Introduction
Acute lymphoblastic leukemia (ALL) is the most
common type of pediatric cancer, accounting for ~25% of all
malignancies diagnosed in children <15 years (1). Although the outcome of childhood ALL has
markedly improved with advancements in risk-adapted chemotherapy
and supportive care (2), between 15
and 20% of patients eventually relapse (3) and recurrent ALL remains the primary
obstacle in improving the cure rate and decreasing mortality
(4).
Minimal residual disease (MRD) in the early stages
of treatment has been widely recognized as one of the most powerful
prognostic indicators. However, not all patients with positive MRD
relapse and certain patients with negative MRD may relapse. To
avoid inadequate therapy for high-risk patients and over-treatment
for low-risk patients (5), novel
prognostic indicators are urgently required for risk
refinement.
An independent study has indicated that microRNA
(miR)-210 is consistently and predominantly upregulated in hypoxic
states (6). By acting on target
genes, miR-210 is involved in a range of physiological and
pathological processes (7–9). In our previous study (10), it was demonstrated that miR-210 is an
independent prognostic factor for pediatric ALL and that low
miR-210 expression (threshold value, 3.8243) is a good predictor
for relapse and induction failure in childhood ALL.
Caspase 8-associated protein 2 (CASP8AP2), a
component of Cajal bodies, is an essential factor in regulating
histone gene transcription, apoptosis and S phase progress
(11–13). Flotho et al (14) demonstrated that decreased
CASP8AP2 expression is markedly associated with increased
rates of MRD and hematological relapse. Kim et al (15) demonstrated that CASP8AP2 is a
target of miR-210 in bone marrow-derived mesenchymal stem cells.
However, an association between expression levels in ALL cells was
not identified.
In the present study, the clinical significance of
CASP8AP2 and the association between CASP8AP2 and
miR-210 was analyzed. In addition, the prognostic value of combined
detection of miR-210 and CASP8AP2 expression was
determined.
Materials and methods
Patients and treatment
Between March 2008 and July 2010, 203 children with
newly diagnosed ALL were enrolled in the Chinese Children's
Leukemia Group (CCLG)-ALL 2008 protocol at Beijing Children's
Hospital. Criteria for patient inclusion were ≥70% leukemic cells
in diagnostic bone marrow (BM) samples (16), treatment according to the CCLG-ALL
2008 protocol (17) and sufficient BM
sample for total RNA/microRNA (miRNA) extraction. A total of 112
children with ALL were excluded from analysis.
On the basis of these criteria, 91 patients (median
age, 5 years; range, 1.0–14 years) were included in the present
study (57 boys and 34 girls), with 81 cases of B cell precursor ALL
(BCP-ALL) and 10 cases of T cell ALL (T-ALL). The median follow-up
time was 37.2 months (range, 1.0–50.0 months). A total of 11
patients suffered from BM relapse or induction failure and all
succumbed between 3 and 11 months after relapse or induction
failure. A further 2 patients succumbed due to severe infection and
the remaining 78 patients were in continuous complete remission
(CCR). BM samples obtained from 5 ALL patients in CCR for >5
years (control group) were used as calibrators (18).
The CCLG-ALL 2008 protocol was approved by the
Beijing Children's Hospital Institutional Ethics Committee and
written informed consent was obtained by the patients'
guardians.
miRNA isolation, reverse transcription
and determination of miR-210 expression
Total miRNA was extracted using the mirVana miRNA
Isolation kit (Ambion; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol as in our previous
study (10). Collected miRNA was
stored at −80°C.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed to determine miR-210 expression.
Laboratory procedures and experimental details were as described in
a previous study (10).
RNA isolation and cDNA synthesis
Mononucleated BM cells were isolated by
Ficoll-Hypaque density-gradient centrifugation (MD Pacific
Biotechnology Co., Ltd., Tianjin, China) and stored at −80°C until
use. Total RNA was extracted within 2 weeks using TRIzol reagent
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). mRNAs were reverse-transcribed into cDNAs using
random hexamers and Moloney murine leukemia virus reverse
transcriptase (Promega Corporation, Madison, WI, USA) according to
the manufacturer's protocol.
