Introduction
In the last years, it was attested that the most
important eukaryotic mechanisms of gene expression regulation
occurs at post-transcriptional level (1). These mechanisms are altered in several
diseases, including cancer, and this could be due to an aberrant
expression and activity of RNA-binding proteins (RBPs) (2,3). These
proteins are key regulators of post-transcriptional gene expression
and among them, one the most known RBP to be implicated in
tumorigenesis is Hu antigen R (HuR) (4–6).
HuR is the ubiquitously expressed member of the Hu
family and it is involved in regulation of mRNA stability and
translation. HuR is located into the nucleus and, in response to
stimuli, can shuttle to the cytoplasm to allow its mRNA target to
be processed. Several studies demonstrated that HuR is
overexpressed and delocalized in the cytoplasm in numerous cancers,
including breast cancer, lung adenocarcinoma, ovarian cancer,
laryngeal squamous cell cancer and colon cancer (6,7). Moreover,
various mRNA of tumorigenesis factors, oncogenes and anti-apoptotic
factors have been identified as HuR targets (5). In a previous study, we demonstrated that
HuR is overexpressed also in thyroid cancer (8).
Thyroid cancer is the most widespread endocrine
malignancy and although it represent only the 1–2% of all human
neoplasms, its incidence is rapidly growing all over the world in
the last decades (9). In most cases,
thyroid carcinomas derived from follicular cells and could be
classified as differentiated carcinomas, that include follicular
thyroid cancer (FTC) and papillary thyroid cancer (PTC), and
undifferentiated carcinomas, also named anaplastic thyroid cancer
(ATC) (10).
ATC constitute one of the most aggressive and lethal
human solid tumor and is characterized by an absence of thyroid
differentiation features, a marked degree of invasiveness and
extensive necrosis (11). ATC
patients have a median survival of 5 months and less than 20%
survive 12 month. Nowadays there are not effective therapies for
ATC, since surgery, traditional chemotherapies and radiation
therapies are mostly ineffective and are not able to improve the
overall survival (10,11). Therefore, innovative approaches for
ATC treatment are needed.
Materials and methods
Cell lines
Nthy-ori-3.1, derived from normal thyroid follicular
epithelial cells and immortalized by the SV40 large T gene, SW1736
and 8505C cell lines, from ATC, were grown in RPMI1640 medium (Euro
Clone, Milan, Italy) supplemented with 10% fetal bovine serum
(Gibco Invitrogen, Milan, Italy), 2 mM L-glutamine (Euro Clone) and
50 mg/ml gentamicin (Gibco Invitrogen). Cultured cells were treated
with either vehicle (DMSO; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) or suberoylanilide hydroxamic acid (SAHA) (Cayman
Chemical, Ann Arbor, MI, USA). Cells were grown in a humidified
incubator (5% CO2 in air at 37°C) (Eppendorf AG,
Hamburg, Germany). All cell lines have been validated by short
tandem repeat and tested for being mycoplasma-free.
Protein extraction and western
blot
Total protein extraction was performed harvesting
Nthy-ori-3.1, SW1736, and 8505C cells by scraping and lysing cells
with total lysis buffer (Tris HCl 50 mM pH8, NaCl 120 mM, EDTA 5
mM, Triton 1%, NP40 1%, DTT 1 mM), supplemented with
phenyl-methylsulphonyl fluoride and protease inhibitors. Lysates,
then, were centrifuged at 13,000 × g for 10 min at 4°C and
supernatants were quantified by Bradford assay.
For western blot analysis, proteins were
electrophoresed either on 7.5, 10 or 12% SDS-PAGE and then
transferred to nitrocellulose membranes (GE Healthcare, Little
Chalfont, UK), saturated with 5% non-fat dry milk in PBS/0.1%
Tween-20. The membranes were then incubated overnight with rabbit
polyclonal anti-HuR antibody 1:500 (EMD Millipore, Billerica, MA,
USA), rabbit anti-PARP antibody 1:400 (Abcam, Cambridge, UK),
rabbit anti-nuclear factor (NF)-κB antibody 1: 200 (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), rabbit anti-actin antibody
1:1,000 (Abcam) or rabbit anti-LSD1 antibody 1:1,000 (Abcam). The
day after, membranes were incubated with anti-rabbit immunoglobulin
coupled to peroxidase 1:4,000 (Sigma-Aldrich) for 2 h. Blots were
developed using UVITEC Alliance LD (UVItec Ltd., Cambridge, UK)
with the SuperSignal Technology (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA).
