Introduction
Glioblastoma (GBM) is a highly aggressive primary
brain cancer that is difficult to treat. The current standard of
care consists of surgery, followed by radiation and chemotherapy
with the alkylating agent temozolomide (TMZ) (1). However, the therapeutic benefit of TMZ
is restricted to the patients presenting with epigenetic silencing
of the O6-methylguanine-DNA methyltransferase (MGMT) DNA repair
gene in the tumor (2). Otherwise,
high expression levels of MGMT allow tumor cells to repair
O6-guanine subsequent to methylation by TMZ, thereby preventing
drug-induced DNA double-strand breaks and tumor cell death. As a
consequence, median overall survival time differs depending on MGMT
promoter methylation status, with 15 months for patients with an
unmethylated promoter and 22 months for those with the methylated
promoter status (3). In either case,
however, (89–97%) of patients succumb to the disease within 5
years, as even for patients initially responding favorably to TMZ,
the effectiveness wanes over time as tumors develop drug resistance
(4).
A newly-approved vaccine-based therapy, rindopepimut
(CDX-110), has demonstrated further extension of median survival
time; however, this regimen is only applicable to the 30% of GBM
patients who are positive for the epidermal growth factor receptor
variant EGFRvIII (5). An approved
treatment for the recurrent setting is the angiogenesis inhibitor
bevacizumab (6). Although it improves
the outcome for the majority of GBM patients, the duration of
benefit is limited and the survival rate from the time of initial
bevacizumab progression is poor (7,8). The
latest therapy to be approved is the alternating electric fields
generator-based NovoTTF therapy, (9)
which has been demonstrated to extend median overall survival time
by ~5 months (10). Regardless of the
treatment regimen, however, patients with GBM continue to exhibit a
dismal prognosis and novel methods of treatment are urgently
required.
In one novel approach, the intranasal delivery of
perillyl alcohol (POH), a naturally occurring monoterpene related
to limonene (11), was investigated
in two clinical studies with grade III and grade IV glioma patients
(12–14). POH was administered via a lightweight
nebulizer and nose mask up to four times daily on a continuous
basis. The results were highly promising, indicating therapeutic
activity along with good tolerance and no long-term central nervous
system or systemic severe adverse events, even following years of
daily administration (12–14). POH activity was also noted in
recurrent patients, i.e., patients who had become unresponsive to
standard of care, and 19% of all patients remained in clinical
remission following 4 years of continuous, exclusive treatment with
intranasal POH (13,14). As of April 2017, a clinical trial is
currently underway in the United States that aims to investigate
the safety and efficacy of intranasal NEO100, a highly purified
version of perillyl alcohol, in patients with recurrent grade IV
glioma (NCT02704858).
Another newly emerging principle being investigated
for the treatment of GBM is the ketogenic diet (KD). Besides being
a lifestyle choice or a method for weight loss, in medicine the KD
is prescribed primarily as a treatment for refractory epilepsy in
children (15). Based on its
high-fat/low-carbohydrate content, the diet forces the body to
metabolize fats and ketone bodies rather than glucose; the state of
ketosis is hypothesized to reduce the frequency of epileptic
seizures (16).
In the context of brain tumor treatment, the KD is
expected to provide two main benefits. First, as epilepsy commonly
develops among GBM patients (17), it
may aid a reduction in the severity and number of epileptic
episodes and alleviate the requirement for anti-epileptic drugs,
which are associated with detrimental neuropsychological effects
and often interfere with the therapeutic regimen (18,19).
Second, KD may increase the efficacy of cancer therapy. Tumor
development is frequently associated with a metabolic alteration,
in which cells switch from aerobic respiration towards increased
glycolysis and lactate production, despite the availability of
oxygen (20). This so-called Warburg
effect (aerobic glycolysis) leads to the increased consumption of
glucose and the inability of the affected cells to utilize ketone
bodies as an energy source (21). In
response to KD, it is hypothesized that tumor cells will starve, or
revert to normal mitochondrial activity and respiration, altogether
resulting in decreased proliferation and metastatic ability, along
with a heightened sensitivity to chemotherapy (22–24).