Quantitative analysis of CASP8AP2
expression
CASP8AP2 expression was detected using
RT-qPCR. The primers and TaqMan probes, which were designed using
Primer Express (version 3.0; Applied Biosystems; Thermo Fisher
Scientific, Inc.) are listed in Table
I. A TaqMan probe of the Abelson (ABL) gene was used as
an internal control and associated primer sequences are described
previously (19).
 | Table I.Sequences of primers and probe for
CASP8AP2 and ABL. |
Table I.
Sequences of primers and probe for
CASP8AP2 and ABL.
| Genes | Sequences of primers
and probe (5′-3′) |
|---|
| CASP8AP2 | CACTTGCCACTTCTACAAGTC
(sense) |
|
| TGGCGGCTAAATATGCAAATG
(antisense) |
|
|
FAM-TGTCAGAAAAGAGGGCCATCATTTAAA-TAMRA
(probe) |
| ABL | ATCCAAGAAGGGGCTGTCC
(sense) |
|
| CCAACGAGCGGCTTCAC
(antisense) |
|
|
FAM-CCTTCAGCGGCCAGTAGCATC-TGA-TAMRA
(probe) |
The reaction mixture contained TaqMan Master ROX mix
(6.25 µl), 10 pmol each primer, 2.5 pmol probe, cDNA template (1
µl) and deionized water to a total volume of 12.5 µl. The reaction
was performed at 95°C for 10 min, followed by 50 cycles of 15 sec
at 95°C and 1 min at 60°C on a 7500 Real Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Each sample was
detected in triplicate. CASP8AP2 expression levels were
calculated using the 2−ΔΔCq method and are presented as
fold change compared with the control group (20).
Detection of MRD
MRD monitoring was performed by qPCR at the end of
induction therapy (day 33). Patient-specific immunoglobulin (Ig)
and T cell receptor (TCR) gene rearrangements, including IgH, IgK,
IgL, Kde, TCRB, TCRG and TCRD, were used as qPCR targets for
quantitative assessment of MRD. Detection methods were as
previously described (21).
Statistical analysis
A receiver operating characteristic (ROC) curve was
used to assess the predictive value of CASP8AP2 expression
for relapse. Relapse-free survival (RFS) was defined as the time
from the diagnostic date through the date of relapse at any site.
Event-free survival (EFS) was estimated from the date of diagnosis
to the date of induction failure, relapse, second tumor or
mortality. Overall survival (OS) was defined as the time between
diagnosis and mortality or last contact with the patient in CCR.
Kaplan-Meier estimator survival analysis was used to determine the
differences in RFS, EFS and OS. Spearman's correlation was used to
determine the association between CASP8AP2 and miR-210. A
Cox's proportional hazards model was utilized to determine an
equation for assessment of the risk of bone marrow relapse. All
analyses were performed with SPSS (version 16.0; SPSS, Inc.,
Chicago, IL, USA) for Microsoft Windows. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinical value of CASP8AP2
expression
No statistically significant differences were
identified between the included and excluded patients regarding age
(P=0.752), sex (P=0.313), immunophenotype (P=0.083), transcription
factor ETV6-AML (P=0.143), breakpoint cluster region-ABL
(P=0.725), transcription factor 3-PBX homeobox 1 (P=0.902),
myeloid/lymphoid or mixed-lineage leukemia rearrangement (P=0.837)
and central nervous system (CNS) involvement (P=0.110).
Median CASP8AP2 expression in the 91 children
evaluated was 0.6591 (range, 0.21–2.05). According to ROC curve
analysis, the optimal threshold value for CASP8AP2
expression was 0.4760 [area under the curve (AUC), 0.865; 95%
confidence interval, 0.725–1.006; P<0.001] with a sensitivity
and specificity of 0.850 and 0.818, respectively.