HuR silencing
For transient silencing of endogenous HuR in SW1736
and 8505c cells, TriFECTa RNAi kit (Integrated DNA Technologies
Inc., Coralville, IA, USA) was used following manufacturer's
instructions. A duplex targeting a site absent in human genome was
used as ‘universal’ negative control. Three different siRNA
oligonucleotides (siRNA1, siRNA2 and siRNA3) were transfected at a
concentration of 1 nM using DharmaFECT 1 Transfection reagent
(Thermo Fisher Scientific, Inc.), according to manufacturer's
instructions. The day before transfection, cells were plated in
antibiotics-free medium. Cells were harvested 72 h after
transfection and gene-silencing efficiency was evaluated by protein
levels analysis.
Cell viability
In order to test cell viability, we applied the
methyl thiazolyl tetrazolium assay (MTT) assay. SW1736 and 8505C
cells (3,000 cells/well) were plated onto 96-well plates in 200 µl
medium/well and were allowed to attach to the plate for 24 h (t0).
Plates were then incubated for 24, 48 and 72 h. 4 mg/ml MTT
(Sigma-Aldrich) was then added to the cell medium and cells were
cultivated for another 4 h darkened in the incubator. The
supernatant was removed, 100 µl/well of DMSO (Sigma-Aldrich) were
added and the absorbance at 570 nm was measured. All experiments
were run in quadruplicate and cell viability was expressed as a
fold-change compared to control.
Soft agar assay
SW1736 and 8505C cells clonogenic activity after HuR
silencing was evaluated by soft agar assay. Briefly, after 72 h HuR
silencing, cells have been collected and 10,000 cells/plate were
suspended in 4 ml of complete medium containing 0.25% agarose and
then seeded to the top of a 1% agarose complete medium layer in 6
cm plates. The colonies were counted on an inverted microscope
Leica DMI-600B (Leica Microsystems Ltd., Heerbrugg, Switzerland).
Data are representative of three independent experiments.
Gene expression assays
500 ng of total RNA of SW1736 and 8505C cells,
treated with SAHA or vehicle, were reverse transcribed to cDNA
using random exaprimers and MMLV reverse transcriptase (Life
Technologies; Thermo Fisher Scientific, Inc.). Real-time PCR was
performed using Platinum SYBR-Green qPCR supermix (Life
Technologies; Thermo Fisher Scientific, Inc.) on the ABI Prism 7300
Sequence Detection Systems (Applied Biosystems). The ∆∆Cq method,
