Introduction
Breast cancer (BC) is one of the most common
malignancies in women, and its incidence is the second highest in
the world (1,2). Despite various treatment methods,
recurrence and metastasis are major obstacles for BC treatments
(3). Studies have shown that about
40% of BC patients have experienced recurrence, and about 60–70% of
patients display distant metastasis (4). Like normal human organs, tumors have a
small number of primitive stem cells that have high proliferation,
self-renewal, and differentiation potential. Moreover, these stem
cells can be resistant to chemoradiotherapy. All of these
characteristics are similar to those of normal stem cells, so
cancer can be considered a type of stem cell disease. These tumor
cells are termed cancer stem cells or tumor stem cells (CSCs or
TSCs) (5,6). These features of breast cancer stem
cells (BCSCs) are also a major reason for the recurrence and
distant metastasis of tumors following conventional therapies
(7).
The formation of a vascular network is extremely
important for tumor growth and metastasis (8,9). If there
are no blood vessels that can provide adequate nutrients for tumor
cells, the tumor volume cannot exceed 2–3 mm3, and organ
structures and functions will also be adversely affected (10,11). As
endothelial cells are necessary to form blood vessels, no
clinically detectable tumors and subsidiary blood vessels will form
in the absence of endothelial cell proliferation (12). Therefore, anti-tumor angiogenesis
therapies are currently of great interest in the field of cancer
treatment research.
Tumor angiogenesis can be functionally divided into
vasculogenesis and angiogenesis (13,14).
Vasculogenesis refers to the process of differentiation and
proliferation of endothelial progenitor cells (EPCs) at tumor
sites. Angiogenesis refers to endotheliosis and the formation of
new blood vessels at or around tumor sites using the original blood
vessels as a template (15). Some
studies have shown that bone marrow-derived EPCs in adults are
involved in the formation of new blood vessels, which challenges
current models in which angiogenesis only occurs during embryonic
development. Additional studies have shown that EPCs also
participate in the regeneration, repair, and angiogenesis of the
heart, brain, and peripheral vessels (16). Tumors are typically rich in new blood
vessels, so researchers have begun to study whether EPCs are
involved in tumor angiogenesis. It has been found that many
patients with cancer exhibit significantly increased numbers of
EPCs in the peripheral blood circulation. As the disease
progresses, EPCs increase as well. This suggests that EPCs may be
involved in tumor angiogenesis. Subsequent animal experiments also
revealed evidence for the contribution of EPCs to angiogenesis in
primary and metastatic tumors. This may be an important reason for
the poor outcomes associated with anti-angiogenic drugs (17).
During the initiation and development of BC,
endothelial cells from different sources participate in the process
of BC tumor angiogenesis. This includes the proliferation of local
microvascular endothelial cells and endothelial cells circulating
in the bone marrow. EPCs may also participate in BC angiogenesis.
Therefore, we hypothesize that endothelial cells derived from BCSCs
may contribute to angiogenesis in BC.
Materials and methods
Sources of tissue samples
In total, 13 clinical BC tissue samples were
collected from BC patients admitted to and diagnosed at the
Clinical Biological Sample Center of the First Affiliated Hospital
of Jinzhou Medical University from January 2015 to May 2015. No
treatments were performed before surgery, and all patients received
and signed the informed consent before sample collection. Eight BC
tissue specimens were successfully dissociated into single-cell
suspensions, but five specimens failed to dissociate. This study
was approved by the ethics committee of the First Affiliated
Hospital of Jinzhou Medical University. Eight specimens were
successfully isolated single cell suspension of BC, but 5 specimens
failed.
Collection of BC tissue samples
Specimens were obtained in the operating room. Each
BC tissue sample was collected in such a way that it did not affect
the pathological diagnosis. Individual samples were placed in
sterile containers containing sterile PBS and streptomycin (500
U/ml) (Gibco, USA), and quickly delivered to the central laboratory
within 1 h. Each specimen was divided into two parts. One part was
used to prepare the single-cell suspension, and the other part was
embedded and processed for paraffin sectioning.
Immunohistochemical staining
Sections were deparaffinized and hydrated according
to routine immunohistochemical procedures. After washing three
times with PBS, sections were blocked with normal serum blocking
solution for 15 min. Subsequently, 100 µl of diluted primary
antibody was carefully added dropwise onto the slides (Santa Cruz,
USA), and incubated overnight in humid boxes at 4°C. After washing
three times with PBS, goat anti-rabbit secondary antibody was added
and incubated for 15 min at room temperature. After washing three
times with PBS, HRP-labeled streptavidin was added and incubated
for 10 min at room temperature. Slides were then washed with PBS
and DAB staining was performed. The colorimetric development
reaction was stopped when the background turned slightly brown.
Sections were counterstained with hematoxylin for 3 min, treated
with 1% HCl alcohol for 30 sec, and rinsed in tap water for 10 min.
Sections were air-dried, mounted with neutral gum, and visualized
using light microscopy. Samples were examined and interpreted by
two or more pathologists.
Immunofluorescence staining
Frozen sections were placed at room temperature for
30 min, fixed in acetone at 4°C for 10 min, washed three times with
PBS buffer, and incubated with 3% H2O2 for 10
min to eliminate endogenous peroxidase activity. After washing
three times, appropriately diluted primary antibody was added and
incubated overnight at 4°C. The next day, samples were rinsed with
PBS and incubated at room temperature for 20 min with reagent 1 of
the kit. Sections were rinsed and then incubated at room
temperature for 30 min with reagent 2 of the kit. After rinsing,
AEC coloration was developed, followed by re-staining. Slides were
mounted with neutral gum and microscopy was performed. The
histological sections were subsequently analyzed by two or more
pathologists.