Using this threshold value, the 91 patients were
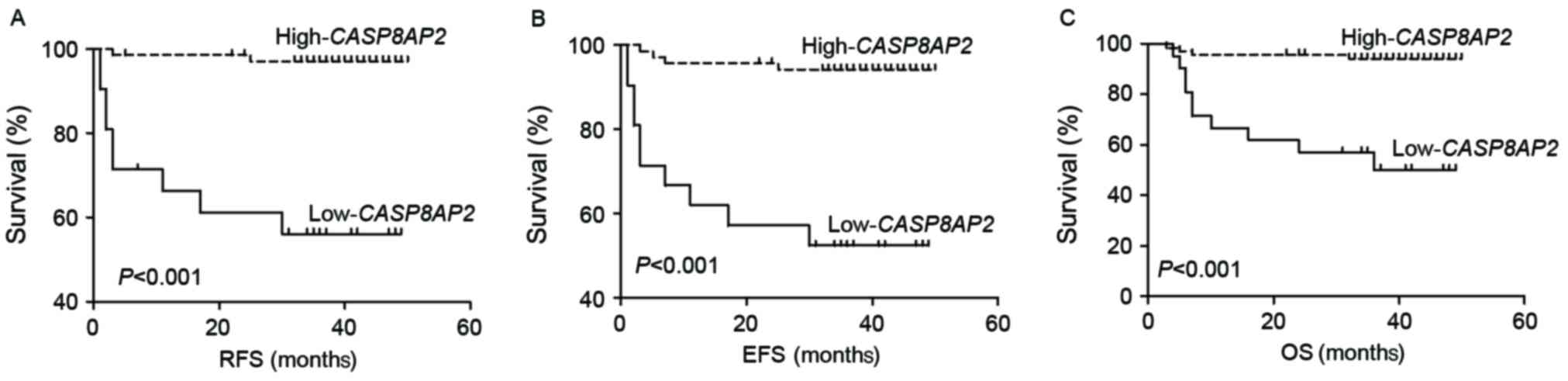
divided into low (n=21) and high (n=70) expression groups. The
relapse rate in the low-CASP8AP2 group (9/21; 42.8%) was
significantly increased compared with that of the
high-CASP8AP2 group (2/70; 2.9%; P<0.001). The
low-CASP8AP2 group exhibited decreased RFS (log-rank:
P<0.001), EFS (log-rank: P<0.001) and OS (log-rank: P=0.005)
compared with the high-CASP8AP2 group (Fig. 1A-C). The results of the present study
indicated that CASP8AP2 expression in patients with newly
diagnosed ALL is a valuable marker for predicting relapse.
Association between miR-210 and
CASP8AP2 expression
Using the threshold value (3.8243) determined in our
previous study, the cohort of 91 patients was divided into low
(n=41) and high (n=50) expression groups. No association was
identified between CASP8AP2 and miR-210 expression in these
groups, regardless of continuous or grouped values (P>0.05).
Associations between CASP8AP2, miR-210 and clinical
characteristics are presented in Table
II.
 | Table II.Association of miR-210 and
CASP8AP2 expression with clinical characteristics. |
Table II.
Association of miR-210 and
CASP8AP2 expression with clinical characteristics.