by means of the SDS software (Applied Biosystems; Thermo Fisher
Scientific, Inc.), was used to calculate mRNA levels.
Oligonucleotide primers were purchased from Sigma-Aldrich and their
sequences are available upon request.
Statistical analysis
All data obtained are expressed as means ± standard
deviation, and significances were analyzed with the Student's
t-test performed with GraphPAD Software for Science (San Diego, CA,
USA).
Results
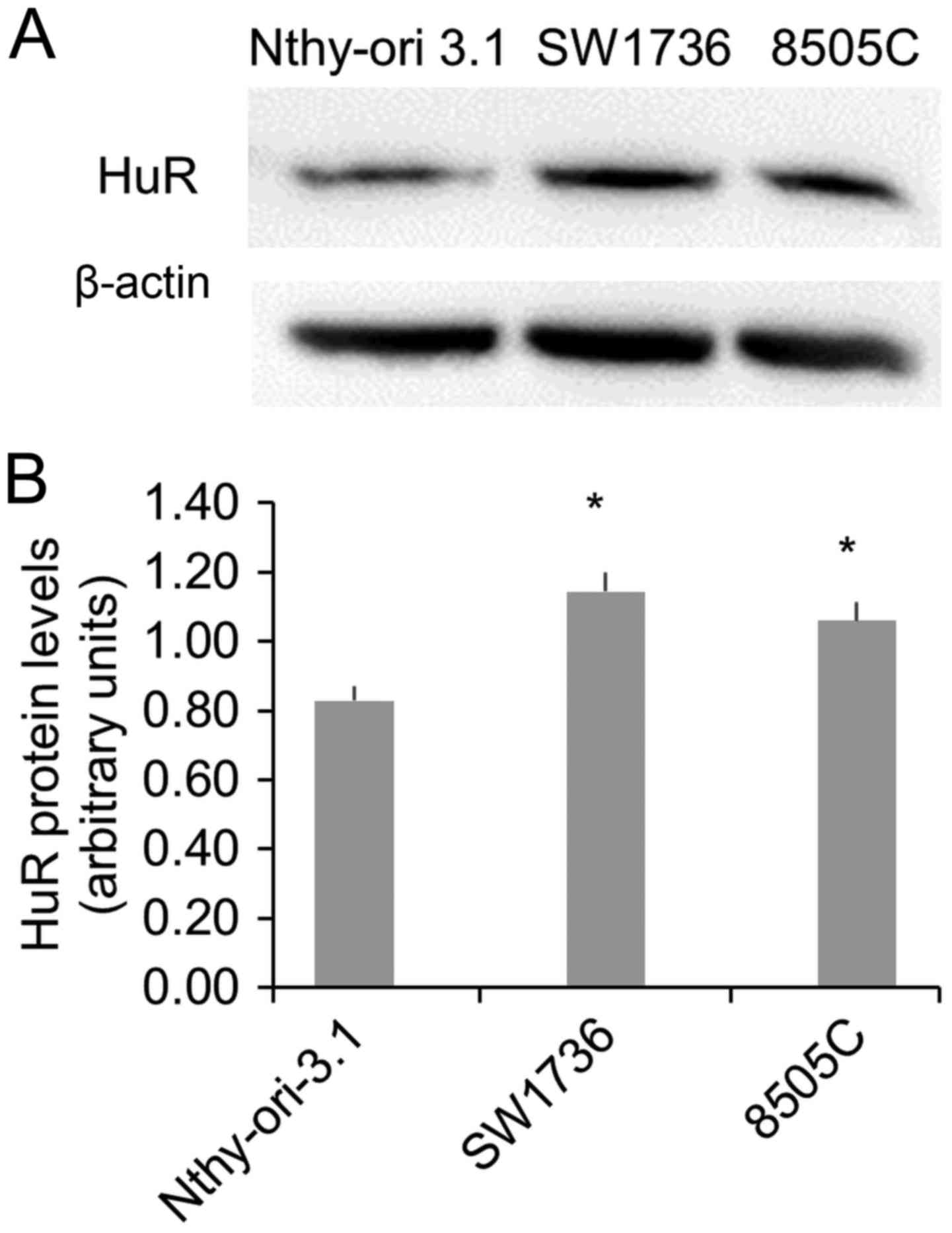
Since we had previously demonstrated the in
vivo HuR overexpression in thyroid cancer and considering the
necessity to identify innovative approaches for ATC treatment, in
this study we focused on the expression and the importance of HuR
in ATC cells. To this purpose, we evaluated HuR protein levels in a
normal (Nthy-ori-3.1) and in two ATC (SW1736 and 8505C) cell lines.
The Western Blot analysis displayed a significant HuR
overexpression in both ATC cells (Fig.
1), confirming data obtained in vivo (8).
In order to evaluate the importance of HuR and its
biological effects in ATC cells, we performed an RNA interference
assay. In a first set of experiments, we performed the silencing
using three different HuR-specific siRNA (1 nM). As shown in
Fig. 2A and B, in both SW1736 and
8505C cell lines, siRNA 1 and 2 induces a strong HuR silencing,
while siRNA 3 seems to have no effects on the RBP expression. Then,
we investigated HuR silencing effects on cell viability, apoptosis
and clonogenic ability. In both cell lines, 1 nM siRNA 1 treatments
induce a slight (about 20%), but significative cell viability
reduction (Fig. 2C). In order to
measure HuR silencing effects on apoptosis, we performed a Western
Blot analysis of PARP, a well-known caspase substrate that is
specifically cleaved during apoptosis (12). As shown in Fig. 2D, siRNA 1 increases apoptosis
phenomena compared to control, in both SW1736 and 8505C cells.
Since mRNA of genes involved in cell aggressiveness have been
identified among known HuR-targets, we investigated the ability of
SW1736 and 8505C to form colonies in soft agar, after
HuR-silencing. We detected a 40% reduction of clonogenic ability
after siRNA 1 treatments in both SW1736 and 8505c cells (Fig. 2E). Therefore, all these data indicate
that HuR plays a positive role in cell proliferation and in
colonies forming ability in ATC-derived cell lines.