HER-2 fluorescent in situ
hybridization (FISH)
Immunofluorescence
Sections were baked at 56°C overnight, and then
placed in xylene at room temperature for 10 min. Sections were
rinsed and then dehydrated for 5 min in absolute ethanol. These
steps were repeated twice, and sections were then air-dried at room
temperature. Next, sections were immersed in pretreatment solution
at 80°C for 10 min, and rinsed in ultrapure water for 3 min. The
residual liquid was aspirated, and the sections were placed into
protease solution at 37°C for 10 min. This was followed by gradient
dehydration, air-drying at room temperature, incubation with an
Abbott probe (the hybridization area, Abbott, USA), mounting,
overnight hybridization using the ThermoBrite hybridization
instrument, DAPI staining, and observation under a fluorescence
microscope.
Interpretation of the results
A ratio <2 indicated that the HER2/NEU
gene was not expressed. A ratio ≤2 indicated that the
HER2/NEU gene was expressed. If the ratio was near the
critical range of 1.8–2.2, 20 more nuclei were counted to calculate
the ratio. Alternatively, conclusions were made using another
counting method in combination with clinical results.
Isolation and culture of BCSCs
BC tissue samples were cut into small pieces, placed
in sterile centrifuge tubes, and digested for 30 min with 0.05%
type II collagenase at 37°C in a sterile incubator. The suspension
was collected after 5 min of centrifugation at 1000 rpm and
filtered. Samples were then incubated with DMEM supplemented with
10% fetal bovine serum and 1% mycillin dual antibodies. The
single-cell suspensions of BC tissues were then examined for the
expression of CD44 and CD24 using flow cytometry.
CD44+/CD24−/low cells were inoculated into
DMEM/F12 serum-free medium containing 20 µg/l EGF, 20 µg/l bFGF,
and 2% B27. The growth of BCSCs was observed, and the medium was
changed 3 days after starting the culture.
Culture and functional testing of endothelial
cells
CD44+/CD24−/low cells were
cultured in the stem cell culture system for 1–2 weeks. After
mammary gland glomus cells formed in the culture plate, they were
collected and digested into single-cell suspensions. Trypan blue
staining was performed to count living cells, and a special culture
medium for endothelial cells (EGM-2) was used to promote
proliferation and observe cell growth. The 3rd-generation
endothelial cells were collected and stained with DiL-Ac-LDL. The
concentration of DiL-Ac-LDL was 10 g/ml, the endothelial cells were
incubated at a temperature of 37°C for 4 h, then washed with PBS.
The cells were fixed with 4% paraformaldehyde fixed cells for 10
min and to take photographed by fluorescence microscope. Positive
cells were considered to be undergoing differentiation. Adipocytes
were used as a control group.
Detection of angiogenesis
A 24-well plate was coated with 300 ml Matrigel (BD,
USA) and gently shaken. The gel was allowed to solidify at 37°C.
The 3rd-generation endothelial cells harvested from the endothelial
cell culture system were then digested with trypsin until the cell
edges became round. After discarding the supernatant, the cells
were repeatedly pipetted in the medium until they formed a
single-cell suspension. The suspension was then inoculated into the
24-well plates. Adipocytes were used as a control. Angiogenesis was
assessed microscopically 24 h after starting the culture.
Detection of CD105 and CD31
CD44+/CD24−/low cells and the
3rd-generation endothelial cells were harvested. Specimens were
prepared and the expression of CD105 and CD31 was assessed by flow
cytometry.
Statistical analysis
SPSS 20.0 software was used to analyze the
experimental results. Data are expressed as the mean ± standard
deviation (x- ± s). The intergroup comparison was performed
by using single-factor analysis of variance, and P<0.05 was
considered statistically significant.
Results
Expression of CD31, VEGF, and mutant
p53 in BC tissue
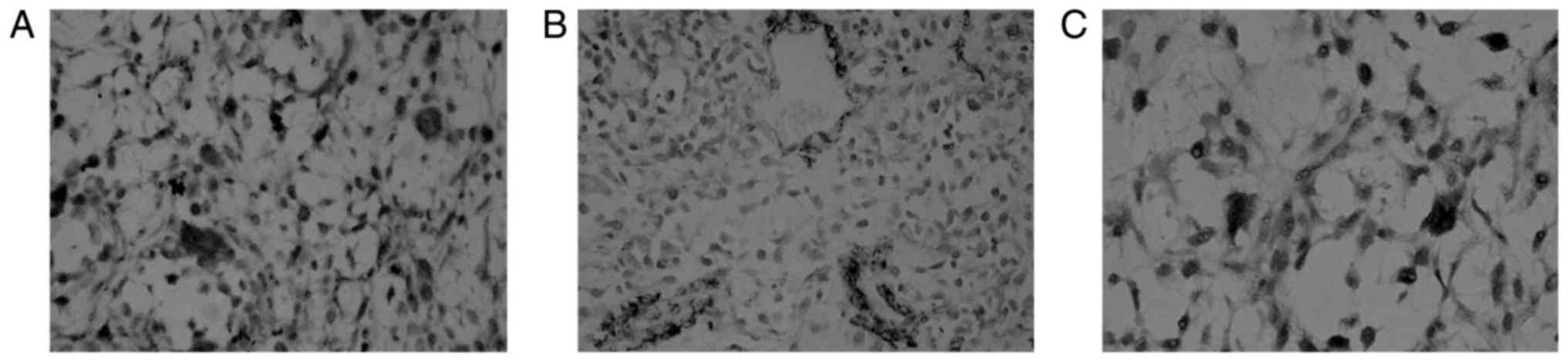
Expression of CD31, VEGF, and mutant p53 was
observed in paraffin sections of BC tissue samples (tan).
