Introduction
Rhabdoid tumors are the most common sarcoma derived
from the soft tissues in patients under the age of 20 years.
Approximately 10% of pediatric solid tumors are rhabdoid tumors,
accounting for ~50% of all rhabdoid tumor malignancies (1,2).
Proliferation and metastasis are two typical characteristics of
rhabdoid tumors and 90% of affected patients are expected to
succumb within 5 years. Surgery combined with systemic radiotherapy
is recommended to promote survival, particularly in pediatric
patients. However, amputation is unavoidable in certain
circumstances and presents a substantial physical and psychological
challenge to patients and their families (3–5). Proton
radiotherapy provides hope to patients with rhabdoid tumors, as it
efficiently inhibits tumor growth and metastasis without damaging
normal tissues or impairing growth or development. However, it is
difficult to widely utilize proton radiotherapy, particularly in
developing countries, due to its limitations and high cost
(6–8).
Chemotherapy drugs, including Adriamycin and cyclophosphamide, have
been trialed in certain cases, but their outcomes remain uncertain
(8). Therefore, identification of
effective and affordable drugs to cure rhabdoid tumors is
required.
Silibinin is a natural extract obtained from milk
thistle (Silybum marianum) that has been widely used in
Asian counties as an anti-oxidant drug (9). One of its known functions is liver
protection, as it stabilizes the cell membrane and reduces reactive
oxygen species in liver cells (10,11). In
addition to its role in normal cell protection, silibinin has
attracted more attention due to its potential antitumor capacities.
Silibinin triggers apoptosis, induces cell cycle arrest or
autophagic cell death to inhibit cell proliferation, and suppresses
cell migration and invasion in a variety of cancer models,
including breast, prostate, bladder and renal cancer types
(12–15). Further investigation is required to
determine whether or not silibinin inhibits rhabdoid tumors,
particularly with regards to tumor cell migration and invasion.
The phosphatidylinositol 3-kinase/protein kinase B
(PI3K/Akt) signaling pathway serves a pivotal function in cell
homeostasis and has been revealed to be involved in cell
proliferation and metastasis regulation (16,17).
Multiple studies have revealed that the PI3K/Akt signaling pathway
is extensively activated in a variety of tumor types, including
breast cancer, prostate cancer and rhabdoid tumors; inactivation of
PI3K/Akt using specific inhibitors or other anticancer drugs leads
to cell proliferation inhibition, migration and invasion
suppression (18–21). Silibinin has been reported to be a
PI3K/Akt suppressor and thus inhibit tumorigenesis and cancer
progression (22,23). Whether or not PI3K/Akt is also
affected by silibinin in rhabdoid tumors is unclear and requires
further clarification.
To the best of our knowledge, the present study was
the first to evaluate the antitumor capacity of silibinin in
rhabdoid tumors, with respect to cell viability, migration and
invasion, with a specific focus on the inactivation of the PI3K/Akt
signaling pathway.
Materials and methods
Reagents
Silibinin (cat. no. S0417), MTT (cat. no. M2128),
protease inhibitor (cat. no. P8340) and phosphatase inhibitor (cat.
no. MSSAFE) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The PI3K inhibitor LY294002 (cat. no. S1105)
and proliferation inhibitor mitomycin C (cat. no. S8146) were
purchased from Selleck Chemicals (Houston, TX, USA). Matrix gel
(cat. no. 356234) was purchased from BD Biosciences (San Jose, CA,
USA). The bicinchoninic acid (BCA) protein qualification kit (cat.
no. 23225) was purchased from Pierce; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). Anti-phosphorylated-Akt (Ser473; cat. no.
4060), anti-total (t)-Akt (cat. no. 4691), anti-GAPDH (cat. no.
5174) primary antibodies, and horseradish peroxidase
(HRP)-conjugated goat anti-rabbit IgG (H+L) (cat. no. 7074) and
HRP-conjugated horse anti-mouse IgG (H+L) (cat. no. 7076) secondary
antibodies were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Dilutions for primary antibodies and secondary
antibodies were 1:1,000 and 1:10,000 respectively.
Cell culture
The human rhabdoid tumor G401 cell line was
purchased from the American Type Culture Collection (cat. no.
CRL-1441; ATCC, Manassas, VA, USA) and cultured in complete medium
which contained ATCC-formulated McCoy's 5A modified medium (cat.
no. 30-2007; ATCC) supplemented with 10% fetal bovine serum (FBS;
cat. no. 10438026; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 0.1 mg/ml streptomycin. Cells were incubated in a
cell culture incubator (cat. no. 3308; Thermo Fisher Scientific,
Inc.) with a humidified atmosphere containing 5% CO2 at
37°C. An inverted microscope at ×40 magnification (Olympus, Tokyo,
Japan) was used to observe cells and to capture images. When cells
achieved 90–100% confluency, cells were digested by 0.25% trypsin
in 0.53 mM EDTA solution and centrifuged at 100 × g for 5 min at
room temperature. Cultured medium was replaced every other day or
according to the experimental design.