Histone deacetylase (HDAC) inhibitors are an
important class of anticancer agents. One of the most known HDAC
inhibitor is the SAHA, that is able to induce cell growth arrest
and apoptosis in different cancer cell lines (13). In a previously study, we have
demonstrated how SAHA 3 µM treatment lead to decrement of cell
viability in the SW1736 cell line (14). Besides, Zhang et al have
demonstrated that the treatment with SAHA induces a reduction of
HuR protein expression in mouse epidermal JB6 Cl41 cells (15).
For this reason, after confirming SAHA-related
reduction cell viability in 8505C cells (data not shown), we
focused on SAHA effects on HuR protein levels in the two ATC cell
lines, in order to determine if SAHA effects in ATC is also due to
HuR downregulation. We evaluated, at different time point, whether
a 3µM SAHA treatment significantly altered HuR mRNA and protein
levels in SW1736 and 8505C cells (Fig.
3). After 48 h SAHA treatment, a reduction about 60 and 50% of
HuR mRNA levels has been detected in SW1736 and 8505C, respectively
(Fig. 3A). Thereafter, HuR protein
levels have been evaluated after 72 h SAHA 3 µM treatment. As shown
in Fig. 3B and C, SAHA induces a
strong HuR protein levels decrease in both cell lines.
To define whether SAHA treatment operate directly on
HuR transcription or involve other factors implicated in its
regulation, we evaluated NF-κB protein levels. Kang et al
have demonstrated that NF-κB, binding HuR promoter, directly
activates its transcription to promote tumorigenesis (16). NF-κB is a transcription factor
implicated in various aspects of tumor biology, such as cell
proliferation, survival, angiogenesis, invasion, metastasis and
drug resistance. Inactivated NF-κB localized in the cytoplasm and
complexed with the inhibitory protein IκBα while activated NF-κB is
translocated into the nucleus where it binds its DNA target
sequences (17,18).
In order to evaluate activated NF-κB levels, we
performed a Western Blot analysis on ATC cells nuclear fraction,
treated or not with SAHA. Data obtained show that SAHA
administration induces a significative reduction of nuclear NF-κB
fraction after 24 and 48 h in both SW1736 and 8505C cells (Fig. 4). These results demonstrate that SAHA
treatment reduces activated NF-κB levels in ATC cells.
Discussion
HuR is a RNA-binding protein that plays a major role
in regulating gene expression (19)
and that can contribute to tumorigenesis (4). HuR transcription is positively
regulated by the NF-κB or by Smad, while the levels of its mRNA and
protein are conditioned upon multiple regulation mechanisms
(16,19). Several studies indicate that HuR
expression and localization are modified in different cancer types,
including thyroid cancer (4,14). ATC, although is a rare thyroid cancer
histotype, is characterized by a very poor prognosis and a complete
absence of differentiation markers (20). Current treatments, based on
combination of surgery, chemotherapy and external radiotherapy, are
not effective. Therefore, new therapies for ATC are particularly
needed.
In order to find new strategies to treat this
aggressive thyroid cancer subtype, we investigated HuR expression
and its biological effects in two ATC-derived cell lines, SW1736
and 8505C. Therefore, we have demonstrated that HuR is
overexpressed in ATC cells and that its silencing determine cell
viability reduction due to an increase in apoptosis processes, in
both cell lines. Focusing on HuR involvement in cell
tumorigenicity, we have established that HuR silencing reduces ATC
cells colony forming ability. In this way, we have proved, for the
first time, the importance of HuR in ATC cell lines in terms of
cell viability, cell death and tumor aggressiveness. Therefore, HuR
could be a possible therapeutic target for ATC treatment.
Consequently, in a second experimental setting, we
focused on substances able to reduce HuR expression in thyroid
cancer. In particular, SAHA, a HDAC inhibitor already FDA-approved
for the treatment of several neoplastic diseases (21), proved to induce HuR downregulation in
mouse epidermal JB6 Cl41 cells (15).
Moreover, in a previous study, we demonstrated SAHA effects on
SW1736 cell viability (14), and in
this study, these data have been confirmed also in 8505C cells. Our
data shown that SAHA induces a strong HuR mRNA and protein levels
reduction, in both ATC cell lines. To better understand the
mechanism through which SAHA induces HuR downregulation, we
investigate the effects of this drug on NF-κB, which is the major
transcription factor of HuR (16). In SW1736 and 8505C cell lines, SAHA
treatment determines a decrease of activated NF-κB protein levels.
This data corroborate the idea that SAHA effects on HuR expression
are not direct, but could be due to its effects of NF-κB.