Expression was enriched in cells lining the vessel lumen or in
vascular spheres (Fig. 1).
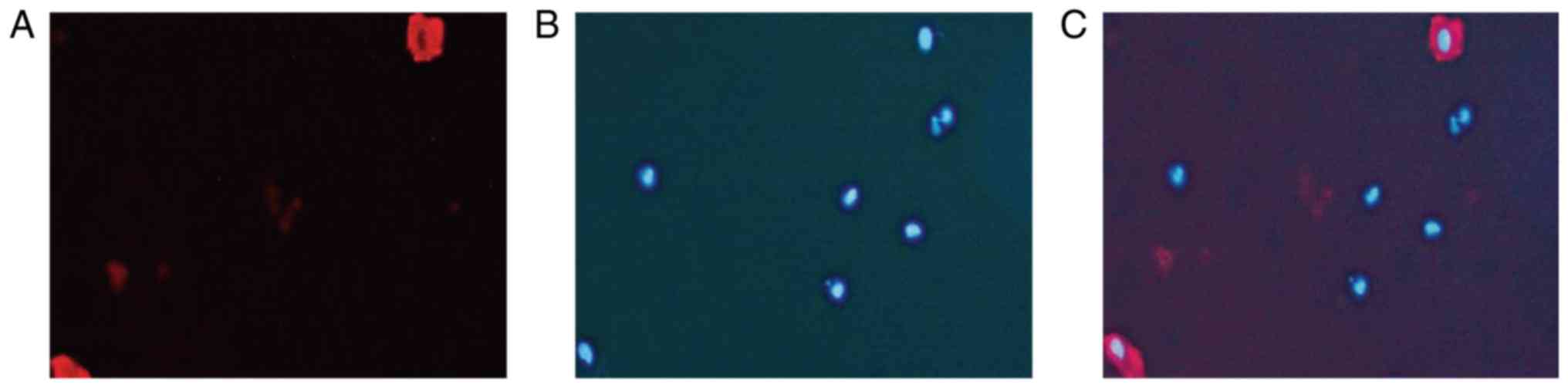
Immunofluorescence showed that CD31 and DAPI co-localized
intravascularly in the BC tissue specimens (Fig. 2).
Expression of HER2 in BC tissue
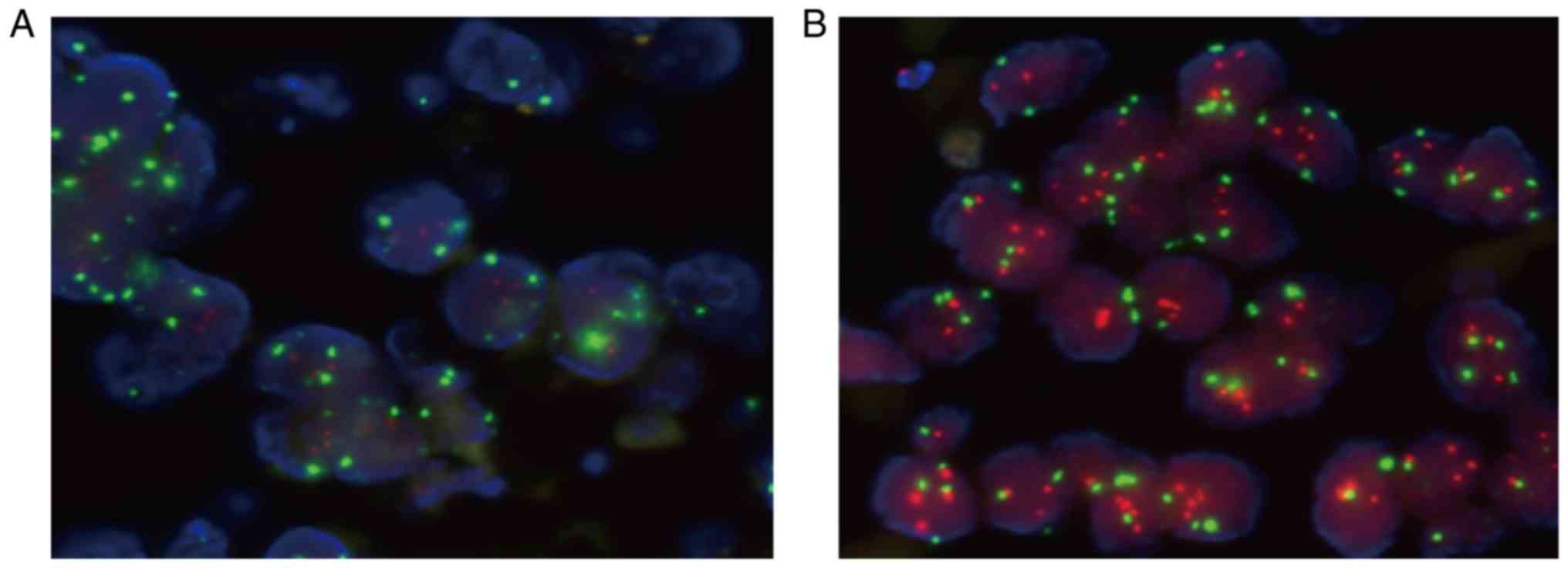
Among the 13 specimens, HER2-positive cells were
detected in 4 of 9 cases. Red FISH signal indicated the
localization of HER2, and green FISH signal stained CSP on
chromosome 17 (Fig. 3).