Cell viability assay
An MTT assay was used to determine cell viability. A
total of 200 µl medium containing 3,000 cells was placed into each
well of a 96-well plate overnight in the cell culture incubator at
37°C. The next day, culture medium was replaced with complete
medium containing 10, 20 or 40 µM silibinin or DMSO and cells were
incubated in the cell culture incubator at 37°C for 24 h. MTT was
added to the medium to obtain a final concentration of 0.5 mg/ml 4
h prior to harvest. The medium was aspirated in each well and 150
ml dimethylsulfoxide (DMSO) per well was added to dissolve the
precipitates. The color change was then measured, and the optical
density (OD) value was read by a plate reader at a wavelength of
490 nm.
Wound healing assay
A 6-well plate was used to perform a wound-healing
assay and each well was seeded with 500,000 cells in 2 ml
ATCC-formulated McCoy's 5A modified medium supplemented with 10%
FBS, 100 U/ml penicillin and 0.1 mg/ml streptomycin. When the cell
density reached 100% confluence, wounds were scratched using 200-µl
pipet tips in each well and were rinsed with 37°C preheated
phosphate-buffered saline (PBS) 3 times. Cells were then cultured
in 2 ml ATCC-formulated McCoy's 5A modified medium supplemented
with 100 U/ml penicillin and 0.1 mg/ml streptomycin containing 20
µM silibinin, 10 µM LY294002, both or vehicle (DMSO), and 1 µg/ml
mitomycin C was added to each well. An inverted microscope at ×40
magnification (Olympus, Tokyo, Japan) was used to image these
wounds at designated time points, 12 and 24 h.
Transwell migration and invasion
assays
Cells were cultured in a 6-well plate and treated
with 20 µM silibinin, 10 µM LY294002, both or vehicle (DMSO), once
they had reached 60% confluence. After 24 h of treatment, the cells
were digested and centrifuged at 100 × g for 5 min at room
temperature. A total of 30,000 cells were seeded onto a Millicell
(cat. no. PSET010R1; Merck KGaA, Darmstadt, Germany) without matrix
gel (migration assay) or 100,000 cells were seeded with matrix gel
(invasion assay) in 200 µl ATCC-formulated McCoy's 5A modified
medium supplemented with 100 U/ml penicillin and 0.1 mg/ml
streptomycin. The Millicells were then placed into the wells of a
12-well plate. The space inside Millicells was considered to be
upper chamber, while the space between the bottom of Millicells and
the bottom of the 12-well plate was considered to be lower chamber.
The lower chamber of each well was filled with 800 µl
ATCC-formulated McCoy's 5A modified medium supplemented with 10%
FBS, 100 U/ml penicillin and 0.1 mg/ml streptomycin, prior to being
placed into the cell culture incubator for 24 h to allow cells to
migrate or invade. When the Millicells were harvested, medium was
aspirated, and the membranes from the inserts were washed with 4°C
PBS 3 times, fixed with 4% paraformaldehyde and stained with 0.1%
crystal violet at room temperature for 15 min respectively. The
membranes were then cut and placed onto slides. Migrated and
invaded cells were then observed and images were captured using a
bright-field light microscope (at ×100 magnification; Olympus,
Tokyo, Japan).
Western blot analysis
Treated cells were washed with pre-chilled (4°C) PBS
3 times prior to being solubilized by radioimmunoprecipitation
assay buffer (cat. no. 89900; Thermo Fisher Scientific, Inc.)
containing protease inhibitor and phosphatase inhibitor. Lysate was
then sonicated and centrifuged at 14,000 × g for 10 min at 4°C. The
supernatant of each lysate was placed into a different tube,
qualified by the BCA qualification system and boiled with 4X
loading buffer (cat. no. NP0007; Thermo Fisher Scientific, Inc.).
In total, 30 µg protein of each sample was loaded onto each column
in 12% SDS-PAGE gel and electrophoresis was performed. Proteins
were subsequently transferred onto a polyvinylidene fluoride
membrane. Membranes were blocked with 5% milk at room temperature
for 1 h and incubated with primary antibodies for 2 h at room
temperature or overnight at 4°C. Membranes were washed with
Tris-buffered saline plus 0.1% Tween (TBST) and subsequently
incubated with secondary antibodies for 1 h at room temperature.
Bands in the membranes were visualized using ECL reagents (cat. no.
1705060; Bio-Rad Laboratories, Inc.) and were detected by ChemiDoc™
XRS+ system (Bio-Rad Laboratories, Inc.). The density of the bands
was quantified by ImageJ software (version 1.50g 13; National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All experiments were performed ≥3 times. GraphPad
Prism 5 (GraphPad software, La Jolla, CA, USA) was utilized to
generate graphs. Data are presented as the mean ± standard
deviation and the differences between experimental groups were
analyzed using one-way analysis of variance by SPSS 17.0 (IBM,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Silibinin inhibits migration and
invasion of G401 cells in a dose-dependent manner
G401 is a well-established cell line derived from
the kidney of a 3-month-old male patient. It was first classified
as a Wilm's tumor cell line, but this was subsequently corrected
and it is now known to be a rhabdoid tumor cell line (24). This cell line has been utilized by
several groups and has been proven to be an ideal in vitro
model for studying rhabdoid tumors (25–27).