|
|
| miR-210
expression |
| CASP8AP2
expression |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Patients
(n=91) | Low | High | P-value | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.083 |
|
| 0.402 |
|
1–9 | 73 | 25 | 48 |
| 16 | 57 |
|
|
>10 | 18 | 10 | 8 | 0.02 | 5 | 13 |
|
| Sex |
|
|
| 0.184 |
|
| 0.434 |
|
Male | 57 | 27 | 30 | 0.056 | 14 | 43 |
|
|
Female | 34 | 8 | 26 |
| 7 | 27 |
|
| WBC count,
cells/l |
|
|
| 0.128 |
|
| 0.582 |
|
<50×109 | 61 | 21 | 40 |
| 14 | 47 |
|
|
≥50×109 | 30 | 14 | 16 | 0.5 | 7 | 23 |
|
| MRD (at day
33) |
|
|
|
|
|
| 0.001 |
|
Positive | 67 | 22 | 45 | 0.624 | 9 | 58 |
|
|
Negative | 24 | 13 | 11 |
| 12 | 12 |
|
|
Immunophenotype |
|
|
|
|
|
| 0.274 |
|
BCP-ALL | 81 | 29 | 52 | 0.151 | 20 | 61 |
|
|
T-ALL | 10 | 6 | 4 |
| 1 | 9 |
|
| Prednisone
response |
|
|
| 0.009 |
|
| 0.227 |
|
Good | 87 | 33 | 54 |
| 19 | 68 |
|
|
Poor | 4 | 2 | 2 | 0.385 | 2 | 2 |
|
| CNS
involvement |
|
|
|
|
|
| 0.41 |
| No | 89 | 34 | 55 |
| 20 | 69 |
|
|
Yes | 2 | 1 | 1 |
| 1 | 1 |
|
| Fusion genes |
|
|
|
|
|
|
|
| BCR-ABL |
|
|
|
|
|
| 0.002 |
|
Positive | 6 | 4 | 2 |
| 5 | 1 |
|
|
Negative | 85 | 31 | 54 |
| 16 | 69 |
|
|
TEL-AML1 |
|
|
|
|
|
| 0.016 |
|
Positive | 27 | 5 | 34 |
| 2 | 25 |
|
|
Negative | 64 | 30 | 22 |
| 19 | 45 |
|
| MLL-AF4 |
|
|
|
|
|
| 0.231 |
|
Positive | 1 | 1 | 0 |
| 1 | 0 |
|
|
Negative | 90 | 34 | 56 |
| 20 | 70 | 0.079 |
|
E2A-PBX1 |
|
|
| 0.366 |
|
|
|
|
Positive | 8 | 4 | 4 |
| 4 | 4 |
|
|
Negative | 83 | 31 | 52 |
| 17 | 66 |
|
Prognostic relevance of miR-210 and
CASP8AP2 expression
miR-210 and CASP8AP2 expression are known
prognostic indicators in pediatric ALL which prompted the
determination in the present study of the efficacy of combining
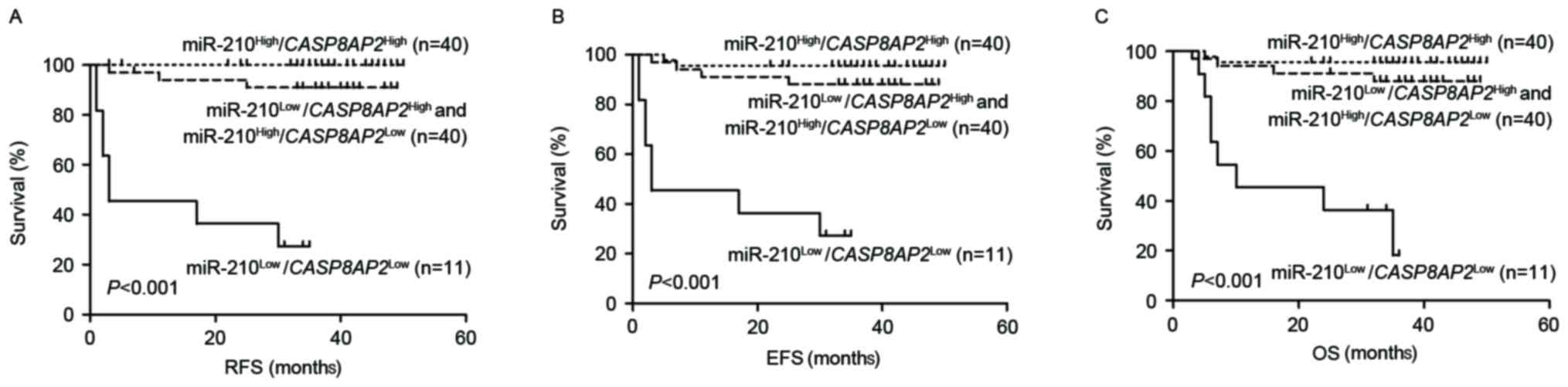
miR-210 and CASP8AP2 expression to predict relapse. The 91
patients were stratified into four groups according to miR-210 and
CASP8AP2 expression. Of the 40 cases in the double
high-expression group
(miR-210high/CASP8AP2high), none of
the patients relapsed, with 3-year EFS and OS values of 93.1±9.9
and 95.7±0.3%, respectively. A total of 11 patients with double
low-expression of the two genes
(miR-210low/CASP8AP2low) exhibited the
poorest outcomes with 3-year RFS, EFS and OS values of 27.3±13.4,
27.3±13.4 and 36.4±14.5%, respectively. No statistically
significant difference was observed in prognosis between patients
with single low-expression of the two genes
(miR-210low/CASP8AP2high, n=30;
miR-210high/CASP8AP2low, n=10) with
3-year RFS, EFS and OS values as follows: 91.7±5.6 vs. 88.9±10.5%;
P=0.830; 91.7±5.6 vs. 80.0±12.6%; P=0.338; and 91.3±5.9 vs.