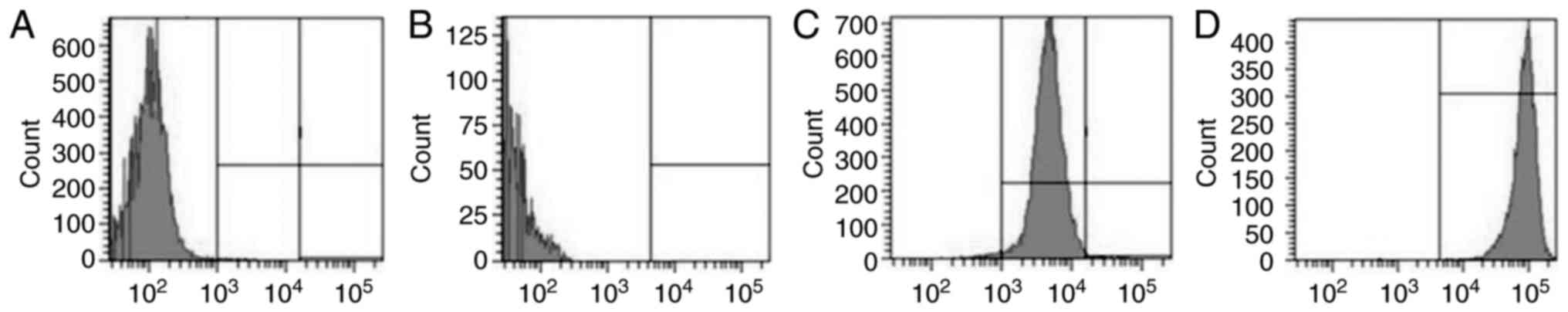
Detection of
CD44+/CD24−/low cells
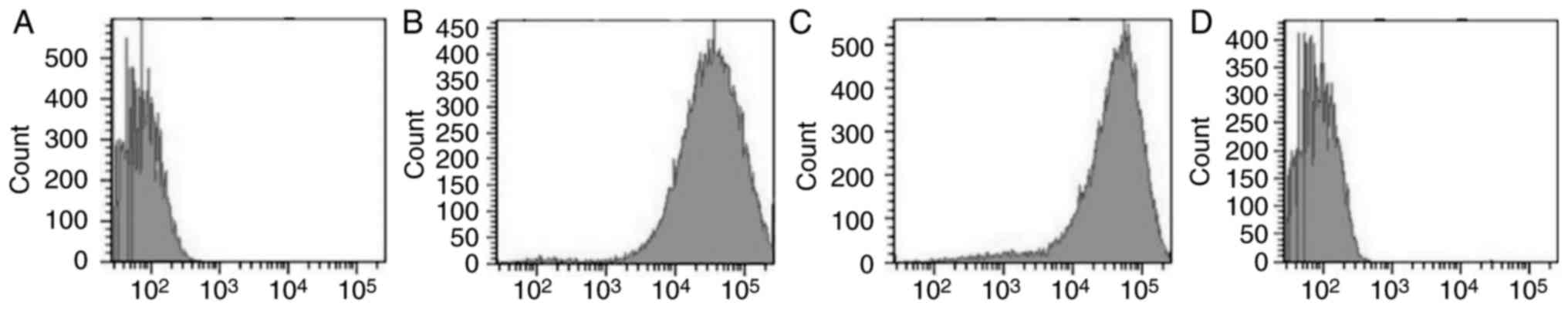
Isolated BCSCs were subjected to flow cytometry
after immunomagnetic sorting to quantify
CD44+/CD24−/low cells. This showed that
CD44+/CD24−/low cells comprised 7.5±2.6 and
94.3±4.7% of the total cell suspension before and after
immunomagnetic sorting, respectively (Fig. 4A and B). CD24+ cells
constituted 48.2±9.4 and 4.3±1.6% of the total cell suspension
before and after immunomagnetic sorting, respectively. This
indicated that lower the proportion of CD24+ cells
observed, higher was the proportion of CD24−/low cells
(Fig. 4C and D).