However, whether or not the oncological behavior of G401 cells can
be affected by silibinin remains unclear.
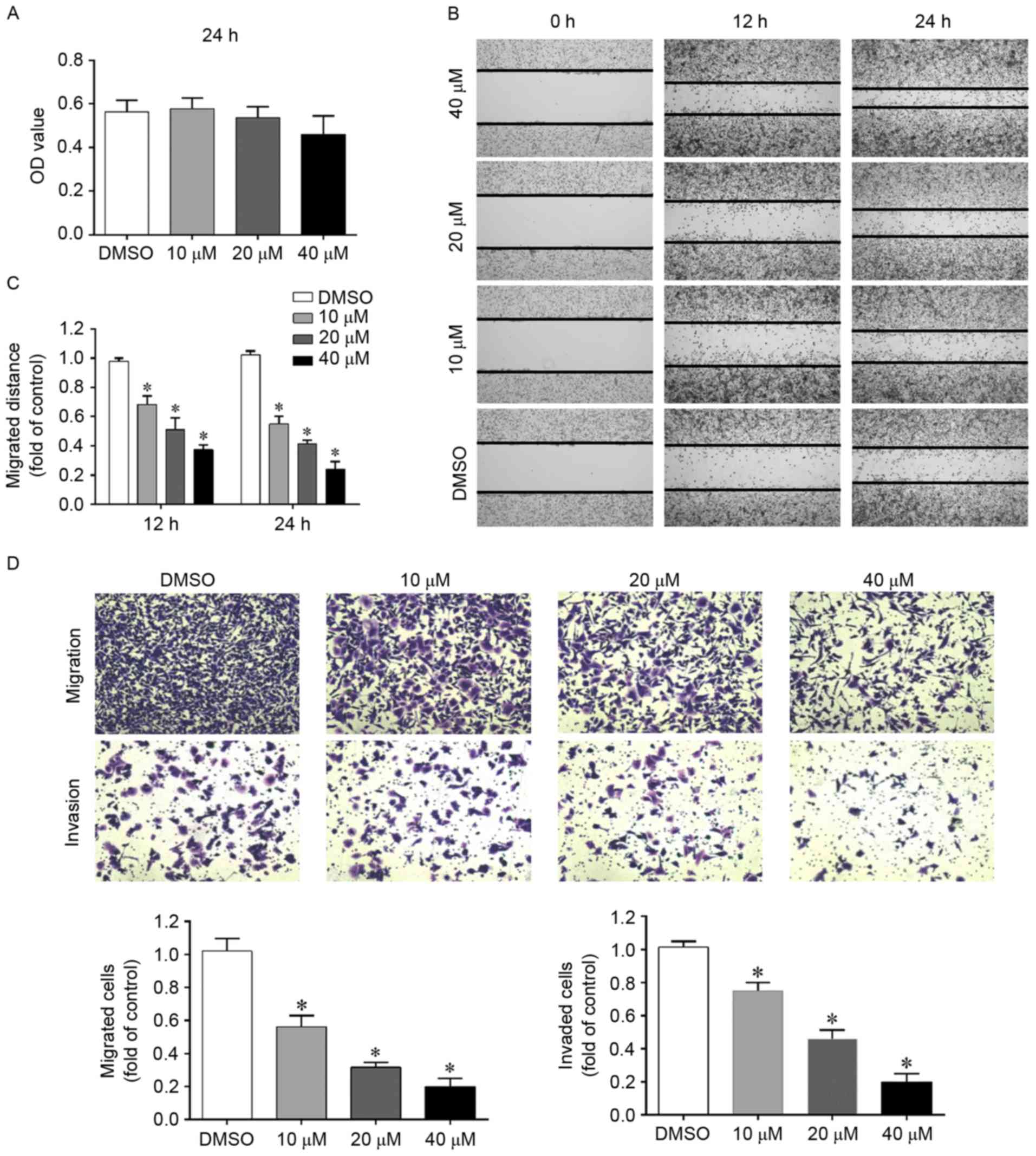
Low doses of silibinin have been demonstrated to
inhibit cell migration and invasion in various tumor models in
vivo and in vitro. In the present study, an MTT assay
was initially used to select the doses that did not significantly
affect cell growth over a treatment period of 24 h. As demonstrated
in Fig. 1A, when cells were treated
with 10, 20 or 40 µM silibinin for 24 h, cell viability was
unaffected compared with the DMSO control and thus, doses were
chosen to be used in subsequent studies. High doses of silibinin
and longer exposure times were revealed to significantly decrease
cell viability (data not shown).