In conclusion, our findings indicate, for the first
time, that the RBP HuR plays an important role in ATC
tumorigenesis. Moreover, we have demonstrated that the HDAC
inhibitor SAHA could be used to obtain a HuR downregulation as
consequences of reduction of NF-κB activation.
Acknowledgements
This study was supported by Associazione Italiana
per la Ricerca sul Cancro (AIRC) (ARIC fellowship Rif. 19481), a
grant from Ministero degli Affari Esteri of Italy (Progetti grande
rilevanza 2016, project no. PGR02954) and from MIUR (PRIN 2015,
project no. 2015HPMLFY-011).
References
|
1
|
Schwanhäusser B, Busse D, Li N, Dittmar G,
Schuchhardt J, Wolf J, Chen W and Selbach M: Global quantification
of mammalian gene expression control. Nature. 473:337–342. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kechavarzi B and Janga SC: Dissecting the
expression landscape of RNA-binding proteins in human cancers.
Genome Biol. 15:R142014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wurth L and Gebauer F: RNA-binding
proteins, multifaceted translational regulators in cancer. Biochim
Biophys Acta. 1849:881–886. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abdelmohsen K and Gorospe M:
Posttranscriptional regulation of cancer traits by HuR. Wiley
Interdiscip Rev RNA. 1:214–229. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wurth L: Versatility of RNA-binding
proteins in cancer. Comp Funct Genomics. 2012:1785252012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Guo Y, Chu H, Guan Y, Bi J and
Wang B: Multiple functions of the RNA-binding protein HuR in cancer
progression, treatment responses and prognosis. Int J Mol Sci.
14:10015–10041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Govindaraju S and Lee BS: Adaptive and
maladaptive expression of the mRNA regulatory protein HuR. World J
Biol Chem. 4:111–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baldan F, Mio C, Allegri L, Conzatti K,
Toffoletto B, Puppin C, Radovic S, Vascotto C, Russo D, Di Loreto C
and Damante G: Identification of tumorigenesis-related mRNAs
associated with RNA-binding protein HuR in thyroid cancer cells.
Oncotarget. 7:63388–63407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lebastchi AH and Callender GG: Thyroid
cancer. Curr Probl Cancer. 38:48–74. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
O'Neill JP and Shaha AR: Anaplastic
thyroid cancer. Oral Oncol. 49:702–706. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaitanya GV, Alexander JS and Babu PP:
PARP-1 cleavage fragments: Signatures of cell-death proteases in
neurodegeneration. Cell Commun Signal. 8:312010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ungerstedt JS, Sowa Y, Xu WS, Shao Y,
Dokmanovic M, Perez G, Ngo L, Holmgren A, Jiang X and Marks PA:
Role of thioredoxin in the response of normal and transformed cells
to histone deacetylase inhibitors. Proc Natl Acad Sci USA. 102:pp.
673–678. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baldan F, Mio C, Allegri L, Puppin C,
Russo D, Filetti S and Damante G: Synergy between HDAC and PARP
inhibitors on proliferation of a human anaplastic thyroid
cancer-derived cell line. Int J Endocrinol. 2015:9783712015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang J, Ouyang W, Li J, Zhang D, Yu Y,
Wang Y, Li X and Huang C: Suberoylanilide hydroxamic acid (SAHA)
inhibits EGF-induced cell transformation via reduction of cyclin D1
mRNA stability. Toxicol Appl Pharmacol. 263:218–224. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang M, Ryu B, Lee M, Han J, Lee JH, Ha
TK, Byun DS, Chae KS, Lee BH, Chun HS, et al: NF-kappaB activates
transcription of the RNA-binding factor HuR, via PI3K-AKT
signaling, to promote gastric tumorigenesis. Gastroenterology.
135:2030–2042.e1-3. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Srikantan S and Gorospe M: HuR function in
disease. Front Biosci (Landmark Ed). 17:189–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blaxall BC, Dwyer-Nield LD, Bauer AK,
Bohlmeyer TJ, Malkinson AM and Port JD: Differential expression and
localization of the mRNA binding proteins, AU-rich element mRNA
binding protein (AUF1) and Hu antigen R (HuR), in neoplastic lung
tissue. Mol Carcinog. 28:76–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mann BS, Johnson JR, Cohen MH, Justice R
and Pazdur R: FDA approval summary: Vorinostat for treatment of
advanced primary cutaneous T-cell lymphoma. Oncologist.
12:1247–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|