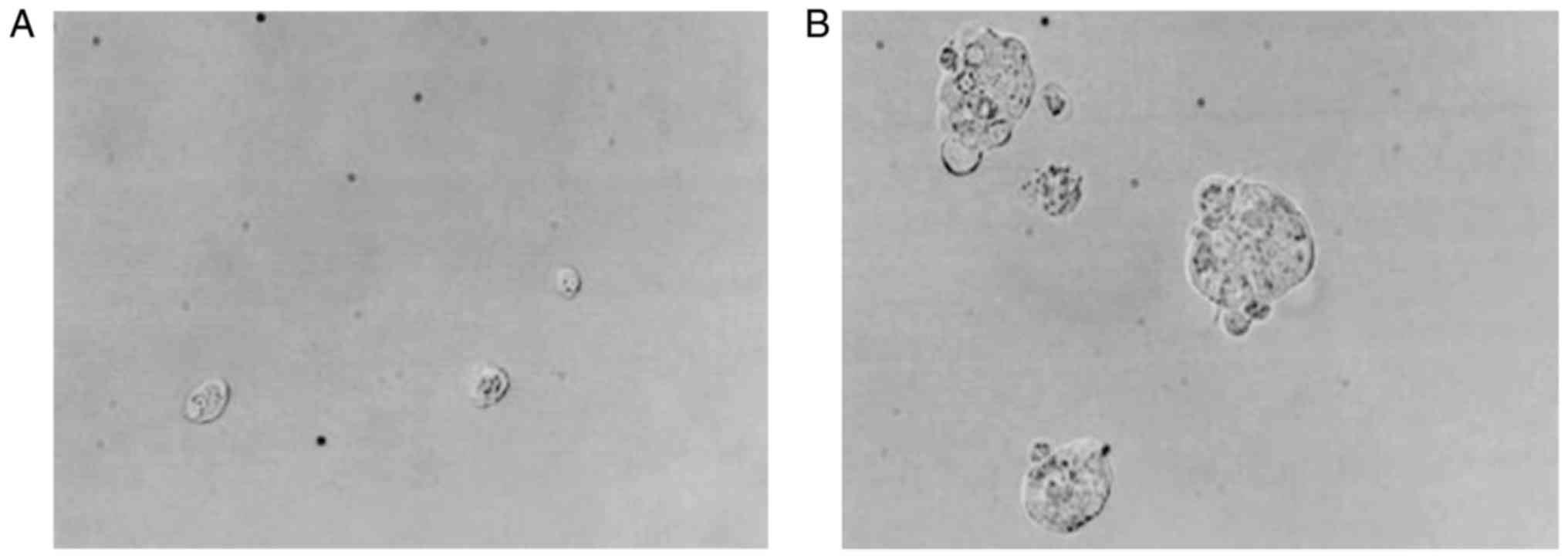
Isolation and culture of BCSCs
Isolated CD44+/CD24−/low
mononuclear cells were round in shape, and they were easily
suspended in the culture medium during the first 3 days of culture.
After 7 days, small colonies could be observed, which increased in
volume significantly after the culture medium was changed once
every day (Fig. 5).
Detection of CD105+ and
CD31+ cells
The percentage of CD105+ and
CD31+ cells in the mammary gland was 4.5±0.9 and
6.2±1.3%, respectively (Fig. 6A and
B). After continuous culturing in the endothelial cell culture
system for three generations, the proportion of CD105+
and CD31+ cells increased to 79.6±9.3 and 84.1±10.7%,
respectively (Fig. 6C a D).
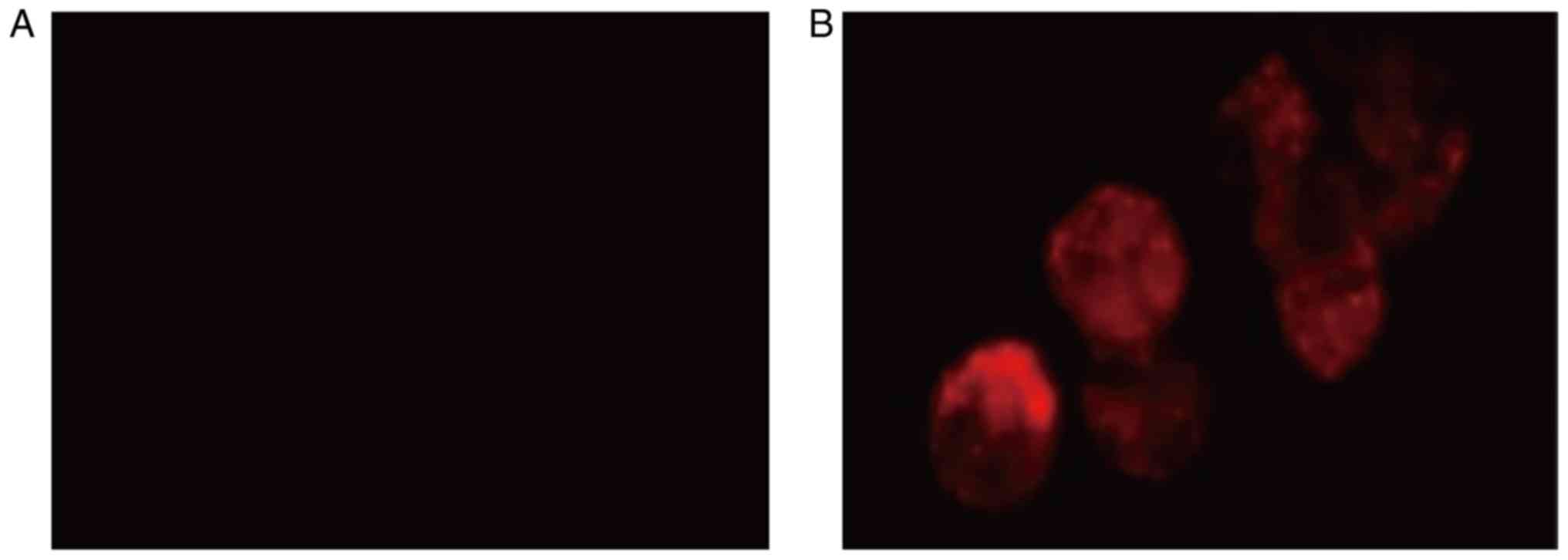
Functional detection of endothelial
cells
Cells cultured in the endothelial cell culture
medium phagocytosed DiL-Ac-LDL, which was demonstrated by the cells
exhibiting red fluorescence. This suggested that the cells were
functional endothelial cells. In contrast, the cells in the control
group did not display red fluorescence (Fig. 7).