A wound healing assay is a convenient, economic and
reliable assay for studying cell migration. Therefore, in order to
evaluate whether silibinin affects the migration of G401 cells, a
wound healing assay was performed. As demonstrated in Fig. 1B and C, when the cells were treated
with silibinin, they migrated more slowly as the wounds had less
closure. To further confirm this finding, a Transwell migration
assay was performed and similar results were obtained (Fig. 1D).
To study the impact of silibinin on cell invasion, a
Transwell invasion assay was performed. In this assay, cells will
invade the other side of the membrane only when the pre-coated
Matrigel is digested by the cells themselves. As demonstrated in
Fig. 1D, silibinin significantly
inhibited cell invasion in a concentration-dependent manner.
These results indicate that silibinin inhibits G401
migration and invasion independent of its growth inhibition
ability.
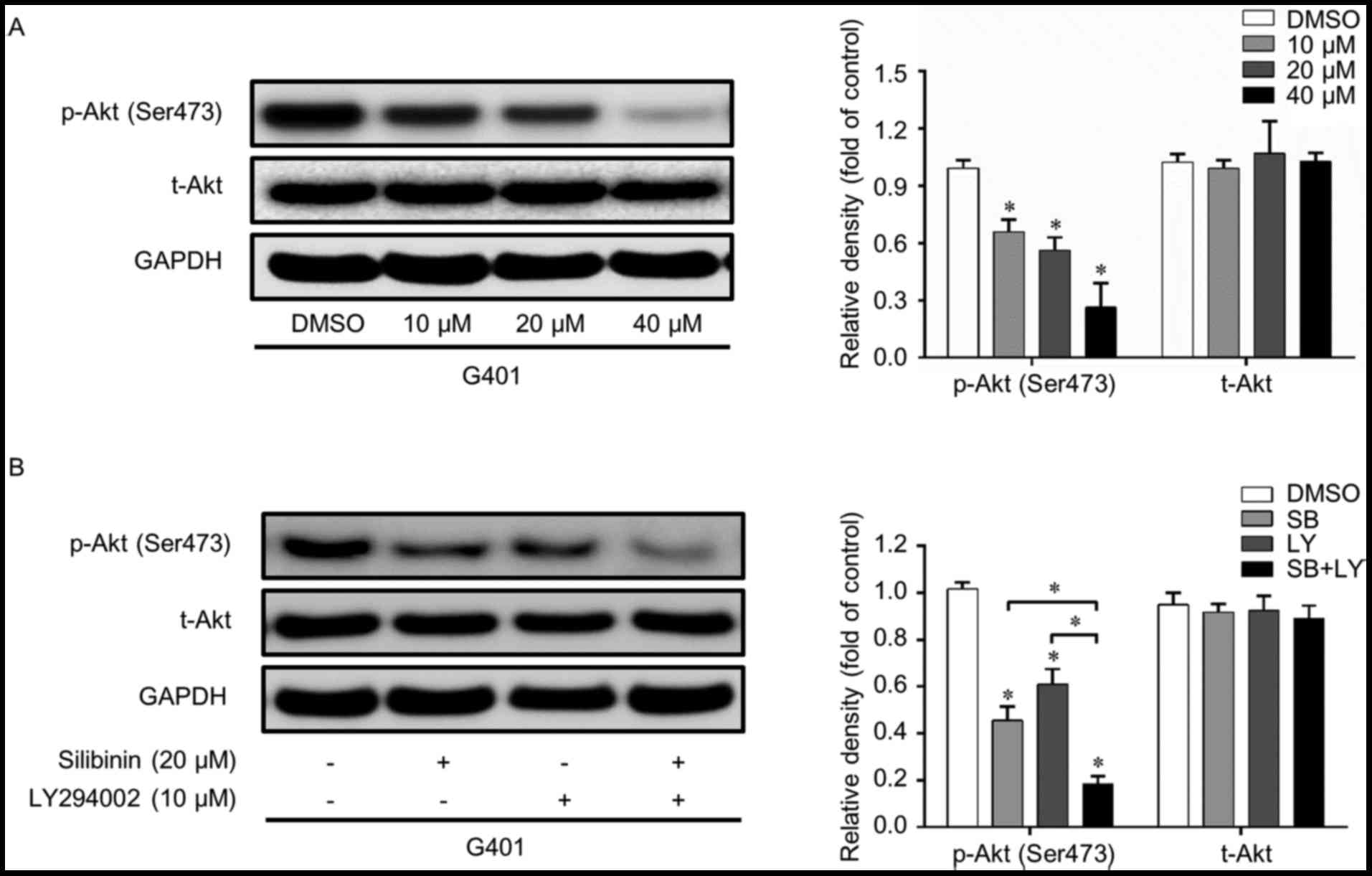
PI3K/Akt signaling pathway is
inactivated by silibinin in G401
The PI3k/Akt signaling pathway serves a pivotal
function in cell homeostasis and is a crucial regulator of cell
growth, migration and invasion. Whether or not this pathway is
involved in the silibinin-induced inhibition of migration and
invasion remains unclear. Therefore, western blot analysis was
performed in order to confirm this hypothesis. As presented in
Fig. 2A, p-Akt (Ser473) was
suppressed by silibinin, while t-Akt was not significantly
affected. To further confirm the function of the PI3k/Akt pathway,
LY294002, a classic PI3K inhibitor, was used. LY294002 and
silibinin independently inhibited the activation of p-Akt without
affecting the total level of Akt. When these two reagents were used
in combination, the PI3K/Akt inhibition effect was further enhanced
(Fig. 2B). These results suggest that
the PI3K/Akt signaling pathway is suppressed when cells are treated
with silibinin.