Previous preclinical studies demonstrated notable
benefits of the KD in a variety of animal tumor models, including
in malignant brain tumors (25) and
in the meta-analysis by Klement et al (26). For example, among groups of mice with
luciferase-labeled GL261 glioma cells implanted into their brains,
median survival time was significantly extended in mice on a KD
compared with mice on a standard diet; in addition, when such mice
received two fractions of radiation (2×4 Gy of whole brain
radiation), the bioluminescent signals from the intracranial tumors
was diminished below the detectable limit in 82% of the mice, and
no signs of tumor recurrence were observed for >200 days
(27).
Encouraging results from the use of a KD were also
reported in patients, and a small number of case studies have
indicated a benefit of the KD in patients with malignant glioma, as
reviewed by Maroon et al (28). It was consistently reported that
patients undergoing the KD presented with reduced blood glucose
levels and beneficial therapeutic responses, including the
transient disappearance of the brain tumor tissue (29–31). Thus,
these case reports, along with numerous anecdotal observations,
presented encouraging signs of metabolic reprogramming by the KD,
along with the increased efficacy of standard of care treatment for
malignant glioma (23,29). This preliminary evidence of the
benefit of KD for cancer therapy is currently being validated in
ongoing, controlled clinical trials, which include patients with
malignant glioma (25).
At the molecular level, reduced glucose availability
may cause endoplasmic reticulum (ER) stress, which triggers the
unfolded protein response (UPR) cellular process, consisting of an
interplay of antagonistic mechanisms; low to moderate activity is
cell protective and supports chemoresistance, but more severe
conditions aggravate these mechanisms to the point where protective
efforts are abandoned and the cell death program is induced instead
(32). As tumor cells frequently
experience chronic stress conditions (due to hypoxia, hypoglycemia,
acidification, oxidative stress, etc.), the protective components
of their ER stress response are continuously engaged and thus, less
able to neutralize additional insults that tax the ER stress
response (33,34). Of note, the ER stress/UPR process has
been described as a potential therapeutic target in GBM (35,36) and
this cellular mechanism has been demonstrated to be targeted by POH
in GBM cells in vitro (37).
It is thus conceivable that the concerted effect of
KD-induced hypoglycemia together with POH-induced responses may
trigger severely aggravated ER stress, resulting in tumor cell
apoptosis (38,39). Therefore, the present study combined a
KD with the intranasal delivery of POH, and investigated the
effects of this novel combination in a cohort of 32 patients who
had previously relapsed from standard of care treatment.
Patients and methods
Patient selection and treatment
The present study was approved by the Fluminense
Federal University (Niteroi, Brazil; UFF-CAAE:
14613313.8.0000.5243), and was performed at the Antonio Pedro
University Hospital, Fluminense Federal University. Each patient
signed a written informed consent prior to enrolling in the
clinical trial of KD with intranasal delivery of POH. POH was
formulated for delivery by inhalation and the preparation was
supplied by the Multidisciplinary Laboratory of Pharmaceutical
Sciences at Rio de Janeiro Federal University (Rio de Janeiro,
Brazil), according to the Brazilian patent no. PI 0107262-5. KD was
administered concomitantly with the daily inhalation of POH for
three months. POH (55 mg; 0.3% v/v) was administered by inhalation
4 times per day, totaling 266.8 mg/day.
For patients to be included in the trial, a
histopathological diagnosis of malignant glioma was required. This
was performed during their initial surgery. Additionally, all
patients presented with relapsed GBM, had no further standard
therapeutic options, were aged >18 years, had measurable
contrast-enhancing tumor on magnetic resonance imaging (MRI),
Karnofsky performance scale of ≥70% or higher, adequate bone marrow
function, white blood cell count of ≥3,000/l, absolute neutrophil
count of ≥1,500/l, platelet count of ≥100,000/l, hemoglobin of ≥8.0
g/dl, bilirubin of ≥0.3 mg.