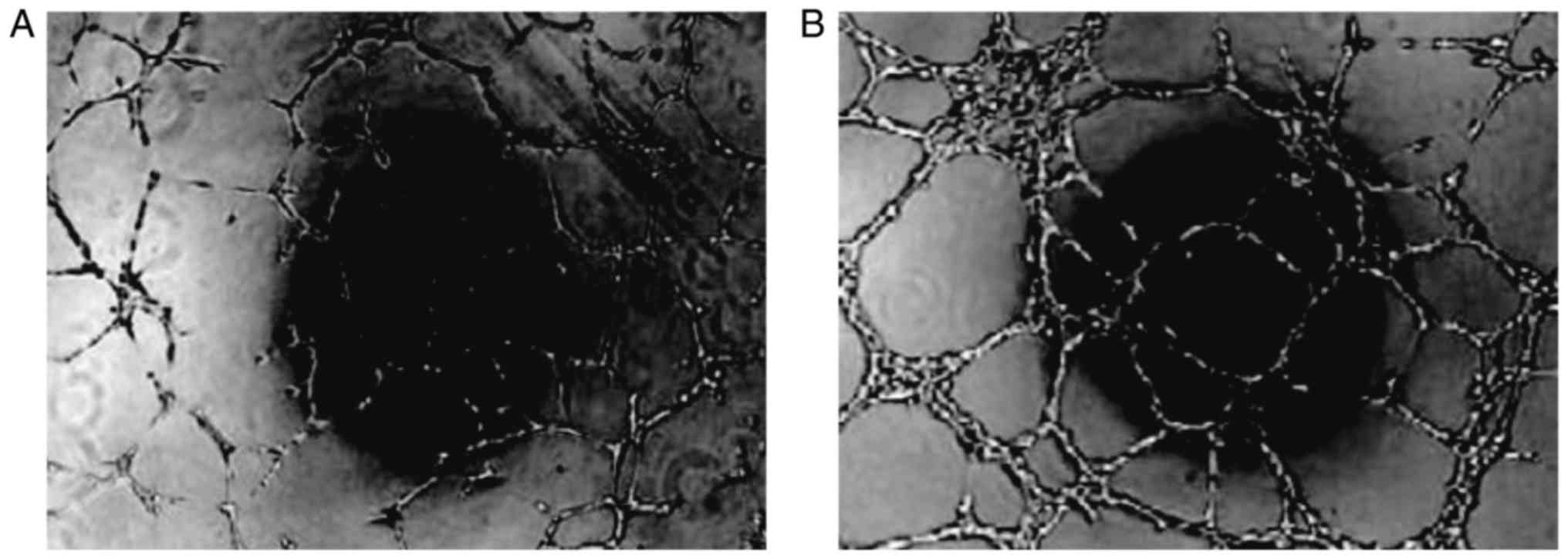
Angiogenesis
Cultured cells were seeded into 24-well plates
together with adipocytes as the control. The endothelial cells
displayed microscopic vascular-like structures that formed 24 h
after culture, but the control cells showed no such morphology
(Fig. 8).
Discussion
BC is one of the most common malignancies in women,
and its incidence rate is the second highest in the world (18–20).
Despite the existence of tumor stem cells in a variety of solid
tumors and hematologic malignancies, there are presently many
problems to be solved (21,22). CSCs has the potential of self-renewal
and multi-directional differentiation, which can differentiate into
tumor parenchyma cells or tumor stromal cells. Recently, it has
been found that the CD133(+) stem-like cell fraction is multipotent
and capable of differentiation along tumor and endothelial
lineages, since EPC was also found in glioma by differentiation of
cancer stem cells. The capacity to generate tumour vasculature of
the cancer stem cells within glioblastoma are novel findings, as
well as the mechanisms of tumor neo-angiogenesis. that provide new
insight into the biology of gliomas and the definition of cancer
stemness, which may be far more than glioblastoma (23). Studies have shown that the endothelial
cells derived from a variety of tissues participate in the process
of tumor angiogenesis in BC (6).
Therefore, we investigated whether BCSCs-derived endothelial cells
likewise participate in tumor angiogenesis.
The results showed that the expression of CD31,
VEGF, and mutant p53 was observable in paraffin-embedded sections
of BC tissues. These cells were arranged along the vascular lumen
or in vascular spheres. Furthermore, immunofluorescence and FISH
also showed that the tumor cells expressed CD31 and HER2. CD31
localizes to the cell junctions of endothelial cells, and it is
widely distributed in vascular cells. CD31 participates in a
variety of physiological processes, such as angiogenesis;
therefore, its expression is an indicator of in vivo
angiogenesis (24). VEGF is a
pro-angiogenic factor that promotes tumor angiogenesis. It also
plays an important role in the proliferation of endothelial cells
(25). Wild-type p53 functions as a
tumor suppressor. However, mutant p53 inactivates the p53
gene, leading to tumorigenesis. At the same time, it has been found
that the high expression of mutant p53 is very important in
intratumoral angiogenesis (26).
Therefore, the expression levels of CD31, VEGF, and p53 can be used
as indicators of tumor vascular endothelial cells. The HER2
gene is a proto-oncogene, which is expressed at low levels in
normal tissues, but is overexpressed or ectopically expressed in
epithelium-derived tumor tissues such as breast cancer. It has been
shown that HER2 expression is closely associated with the
recurrence, metastasis, and poor prognosis of BC (27). FISH can detect the expression of the
HER2 gene in BC tissue, which can help clarify the
relationship of chromosomes with gene and protein expression
levels. The results of this study showed that the expressions of
CD31 and HER2 in BC vascular cells confirmed the presence of
tumor-derived endothelial cells in BC vessels. Moreover, some
endothelial cells in BC have tumor homology.
CD24 is typically expressed in a variety of tumor
cells. CD24+ cells display significantly enhanced
tumorigenesis and metastasis, but the expression is downregulated
in invasive BC. In contrast, CD24− cells show biological
characteristics associated with BCSCs (28). CD44 is upregulated in various tumor
cells, including BC cells. Studies have shown that the
overexpression of CD44 promotes tumor invasion and metastasis.