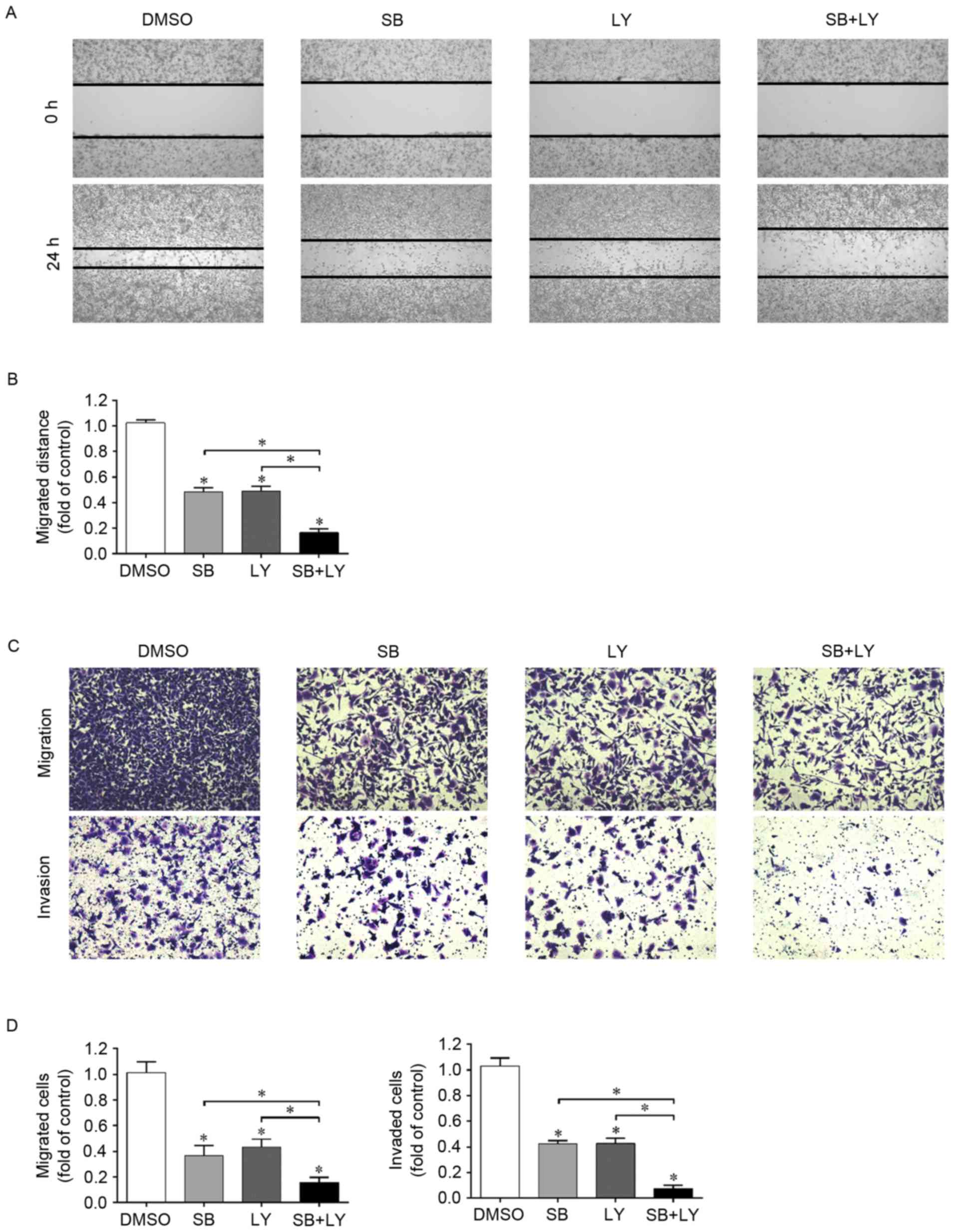
PI3K/Akt signaling pathway is involved
in the inhibition of migration and invasion induced by
silibinin
Since silibinin was confirmed to be a PI3K/Akt
suppressor in the G401 cell line and as silibinin inhibits the
migration and invasion of G401 cells, the role of PI3K/Akt
inactivation in the silibinin-induced inhibition of migration and
invasion requires further investigation. In the wound healing
assay, silibinin and LY294002 independently suppressed wound
closure, and when these two reagents were used in combination, the
inhibitory effect was enhanced (Fig. 3A
and B). These results were further confirmed using a Transwell
migration assay (Fig. 3C and D).