KD details and monitoring
The anthropometric and biochemical status of each
patient was assessed at the time of inclusion in the study. The KD
was prescribed according to the following distribution: Energy, 25
kcal/kg; protein, 1.5 g/kg; 25% of total calories from
carbohydrate, 50% from lipids; cholesterol, ≤200 mg/day; saturated
fat, <7%; polyunsaturated fat, <10%; monounsaturated fat,
<20% and fiber, 20–30 g/day. Analysis was performed with urinary
ketones as it was cheaper and simple for the patients to perform.
Participants received urinalysis strips and instructions for use of
the test strip. They were instructed to perform the measurement 3
times a week and report the results to the researchers weekly.
Levels of ketone bodies in the urine of 5–15 mg/dl were considered
to confirm adherence to the KD. Adverse effects, including nausea,
weakness, tremors, mood swings, headaches, dizziness and drowsiness
were monitored through patient/family reporting by phone contact,
and reporting on medical records during the visit.
Patient imaging
Imaging examinations were performed at a range of
institutions, using a 1.5 Tesla magnetic resonance imaging (MRI)
scanner with a brain coil device. All examinations were included in
the complete routine for MRI evaluation with multiplanar T1 W
acquisitions prior and subsequent to the venous administration of
paramagnetic contrast with volumetric series in 3D, T2,
T2/fluid-attenuated inversion recovery (FLAIR), diffusion and
susceptibility weighted imaging. Magnetic resonance (MR) proton
spectroscopy and MR perfusion were also performed. Images of the MR
examinations were analyzed by two radiologists with experience in
neuroradiology that produced a consensus diagnosis using a
workstation with a picture archiving and communication system. The
observing radiologists were aware of the GBM diagnosis and
follow-up data. The image analysis focused on the following
characteristics: Lesion distribution and volume, signal intensity
and T2/FLAIR imaging characteristics, perilesional edema, diffusion
restriction patterns, and contrast enhancement.
Assessment of outcome
At the beginning of an evaluation, lesions were
measured in order to provide bases for comparison. Response
assessment and evaluation criteria for target lesions were as
follows: Complete response (CR), all target lesions disappeared
during the course of treatment; partial response (PR), decreases of
≥30% were noted in the lesion with the largest diameter; stable
disease (SD), there was no significant decrease or increase in the
size of target lesions; progressive disease (PD), there was an
increase of ≥20% in the largest diameter of targeted lesions or the
appearance of a new lesion. Other parameters evaluated were:
Peritumoral edema, neurological stability, corticosteroid
requirement and general improvement of condition, including reduced
frequency of seizures, weight loss, reduced serum lipid levels and
low density lipoprotein cholesterol (LDL-C), reduced body fat and
increase in lean muscle mass.
Statistical methods
To evaluate the tumor area associated with brain
edema before and after dietary intervention in the groups,
statistical analysis was performed using a unilateral signal test.
A paired Student's t-test was performed to compare normally
distributed biochemical parameters, whereas a signal test was used
for non-normal parameters, within each treatment group. Comparisons
between groups at equivalent timepoints were made with a Student's
t-test for normally distributed data and a Mann-Whitney test for
non-normal parameters. Statistical tests were performed using SPSS
software version 18.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference. The
data were graphically presented using box plots.
Results
Patient characteristics
A total of 32 patients with recurrent GBM were
enrolled. A total of 17 were allocated to the KD treatment group
(range, 31–61; median, 53 years old) and 15 to the control group
(standard diet; range, 27–55 years; median, 48 years old). A total
of 17 patients completed the study, including 9 patients in the KD
group and 8 patients in the control group. Of the 15 patients that
did not complete the study, 12 succumbed to their disease and 3
were excluded due to diet non-adherence. Before entering the
clinical trial, all patients had received prior conventional
therapy (including surgery, chemotherapy and radiotherapy; Table I). No adverse effects, including
nausea, weakness, tremors, mood swings, headaches, dizziness or
drowsiness, were observed in the study participants.
 | Table I.General characteristics of KD and
control groups. |
Table I.