80.0±12.6%; P=0.351, respectively. The two subgroups were combined
into a single group (n=40) and an intermediate prognosis was
determined with 3-year RFS, EFS and OS values of 91.0±5.0, 88.0±5.6
and 83.3±6.9%, respectively (Fig.
2A-C). The results of the present study indicate that combined
detection of miR-210 and CASP8AP2 expression may accurately
predict ALL relapse.
Estimation of relapse risk based on
clinical features, miR-210 and CASP8AP2 expression
In COX regression analysis, white blood cell counts,
MRD at day 33, prednisone response, CNS involvement, BCR-ABL1,
TEL-AML, E2A-PBX1, MLL rearrangement, and miR-210 and
CASP8AP2 expression were considered covariates. Results of
the present study indicated that MRD at day 33, miR-210 and
CASP8AP2 expression are all independent prognostic
indicators (Table III). On the
basis of the final Cox's proportional hazards model for RFS, an
equation, composed of the three factors, was devised to estimate
the risk of relapse as follows: Risk index =3.393× MRD-3.549×
miR-210-2.855× CASP8AP2
 | Table III.Prognostic significance of miR-210
and CASP8AP2 expression levels and other common clinical
features analyzed by Cox's proportional hazards model. |
Table III.
Prognostic significance of miR-210
and CASP8AP2 expression levels and other common clinical
features analyzed by Cox's proportional hazards model.
|
|
|
| 95% confidence
intervals for HR |
|---|
|
|
|
|
|
|---|
| Features | Hazard ratio
(HR) | P-value | Lower | Upper |
|---|
| miR-210 | 0.029 | 0.012 | 0.002 | 0.461 |
|
CASP8AP2 | 0.058 | 0.015 | 0.006 | 0.575 |
| MRD (at day
33) | 29.742 | 0.026 | 1.498 | 590.316 |
| Prednisone
response | 1.352 | 0.796 | 0.138 | 13.259 |
| CNS
involvement | 4.246 | 0.450 | 0.100 | 180.757 |
| BCR-ABL | 0.467 | 0.534 | 0.042 | 5.147 |
| TEL-AML | 0.310 | 0.410 | 0.019 | 5.043 |
|
E2A-PBX1 | 3.151 | 0.436 | 0.176 | 56.447 |
| MLL
rearrangements | 1.914 | 0.658 | 0.108 | 33.988 |
In the aforementioned equation, MRD represents MRD