To illustrate the role served by PI3K/Akt in
silibinin-induced invasion inhibition, the Transwell invasion assay
was performed again. As presented in Fig.
3C and D, silininin and LY294002 suppressed cell invasion, as
there were fewer invaded cells in these groups than in the control
group. Furthermore, when these two compounds were used in
combination, cell invasion was further inhibited as this group had
the least invaded cells. These data suggest that the inactivation
of PI3K/Akt by silibinin positively contributes to the inhibition
of cell migration and invasion.
Discussion
Rhabdoid tumors, which usually occur in individuals
under the age of 2 years, are one of the most aggressive
malignancies and present with a poor prognosis. Surgery combined
with systemic radiotherapy is currently the recommended treatment,
but the efficiency of this method is limited due to the high
metastatic capacity of rhabdoid tumors (3–5).
Therefore, metastasis suppression, together with proliferation
inhibition, is the key to curing rhabdoid tumors. Silibinin, a
natural extract, has been approved as a potential tumor suppressor
in various in vivo and in vitro studies (13). However, whether or not silibinin also
exerts its antitumor capacity in rhabdoid tumors is unclear. To the
best of our knowledge, the present in vitro study was the
first to identify that silibinin inhibits proliferation, migration
and invasion in the rhabdoid tumor cell line, G401, via
inactivation of the PI3K/Akt signaling pathway.
Numerous studies have indicated that silibinin is a
powerful antimetastatic drug; it inhibits β-catenin/ZEB1 signaling,
epithelial-mesenchymal transition and stemness in bladder cancer in
order to suppress metastasis (28).
In renal cancer, mitogen-activated protein kinases are inactivated
by silibinin, thereby suppressing cell migration and invasion
(29,30). In the present study, in G401, a
rhabdoid tumor cell line, silibinin was revealed to significantly
inhibit cell migration in a wound healing assay and a Transwell
migration assay, and cell invasion in a Transwell invasion assay in
a concentration-dependent manner.
The PI3K/Akt pathway is an important intracellular
signaling pathway that is directly associated with cellular
quiescence, proliferation and metastasis. PI3K is enhanced by
various factors, including, epidermal growth factor and insulin,
and is antagonized by several oncoproteins, including phosphatase
and tensin homolog (17,21). PI3K activation facilities Akt
phosphorylation and activation, localizes it at the plasma
membrane, and thereby genetically and epigenetically affects its
downstream target genes (31–34). This pathway is known to be overactive
in a number of cancer types, resulting in reduced apoptosis and
facilitated proliferation and metastasis, and is therefore
considered to be a potential therapeutic target. Inactivation of
the PI3K/Akt pathway in breast cancer induces apoptosis, cell cycle
arrest and autophagy to suppress cell proliferation (19,35).
Meanwhile, epithelial-mesenchymal transition and matrix
metalloproteinase synthesis are reduced by PI3K antagonists, and
migration and invasion are inhibited as a result (36,37).
Additionally, inhibition of the PI3K/Akt pathway sensitizes cancer
cells to chemotherapeutic drugs, including, doxorubicin (38).
The precise function served by the PI3K/Akt pathway
in rhabdoid tumors is unclear since studies regarding this are
limited, but it is likely to be overactivated, as it has been
reported to be a dependent factor for cell survival (39). Silibinin has been proven to be an
efficient PI3K/Akt pathway inhibitor; however, whether or not it
affects this signaling pathway in rhabdoid tumors and contributes
to their anti-metastatic ability requires further elucidation. The
present study revealed that, following administration of silibilin,
the PI3K/Akt signaling pathway was inactivated and this positively
contributed towards the inhibition of metastasis. Silibinin and
LY294002 independently inhibited the PI3K/Akt pathway, migration
and invasion. When these two compounds were used in combination,
PI3K/Akt signaling suppression and metastasis inhibition were
further enhanced. Nevertheless, the present study did not
investigate the manner through which silibinin affects PI3K/Akt
signaling and thus, further studies are required in order to
determine this.
In addition to inhibiting metastasis, the present
study also observed that silibinin suppressed G401 cell viability
at high doses and longer exposure times. Silibinin has been
demonstrated to be a strong proliferation suppressor via its
ability to initiate and activate multiple programs, including,
apoptosis and cell cycle arrest, in various cancer models, but
whether or not these are involved in silibinin-induced growth
inhibition in rhabdoid tumors requires further investigation.
Taken together, the results of the present in
vitro study indicate that silibinin inhibits migration and
invasion of the rhabdoid tumor cell line, G401, and that it
inactivates the PI3K/Akt signaling pathway. These findings
contribute toward the understanding of the antitumor capacity of
silibinin and suggest that silibinin may be a potential
chemotherapeutic drug for combating rhabdoid tumors in the
future.