Another study has shown that CD44 is an important marker of BCSCs
(29). Therefore, many researchers in
China and abroad isolate CD44+/CD24− cells
during sorting, which are considered bona fide BCSCs. In this
study, BCSCs were sorted using the immunomagnetic sorting method.
The results showed that CD44+ cells comprised about
7.5±2.6% of the total cell population before sorting, and this
increased to about 94.3±4.7% after sorting. In contrast,
CD24+ cells constituted 48.2±9.4% of the total cell
population before sorting and 4.3±1.6% after sorting, indicating
that lower the abundance of CD24+ cells, higher is the
amount of CD24−/low cells. These results showed that
immunomagnetic sorting enriched for
CD44+/CD24−/low cells with high purity. The
sorted cell suspension could form BC cell clusters after in
vitro culture, indicating that they possessed tumor stem
cell-like proliferation abilities. Meanwhile, the cells cultured in
the endothelial differentiation culture system could internalize
DiL-Ac-LDL, demonstrating their endothelial cell-like
physiology.
Like CD34, CD31 is a pan-vascular endothelium
marker. It cannot distinguish whether vascular endothelial cells
are proliferating or not. In contrast, CD105 is present on the
surface of endothelial cells and is specifically expressed in
proliferating neovascular endothelial cells. Thus, CD105 is
considered the best marker of neovascularization (30). In this study, mammary gland cells were
digested, cultured in the EGM-2 endothelial cell culture system,
and the 3rd-generation cells were collected for flow cytometry. The
results showed that the CD105+ and CD31+
cells from the mammary gland comprised 4.5±0.9 and 6.2±1.3% of the
total population, respectively. In the 3rd-generation cells, this
increased significantly to 79.6±9.3 and 84.1±10.7%, respectively,
suggesting that BCSCs may differentiate into endothelial cells in
the endothelial cell culture system. Meanwhile, the in vitro
3D gel culture experiments revealed vascular-like structures in
cells differentiated and cultured from endothelial cells,
confirming that endothelial cells differentiated from BCSCs have
the ability to form vessels.
In summary, we believe that BCSCs-derived
endothelial cells can differentiate into vascular endothelial cells
and participate in tumor angiogenesis in vitro.
Acknowledgements
This study was supported by the President Fund of
Liaoning Medical College-Clinical Medicine Construction Special
Fund (No. XZJJ20140215).
References
|
1
|
Hong CS, Graham NA, Gu W, Camacho
Espindola C, Mah V, Maresh EL, Alavi M, Bagryanova L, Krotee PA,
Gardner BK, et al: MCT1 modulates cancer cell pyruvate export and
growth of tumors that co-express MCT1 and MCT4. Cell Rep.
14:1590–1601. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu R, Shi P, Nie Z, Liang H, Zhou Z, Chen
W, Chen H, Dong C, Yang R, Liu S and Chen C: Mifepristone
suppresses basal triple-negative breast cancer stem cells by
down-regulating KLF5 expression. Theranostics. 6:533–544. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang J, Liu D, Feng Z, Mao J, Zhang C, Lu
Y, Li J, Zhang Q, Li Q and Li L: MicroRNA-138 modulates metastasis
and EMT in breast cancer cells by targeting vimentin. Biomed
Pharmacother. 77:135–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Telang N: Putative cancer-initiating stem
cells in cell culture models for molecular subtypes of clinical
breast cancer. Oncol Lett. 10:3840–3846. 2015.PubMed/NCBI
|
|
5
|
Zhu A, Li Y, Song W, Xu Y, Yang F, Zhang
W, Yin Y and Guan X: Antiproliferative Effect of Androgen Receptor
Inhibition in Mesenchymal Stem-Like Triple-Negative Breast Cancer.
Cell Physiol Biochem. 38:1003–1014. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schulenburg A, Blatt K, Cerny-Reiterer S,
Sadovnik I, Herrmann H, Marian B, Grunt TW, Zielinski CC and Valent
P: Cancer stem cells in basic science and in translational
oncology: can we translate into clinical application? J Hematol
Oncol. 8:162015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Zhang L, Hu C, Liang S, Fei X, Yan
N, Zhang Y and Zhang F: WNT pathway inhibitor pyrvinium pamoate
inhibits the self-renewal and metastasis of breast cancer stem
cells. Int J Oncol. 48:1175–1186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu J, Zhang Y, Leung LH, Liu L, Yang F and
Yao X: Efficacy and safety of angiogenesis inhibitors in advanced
gastric cancer: A systematic review and meta-analysis. J Hematol
Oncol. 18:1112016. View Article : Google Scholar
|
|
9
|
Goel G and Sun W: Ramucirumab, another
anti-angiogenic agent for metastatic colorectal cancer in
second-line setting-its impact on clinical practice. J Hematol
Oncol. 8:922015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trivanović D, Jauković A, Krstić J,
Nikolić S, Okić Djordjević I, Kukolj T, Obradović H, Mojsilović S,
Ilić V, Santibanez JF and Bugarski D: Inflammatory cytokines prime
adipose tissue mesenchymal stem cells to enhance malignancy of
MCF-7 breast cancer cells via transforming growth factor-β1. IUBMB
Life. 68:190–200. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kasimir-Bauer S, Bittner AK, König L,
Reiter K, Keller T, Kimmig R and Hoffmann O: Does primary
neoadjuvant systemic therapy eradicate minimal residual disease?