at day 33 (1 for MRD <10−4 and 2 for MRD
≥10−4), miR-210 represents miR-210 expression levels (1
for low-miR-210 and 2 for high-miR-210) and CASP8AP2
represents CASP8AP2 expression levels (1 for
low-CASP8AP2 and 2 for high-CASP8AP2). The predictive
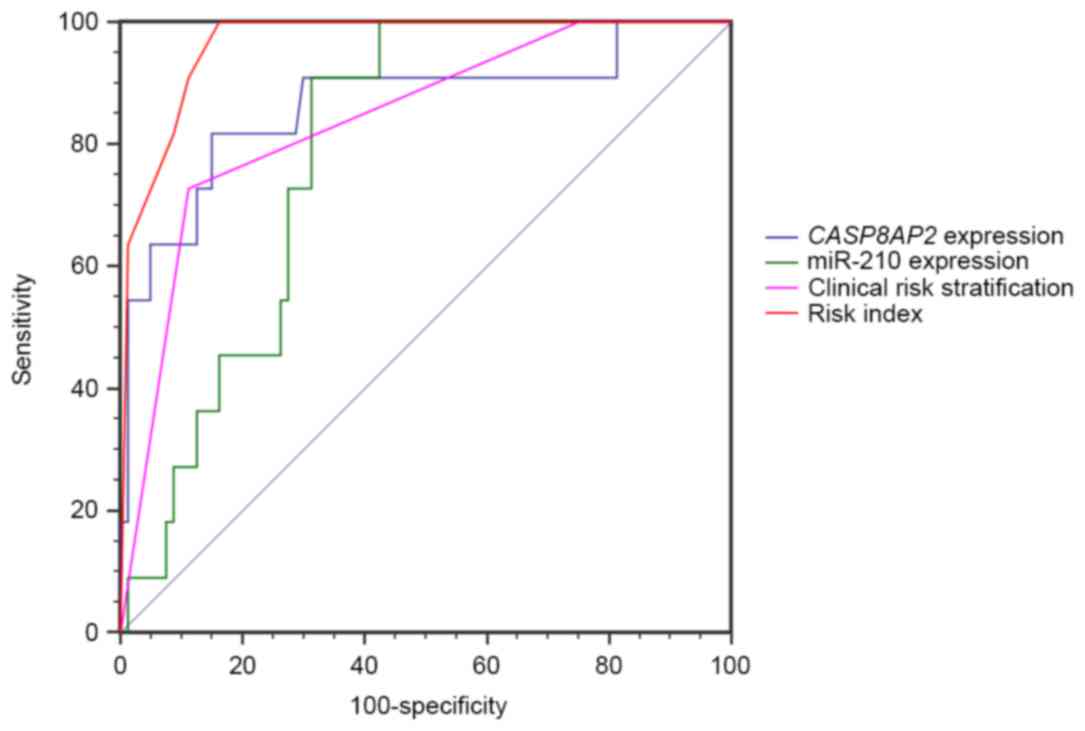
value of this algorithm was tested using an ROC curve. The AUC was
0.965 (P<0.001), which was improved compared with miR-210 and
CASP8AP2 expression or clinical risk stratification alone (0.789,
0.865 and 0.841, respectively; Fig.
3), indicating that combined assessment of miR-210 and
CASP8AP2 expression may identify patients at increased risk
of relapse.
Discussion
In the present study, the AUC of the ROC curve of
the current clinical risk stratification was 0.841, suggesting that
improvement is required. The prognostic value of miR-210 and
CASP8AP2 has been demonstrated in previous studies, and
CASP8AP2 has been demonstrated as a target of miR-210 in
stem cells. The present study evaluated the association between
miR-210 and CASP8AP2 in pediatric ALL at the mRNA level and
explored the prognostic significance of joint detection. The
results of the present study identified that decreased miR-210 or
CASP8AP2 expression in newly diagnosed ALL patient BM
samples was associated with increased MRD, increased BM relapse
rate and poor RFS, EFS and OS. Multivariate analyses indicated that
miR-210 and CASP8AP2 expression are independent prognostic
factors following adjustment for other risk factors. Combined
assessment of miR-210 and CASP8AP2 expression is considered
an improved method, compared with a single assessment or the
current clinical risk stratification, in identifying patients at
increased risk of relapse. Furthermore, an equation was devised for
estimating bone marrow relapse risk, based on MRD at day 33 and
miR-210 and CASP8AP2 expression. As expected, the equation
predicted treatment outcome more precisely than clinical risk
stratification alone.