Acknowledgements
The present study was supported by the Foundation of
Health and Family Planning Commission of Zhejiang Province (grant
no. 20146242) and the Foundation of Wenzhou Science and Technology
Bureau (grant no. Y20140248).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016: CA Cancer J Clin. 66:7–30. 2016.
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geller JI: Current standards of care and
future directions for ‘high-risk’ pediatric renal tumors:
Anaplastic Wilms tumor and Rhabdoid tumor. Urol Oncol. 34:50–56.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuroda N, Karashima T, Inoue K, Kasajima
A, Ohe C, Kawakami F, Mikami S, Matsuura K, Moriyama M, Nagashima
Y, et al: Review of renal cell carcinoma with rhabdoid features
with focus on clinical and pathobiological aspects. Pol J Pathol.
66:3–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
National Cancer Institute: Childhood
Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment
(PDQ®). Health professional version. 2002.
|
|
6
|
McGovern SL, Okcu MF, Munsell MF,
Kumbalasseriyil N, Grosshans DR, McAleer MF, Chintagumpala M,
Khatua S and Mahajan A: Outcomes and acute toxicities of proton
therapy for pediatric atypical teratoid/rhabdoid tumor of the
central nervous system. Int J Radiat Oncol Biol Phys. 90:1143–1152.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kralik SF, Ho CY, Finke W, Buchsbaum JC,
Haskins CP and Shih CS: Radiation necrosis in pediatric patients
with brain tumors treated with proton radiotherapy. AJNR Am J
Neuroradiol. 36:1572–1578. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elsayad K, Kriz J, Samhouri L, Haverkamp
U, Straeter R, Stummer W and Eich HT: Long-term survival following
additive radiotherapy in patients with atypical teratoid rhabdoid
tumors. Strahlenther Onkol. 192:569–581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wellington K and Jarvis B: Silymarin: A
review of its clinical properties in the management of hepatic
disorders. BioDrugs. 15:465–489. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sozen H, Celik OI, Cetin ES, Yilmaz N,
Aksozek A, Topal Y, Cigerci IH and Beydilli H: Evaluation of the
protective effect of silibinin in rats with liver damage caused by
itraconazole. Cell Biochem Biophys. 71:1215–1223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Flora K, Hahn M, Rosen H and Benner K:
Milk thistle (Silybum marianum) for the therapy of liver disease.
Am J Gastroenterol. 93:139–143. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dastpeyman M, Motamed N, Azadmanesh K,
Mostafavi E, Kia V, Jahanian-Najafabadi A and Shokrgozar MA:
Inhibition of silibinin on migration and adhesion capacity of human
highly metastatic breast cancer cell line, MDA-MB-231, by
evaluation of β1-integrin and downstream molecules, Cdc42, Raf-1
and D4GDI. Med Oncol. 29:2512–2518. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vue B, Zhang S, Zhang X, Parisis K, Zhang
Q, Zheng S, Wang G and Chen QH: Silibinin derivatives as
anti-prostate cancer agents: Synthesis and cell-based evaluations.
Eur J Med Chem. 109:36–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zeng J, Sun Y, Wu K, Li L, Zhang G, Yang
Z, Wang Z, Zhang D, Xue Y, Chen Y, et al: Chemopreventive and
chemotherapeutic effects of intravesical silibinin against bladder
cancer by acting on mitochondria. Mol Cancer Ther. 10:104–116.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li F, Ma Z, Guan Z, Chen Y, Wu K, Guo P,
Wang X, He D and Zeng J: Autophagy induction by silibinin
positively contributes to its anti-metastatic capacity via
AMPK/mTOR pathway in renal cell carcinoma. Int J Mol Sci.
16:8415–8429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Massacesi C, Di Tomaso E, Urban P, Germa
C, Quadt C, Trandafir L, Aimone P, Fretault N, Dharan B, Tavorath R
and Hirawat S: PI3K inhibitors as new cancer therapeutics:
Implications for clinical trial design. Onco Targets Ther.
9:203–210. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Faes S and Dormond O: PI3K and AKT:
Unfaithful partners in cancer. Int J Mol Sci. 16:21138–21152. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mundi PS, Sachdev J, McCourt C and
Kalinsky K: AKT in cancer: New molecular insights and advances in
drug development. Br J Clin Pharmacol. 82:943–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/AKT pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen H, Zhou L, Wu X, Li R, Wen J, Sha J
and Wen X: The PI3K/AKT pathway in the pathogenesis of prostate
cancer. Front Biosci (Landmark Ed). 21:1084–1091. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Foster K, Wang Y, Zhou D and Wright C:
Dependence on PI3K/Akt signaling for malignant rhabdoid tumor cell
survival. Cancer Chemother Pharmacol. 63:783–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Hai J, Cao M, Zhang Y, Pei S,
Wang J and Zhang Q: Silibinin ameliorates steatosis and insulin
resistance during non-alcoholic fatty liver disease development
partly through targeting IRS-1/PI3K/Akt pathway. Int
Immunopharmacol. 17:714–720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng N, Luo J and Guo X: Silybin
suppresses cell proliferation and induces apoptosis of multiple
myeloma cells via the PI3K/Akt/mTOR signaling pathway. Mol Med Rep.