General characteristics of KD and
control groups.
| Characteristic | KD group | Control group |
|---|
| Total, n | 9 | 8 |
| Age, years |
|
|
|
Median | 53 | 48 |
|
Range | 31–61 | 27–55 |
| Sex, n (%) |
|
|
|
Male | 4 (44) | 6 (75) |
|
Female | 5 (56) | 2 (25) |
| Previous therapy,
n |
|
|
|
Radiotherapy | 9 | 8 |
|
Chemotherapy | 9 | 8 |
|
Anti-epileptic
druga | 9 | 8 |
|
Corticosteroid | 9 | 8 |
| Karnofsky
performance status |
|
|
|
100 | 0 | 0 |
| 90 | 1 | 1 |
| 80 | 6 | 4 |
| 70 | 2 | 3 |
Clinical outcomes
Following three months of adherence to the treatment
in the KD group, PR was observed in 77.8% (7/9) patients,
characterized by the reduction of MRI tumor size and peritumoral
edema (Fig. 1), neurological
stability, reduced corticosteroid requirement, and the general
improvement of condition. The corticosteroid was maintained during
the trial for all patients, but the dose was decreased in the
patients who presented with an improvement of clinical status (5/9
patients). PD was observed in 11.1% (1/9) patients, with an
increased tumor size or the appearance of a new lesion in MRI.
Among the patients of the control group, PR was noted in 25.0% (2/8
patients), and PD was observed in 50.0% (4/8 patients). The imaging
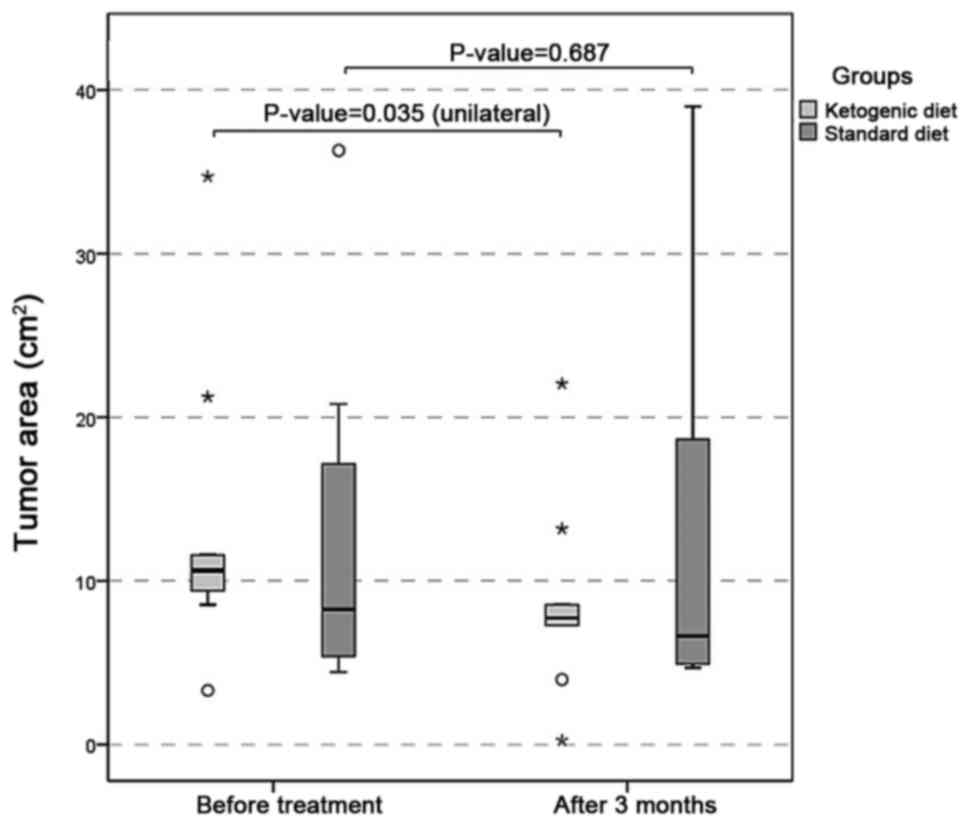
data demonstrated that tumor area was significantly smaller in the
KD group at 90 days compared with the baseline (P=0.035), and not
in the control group (P=0.687; Fig.