Functioning as a hypoxamir, miR-210 participates in
regulation of a number of physiological and pathological processes
including cell survival, proliferation, differentiation, apoptosis
and development (7–9). A previous study has indicated that
leukemic bone marrow is likely in a hypoxic microenvironment at the
initial diagnosis, due to the increased proliferation and oxygen
consumption of leukemic cells (22).
This is consistent with the results of the present study indicating
that increased miR-210 expression is prevalent in BM samples at
initial diagnosis. In our previous study, the prognostic
significance of decreased miR-210 expression in pediatric ALL was
demonstrated (10). However, Zhang
et al (23) identified that
miR-210 expression in a high-risk group (HR) was significantly
increased, compared with that in intermediate risk (IR) or standard
risk (SR) groups, indicating that increased miR-210 expression is
associated with an poorer outcome in pediatric ALL, which is in
contrast with the results of the present study. The reasons for the
conflicting results may be a substantial difference in the risk
classification between the two groups. Zhang et al (23) conducted a study in which the
proportion of HR patients was increased compared with that in the
present study (36.7%, 18/49 vs. 17.5%, 16/91, respectively). In
addition, Zhang et al (23)
extracted total miRNAs using TRIzol reagent and detected miRNA
levels using an miRNA chip, whereas the present study used mirVana
miRNA Isolation kit, TaqMan MicroRNA Assay and RT-qPCR.
Kim et al (15)
demonstrated that CASP8AP2 is the target of miR-210 in bone
marrow-derived mesenchymal stem cells. However, the present study
did not identify an association between these factors; this may be
due to the fact that miRNAs regulate gene expression
post-transcriptionally which failed to demonstrate a negative
association at the mRNA level (24).
The complex regulatory network, including miR-210 and its target
genes, varies in distinct cell types and further studies are
required to explore additional possible associations.
Target genes of miR-210 in pediatric ALL have not
been studied and the underlying molecular mechanisms of decreased
miR-210 expression associated with a poor prognosis remain unclear.
Additional studies are required to elucidate the underlying
molecular mechanisms of poor prognosis linked to decreased miR-210
and CASP8AP2 expression in pediatric ALL. Studies of the
role that these indicators serve in drug resistance may provide
insight into their prognostic value in treating pediatric ALL.
Acknowledgements
The present study was supported by the Priming
Scientific Research Foundation for the Junior Researcher in Beijing
Tongren Hospital, Capital Medical University (grant no.
2014-YJJ-ZZL-011), the Key Scientific Research Training Fund of
Beijing Tongren Hospital, Capital Medical University (grant no.
2015-YJJ-GGL-009).
References
|
1
|
Pui CH, Carroll WL, Meshinchi S and Arceci
RJ: Biology, risk stratification, and therapy of pediatric acute
leukemias: An update. J Clin Oncol. 29:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hunger SP, Lu X, Devidas M, Camitta BM,
Gaynon PS, Winick NJ, Reaman GH and Carroll WL: Improved survival
for children and adolescents with acute lymphoblastic leukemia
between 1990 and 2005: A report from the children's oncology group.
J Clin Oncol. 30:1663–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Locatelli F, Schrappe M, Bernardo ME and
Rutella S: How I treat relapsed childhood acute lymphoblastic
leukemia. Blood. 120:2807–2816. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhojwani D and Pui CH: Relapsed childhood
acute lymphoblastic leukaemia. Lancet Oncol. 14:e205–e217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pui CH, Mullighan CG, Evans WE and Relling
MV: Pediatric acute lymphoblastic leukemia: Where are we going and
how do we get there? Blood. 120:1165–1174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang X, Le QT and Giaccia AJ:
MiR-210-micromanager of the hypoxia pathway. Trends Mol Med.
16:230–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Devlin C, Greco S, Martelli F and Ivan M:
miR-210: More than a silent player in hypoxia. IUBMB Life.
