Ibrahim-Hashim
A, Balagurunathan Y, Gatenby RA and Gillies RJ: Intermittent
hypoxia selects for genotypes and phenotypes that increase
survival, invasion, and therapy resistance. PLoS One.
10:e1209582015. View Article : Google Scholar
|
|
18
|
Bhaskara VK, Mohanam I, Rao JS and Mohanam
S: Intermittent hypoxia regulates stem-like characteristics and
differentiation of neuroblastoma cells. PLoS One. 7:e309052012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi H, Gillespie DL, Berg S, Rice C,
Couldwell S, Gu J, Colman H, Jensen RL and Huang LE: Intermittent
induction of HIF-1α produces lasting effects on malignant
progression independent of its continued expression. PLoS One.
10:e1251252015.
|
|
20
|
Shen C, Beroukhim R, Schumacher SE, Zhou
J, Chang M, Signoretti S and Kaelin WG Jr: Genetic and functional
studies implicate HIF1α as a 14q kidney cancer suppressor gene.
Cancer Discov. 1:222–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miao ZF, Zhao TT, Wang ZN, Xu YY, Mao XY,
Wu JH, Liu XY, Xu H, You Y and Xu HM: Influence of different
hypoxia models on metastatic potential of SGC-7901 gastric cancer
cells. Tumour Biol. 35:6801–6808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi J, Wan Y and Di W: Effect of hypoxia
and re-oxygenation on cell invasion and adhesion in human ovarian
carcinoma cells. Oncol Rep. 20:803–807. 2008.PubMed/NCBI
|
|
23
|
Zhou W, Wang G, Zhao X, Xiong F, Zhou S,
Peng J, Cheng Y, Xu S and Xu X: A multiplex qPCR gene dosage assay
for rapid genotyping and large-scale population screening for
deletional α-thalassemia. J Mol Diagn. 15:642–651. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu H, Wang D, Zhang L, Xie X, Wu Y, Liu
Y, Shao G and Su Z: Upregulation of autophagy by hypoxia-inducible
factor-1α promotes EMT and metastatic ability of CD133+ pancreatic
cancer stem-like cells during intermittent hypoxia. Oncol Rep.
32:935–942. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Almendros I, Wang Y and Gozal D: The
polymorphic and contradictory aspects of intermittent hypoxia. Am J
Physiol Lung Cell Mol Physiol. 307:L129–L140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pires IM, Bencokova Z, Milani M, Folkes
LK, Li JL, Stratford MR, Harris AL and Hammond EM: Effects of acute
versus chronic hypoxia on DNA damage responses and genomic
instability. Cancer Res. 70:925–935. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zepeda AB, Pessoa A Jr, Castillo RL,
Figueroa CA, Pulgar VM and Farías JG: Cellular and molecular
mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell
Biochem Funct. 31:451–459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noh MY, Kim YS, Lee KY, Lee YJ, Kim SH, Yu
HJ and Koh SH: The early activation of PI3K strongly enhances the
resistance of cortical neurons to hypoxic injury via the activation
of downstream targets of the PI3K pathway and the normalization of
the levels of PARP activity, ATP, and NAD+. Mol Neurobiol.
47:757–769. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen PY, Ho YR, Wu MJ, Huang SP, Chen PK,
Tai MH, Ho CT and Yen JH: Cytoprotective effects of fisetin against
hypoxia-induced cell death in PC12 cells. Food Funct. 6:287–296.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toffoli S, Feron O, Raes M and Michiels C:
Intermittent hypoxia changes HIF-1alpha phosphorylation pattern in
endothelial cells: Unravelling of a new PKA-dependent regulation of
HIF-1alpha. Biochim Biophys Acta. 1773:1558–1571. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mottet D, Dumont V, Deccache Y, Demazy C,
Ninane N, Raes M and Michiels C: Regulation of hypoxia-inducible
factor-1alpha protein level during hypoxic conditions by the
phosphatidylinositol 3-kinase/akt/glycogen synthase kinase 3beta
pathway in HepG2 cells. J Biol Chem. 278:31277–31285. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Monti E and Gariboldi MB: HIF-1 as a
target for cancer chemotherapy, chemosensitization and
chemoprevention. Curr Mol Pharmacol. 4:62–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dewhirst MW: Intermittent hypoxia furthers
the rationale for hypoxia-inducible factor-1 targeting. Cancer Res.
67:854–855. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Moeller BJ, Cao Y, Li CY and Dewhirst MW:
Radiation activates HIF-1 to regulate vascular radiosensitivity in
tumors: Role of reoxygenation, free radicals, and stress granules.
Cancer Cell. 5:429–441. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang IA, Oh MJ, Kim MH, Park SK, Kim BG
and Namgung U: Vimentin phosphorylation by Cdc2 in schwann cell
controls axon growth via β1-integrin activation. FASEB J.
26:2401–2413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen WC, Hsu KY, Hung CM, Lin YC, Yang NS,
Ho CT, Kuo SC and Way TD: The anti-tumor efficiency of
pterostilbene is promoted with a combined treatment of Fas
signaling or autophagy inhibitors in triple negative breast cancer
cells. Food Funct. 5:1856–1865. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakajima E, Hammond KB, Rosales JL,
Shearer TR and Azuma M: Calpain, not caspase, is the causative
protease for hypoxic damage in cultured monkey retinal cells.
Invest Ophthalmol Vis Sci. 52:7059–7067. 2011. View Article : Google Scholar : PubMed/NCBI
|