13:3243–3248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Garvin AJ, Re GG, Tarnowski BI,
Hazen-Martin DJ and Sens DA: The G401 cell line, utilized for
studies of chromosomal changes in Wilms' tumor, is derived from a
rhabdoid tumor of the kidney. Am J Pathol. 142:375–380.
1993.PubMed/NCBI
|
|
25
|
Krust B, El Khoury D, Soundaramourty C,
Nondier I and Hovanessian AG: Suppression of tumorigenicity of
rhabdoid tumor derived G401 cells by the multivalent HB-19
pseudopeptide that targets surface nucleolin. Biochimie.
93:426–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Unland R, Borchardt C, Clemens D, Kool M,
Dirksen U and Frühwald MC: Analysis of the antiproliferative
effects of 3-deazaneoplanocin A in combination with standard
anticancer agents in rhabdoid tumor cell lines. Anticancer Drugs.
26:301–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Megison ML, Gillory LA, Stewart JE, Nabers
HC, Mrozcek-Musulman E and Beierle EA: FAK inhibition abrogates the
malignant phenotype in aggressive pediatric renal tumors. Mol
Cancer Res. 12:514–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu K, Ning Z, Zeng J, Fan J, Zhou J, Zhang
T, Zhang L, Chen Y, Gao Y, Wang B, et al: Silibinin inhibits
β-catenin/ZEB1 signaling and suppresses bladder cancer metastasis
via dual-blocking epithelial-mesenchymal transition and stemness.
Cell Signal. 25:2625–2633. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chang HR, Chen PN, Yang SF, Sun YS, Wu SW,
Hung TW, Lian JD, Chu SC and Hsieh YS: Silibinin inhibits the
invasion and migration of renal carcinoma 786-O cells in vitro,
inhibits the growth of xenografts in vivo and enhances
chemosensitivity to 5-fluorouracil and paclitaxel. Mol Carcinog.
50:811–823. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang L, Li L, Zeng J, Gao Y, Chen YL,
Wang ZQ, Wang XY, Chang LS and He D: Inhibitory effect of silibinin
on EGFR signal-induced renal cell carcinoma progression via
suppression of the EGFR/MMP-9 signaling pathway. Oncol Rep.
28:999–1005. 2012.PubMed/NCBI
|
|
31
|
King D, Yeomanson D and Bryant HE: PI3King
the lock: Targeting the PI3K/Akt/mTOR pathway as a novel
therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol.
37:245–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wyatt LA, Filbin MT and Keirstead HS: PTEN
inhibition enhances neurite outgrowth in human embryonic stem
cell-derived neuronal progenitor cells. J Comp Neurol.
522:2741–2755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen X, Wang YW, Xing AY, Xiang S, Shi DB,
Liu L, Li YX and Gao P: Suppression of SPIN1-mediated PI3K-Akt
pathway by miR-489 increases chemosensitivity in breast cancer. J
Pathol. 239:459–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pulido R: PTEN: A yin-yang master
regulator protein in health and disease. Methods. 77–78:3–10. 2015.
View Article : Google Scholar
|
|
35
|
Grunt TW and Mariani GL: Novel approaches
for molecular targeted therapy of breast cancer: Interfering with
PI3K/AKT/mTOR signaling. Curr Cancer Drug Targets. 13:188–204.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rafael D, Doktorovová S, Florindo HF,
Gener P, Abasolo I, Schwartz S Jr and Videira MA: EMT blockage
strategies: Targeting Akt dependent mechanisms for breast cancer
metastatic behaviour modulation. Curr Gene Ther. 15:300–312. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou R, Xu L, Ye M, Liao M, Du H and Chen
H: Formononetin inhibits migration and invasion of MDA-MB-231 and
4T1 breast cancer cells by suppressing MMP-2 and MMP-9 through
PI3K/AKT signaling pathways. Horm Metab Res. 46:753–760. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen WC, Lai YA, Lin YC, Ma JW, Huang LF,
Yang NS, Ho CT, Kuo SC and Way TD: Curcumin suppresses
doxorubicin-induced epithelial-mesenchymal transition via the
inhibition of TGF-β and PI3K/AKT signaling pathways in
triple-negati breast cancer cells. J Agric Food Chem.
61:11817–11824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Saletta F, Wadham C, Ziegler DS, Marshall
GM, Haber M, McCowage G, Norris MD and Byrne JA: Molecular
profiling of childhood cancer: Biomarkers and novel therapies. BBA
Clin. 1:59–77. 2014. View Article : Google Scholar : PubMed/NCBI
|