Analysis of disseminated and circulating tumor cells before and
after therapy. Breast Cancer Res. 18:202016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mori M, Ito F, Shi L, Wang Y, Ishida C,
Hattori Y, Niwa M, Hirayama T, Nagasawa H, Iwase A, et al: Ovarian
endometriosis-associated stromal cells reveal persistently high
affinity for iron. Redox Biol. 6:578–586. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanwar JR, Kamalapuram SK, Krishnakumar S
and Kanwar RK: Multimodal iron oxide (Fe3O4)-saturated lactoferrin
nanocapsules as nanotheranostics for real-time imaging and breast
cancer therapy of claudin-low, triple-negative
(ER(−)/PR(−)/HER2(−)). Nanomedicine (Lond). 11:249–268. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharma B, Varney ML, Saxena S, Wu L and
Singh RK: Induction of CXCR2 ligands, stem cell-like phenotype, and
metastasis in chemotherapy-resistant breast cancer cells. Cancer
Lett. 372:192–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie J, Liu J, Liu H, Liang S, Lin M, Gu Y,
Liu T, Wang D, Ge H and Mo SL: The antitumor effect of tanshinone
IIA on anti-proliferation and decreasing VEGF/VEGFR2 expression on
the human non-small cell lung cancer A549 cell line. Acta Pharm Sin
B. 5:554–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Botelho MC and Alves H: Endothelial
progenitor cells in breast cancer. Int J Immunother Cancer Res.
2:1–2. 2016.PubMed/NCBI
|
|
17
|
Teng L, Peng S, Guo H, Liang H, Xu Z, Su Y
and Gao L: Conditioned media from human ovarian cancer endothelial
progenitor cells induces ovarian cancer cell migration by
activating epithelial-to-mesenchymal transition. Cancer Gene Ther.
22:518–523. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amoury M, Kolberg K, Pham AT, Hristodorov
D, Mladenov R, Di Fiore S, Helfrich W, Kiessling F, Fischer R,
Pardo A, et al: Granzyme B-based cytolytic fusion protein targeting
EpCAM specifically kills triple negative breast cancer cells in
vitro and inhibits tumor growth in a subcutaneous mouse tumor
model. Cancer Lett. 372:201–209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong L, Guo S, Liu C, Zhao Y, Feng C, Liu
Y, Wang T and Li C: Overexpression of SDF-1 activates the NF-κB
pathway to induce epithelial to mesenchymal transition and cancer
stem cell-like phenotypes of breast cancer cells. Int J Oncol.
48:1085–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Zou T, Wang S, Chen H, Su D, Fu X,
Zhang Q and Kang X: Genistein-induced differentiation of breast
cancer stem/progenitor cells through a paracrine mechanism. Int J
Oncol. 48:1063–1072. 2016.PubMed/NCBI
|
|
21
|
Yin X, Zhang BH, Zheng SS, Gao DM, Qiu SJ,
Wu WZ and Ren ZG: Coexpression of gene Oct4 and Nanog initiates
stem cell characteristics in hepatocellular carcinoma and promotes
epithelial-mesenchymal transition through activation of Stat3/Snail
signaling. J Hematol Oncol. 8:232015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Y, Cang S, Han L, Liu C, Yang P,
Solangi Z, Lu Q, Liu D and Chiao JW: Establishment of prostate
cancer spheres from a prostate cancer cell line after phenethyl
isothiocyanate treatment and discovery of androgen-dependent
reversible differentiation between sphere and neuroendocrine cells.
Oncotarget. 7:26567–26579. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Glioblastoma stem-like cells give rise to tumour
endothelium. Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Tong Q, Liu S, Cui J, Zhang Q, Sun
W and Yang S: ZEB1 Upregulates VEGF Expression and Stimulates
Angiogenesis in Breast Cancer. PLoS One. 11:e01487742016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
De Francesco EM, Pellegrino M, Santolla
MF, Lappano R, Ricchio E, Abonante S and Maggiolini M: GPER
mediates activation of HIF1α/VEGF signaling by estrogens. Cancer
Res. 74:4053–4064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jaeger S, Min J, Nigsch F, Camargo M, Hutz
J, Cornett A, Cleaver S, Buckler A and Jenkins JL: Causal Network
Models for Predicting Compound Targets and Driving Pathways in
Cancer. J Biomol Screen. 19:791–802. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lekovic G, Drazin D, Mak AC and Schwartz
MS: Cyberknife radiosurgery and concurrent intrathecal chemotherapy
for leptomeningeal metastases: Case report of prolonged survival of
a HER-2+ breast cancer patient status-post craniospinal
irradiation. Cureus. 8:e4532016.PubMed/NCBI
|
|
28
|
Suyama K, Onishi H, Imaizumi A, Shinkai K,
Umebayashi M, Kubo M, Mizuuchi Y, Oda Y, Tanaka M, Nakamura M and
Katano M: CD24 suppresses malignant phenotype by downregulation of
SHH transcription through STAT1 inhibition in breast cancer cells.
Cancer Lett. 374:44–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gangopadhyay S, Nandy A, Hor P and
Mukhopadhyay A: Breast cancer stem cells: A novel therapeutic
target. Clin Breast Cancer. 13:7–15. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park MT, Oh ET, Song MJ, Kim WJ, Cho YU,
Kim SJ, Han JY, Suh JK, Choi EK, Lim BU, et al: The
radiosensitivity of endothelial cells isolated from human breast
cancer and normal tissue in vitro. Microvasc Res. 84:140–148. 2012.
View Article : Google Scholar : PubMed/NCBI
|