63:94–100. 2011.PubMed/NCBI
|
|
8
|
Crosby ME, Kulshreshtha R, Ivan M and
Glazer PM: MicroRNA regulation of DNA repair gene expression in
hypoxic stress. Cancer Res. 69:1221–1229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chan SY and Loscalzo J: MicroRNA-210: A
unique and pleiotropic hypoxamir. Cell Cycle. 9:1072–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mei Y, Gao C, Wang K, Cui L, Li W, Zhao X,
Liu F, Wu M, Deng G, Ding W, et al: Effect of microRNA-210 in
prognosis and response to chemotherapeutic drugs in pediatric acute
lymphoblastic leukemia. Cancer Sci. 105:463–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barcaroli D, Dinsdale D, Neale MH,
Bongiorno-Borbone L, Ranalli M, Munarriz E, Sayan AE, McWilliam JM,
Smith TM, Fava E, et al: FLASH is an essential component of Cajal
bodies. Proc Natl Acad Sci USA. 103:pp. 14802–14807. 2006,
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Barcaroli D, Bongiorno-Borbone L,
Terrinoni A, Hofmann TG, Rossi M, Knight RA, Matera AG, Melino G
and De Laurenzi V: FLASH is required for histone transcription and
S-phase progression. Proc Natl Acad Sci USA. 103:pp. 14808–14812.
2006, View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Evans HG and Evans DR: FLASH
knockdown sensitizes cells to Fas-mediated apoptosis via
down-regulation of the anti-apoptotic proteins, MCL-1 and Cflip
short. PLoS One. 7:e329712012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flotho C, Coustan-Smith E, Pei D, Iwamoto
S, Song G, Cheng C, Pui CH, Downing JR and Campana D: Genes
contributing to minimal residual disease in childhood acute
lymphoblastic leukemia: Prognostic significance of CASP8AP2. Blood.
108:1050–1057. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HW, Haider HK, Jiang S and Ashraf M:
Ischemic preconditioning augments survival of stem cells via
miR-210 expression by targeting caspase-8-associated protein 2. J
Biol Chem. 284:33161–33168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carroll WL, Bhojwani D, Min DJ, Raetz E,
Relling M, Davies S, Downing JR, Willman CL and Reed JC: Pediatric
acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ
Program. 102–131. 2003.PubMed/NCBI
|
|
17
|
Wang KL, Mei YY, Cui L, Zhao XX, Li WJ,
Gao C, Liu SG, Jiao Y, Liu FF, Wu MY, et al: E2F3a gene expression
has prognostic significance in childhood acute lymphoblastic
leukemia. Eur J Haematol. 93:281–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rieu I and Powers SJ: Real-time
quantitative RT-PCR: Design, calculations, and statistics. Plant
Cell. 21:1031–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pallisgaard N, Clausen N, Schroder H and
Hokland P: Rapid and sensitive minimal residual disease detection
in acute leukemia by quantitative real-time RT-PCR exemplified by
t(12;21) TEL-AML1 fusion transcript. Genes Chromosomes Cancer.
26:355–365. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao C, Zhao XX, Li WJ, Cui L, Zhao W, Liu
SG, Yue ZX, Jiao Y, Wu MY and Li ZG: Clinical features, early
treatment responses, and outcomes of pediatric acute lymphoblastic
leukemia in China with or without specific fusion transcripts: A
single institutional study of 1,004 patients. Am J Hematol.
87:1022–1027. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mortensen BT, Jensen PO, Helledie N,
Iversen PO, Ralfkiaer E, Larsen JK and Madsen MT: Changing bone
marrow micro-environment during development of acute myeloid
leukaemia in rats. Br J Haematol. 102:458–464. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Luo XQ, Zhang P, Huang LB, Zheng
YS, Wu J, Zhou H, Qu LH, Xu L and Chen YQ: MicroRNA patterns
associated with clinical prognostic parameters and CNS relapse
prediction in pediatric acute leukemia. PLoS One. 4:e78262009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qin Q, Furong W and Baosheng L: Multiple
functions of hypoxia-regulated miR-210 in cancer. J Exp Clin Cancer
Res. 33:502014. View Article : Google Scholar : PubMed/NCBI
|