2).
There was a statistically significant difference
(P=0.003) between the total cholesterol level in the initial and
final measurements of the KD group, with values significantly lower
at 90 days (Fig. 3). There was a
statistically significant difference (P=0.0002) between LDL-C at
the initial and final time in treated patients, with higher values
at the onset. Test levels of triglycerides also exhibited
statistically significant differences (P=0.004) between the initial
and final time in the KD group, with higher values at baseline. The
same comparisons of total cholesterol, LDL-C and triglycerides in
the standard diet group revealed no statistically significant
differences between the baseline and 90 days. In the comparisons
between the groups (KD and control) at equivalent timepoints,
significant differences were observed with regard to total
cholesterol and triglycerides between the groups at the initial
time (P=0.013 and P=0.036, respectively); however, there was no
significant difference in LDL-C levels. At 90 days, all three
biochemical parameters exhibited no significant statistical
difference between the groups (Table
II).
 | Table II.Statistical comparison of biochemical
parameters within and between the KD and control treatment
groups. |
Table II.
Statistical comparison of biochemical
parameters within and between the KD and control treatment
groups.
|
| KD group | Control group |
|
|---|
|
|
|
|
|
|---|
| Parameter | Mean ± SD | Range | P-value | Mean ± SD | Range | P-value | P-value between KD
and control |
|---|
| Total
cholesterol |
|
|
|
|
|
|
|
|
Baseline |
260.9±51.5 | 211–360 | 0.003a |
195.6±42.7 | 131–253 | 0.663 | 0.013b |
| After
90 days |
220.7±45.5 | 170–320 |
|
192.6±48.0 | 140–268 |
| 0.235 |
| Low-density
lipoprotein cholesterol |
|
|
|
|
|
|
|
|
Baseline |
148.8±34.1 | 103–200 | 0.0002a |
118.6±36.8 | 60–160 | 0.642 | 0.100 |
| After
90 days |
122.1±27.9 | 89–172 |
|
116.5±36.0 | 73–167 |
| 0.722 |
| Triglycerides |
|
|
|
|
|
|
|
|
Baseline |
180.4±100.9 | 81–413 | 0.004a |
100.4±64.8 | 40–251 | 1.000 | 0.036b |
| After
90 days |
154.4±93.3 | 71–373 |
|
99.1±42.0 | 55–173 |
| 0.114 |
Additionally, all patients on the KD exhibited a
moderate decrease in fasting glucose levels, although this was
without statistical significance. At the end of the study, all
patients on KD were within normoglycemic levels (Table III).
 | Table III.Biochemical parameters of the KD
group prior and subsequent to 90 days of the KD. |
Table III.
Biochemical parameters of the KD
group prior and subsequent to 90 days of the KD.
| A, Prior to the
KD |
|---|
|
|---|
|
| Parameter,
mg/dl |
|---|
|
|
|
|---|
| PI | FG | TC | LDL | HDL | TG |
|---|
| 1 | 77 | 211 | 113 | 80 | 92 |
| 2 | 71 | 295 | 189 | 79 | 136 |
| 3 | 87 | 214 | 103 | 65 | 230 |
| 4 | 96 | 306 | 147 | 145 | 81 |
| 5 | 86 | 235 | 132 | 67 | 178 |
| 6 | 80 | 360 | 200 | 69 | 413 |
| 7 | 112 | 215 | 134 | 39 | 211 |
| 8 | 90 | 275 | 182 | 70 | 116 |
| 9 | 90 | 237 | 139 | 65 | 167 |
| Mean ± SD |
87.7±11.2 |
260.9±49.0 |
148.8±32.2 |
75.4±27.0 |
180±95.2 |
|
| B, 3 months from
the start of the KD |
|
|
| Parameter,
mg/dl |
|
|
|
| PI | FG | TC | LDL | HDL | TG |
|
| 1 | 74 | 170 | 93 | 61 | 81 |
| 2 | 68 | 210 | 135 | 70 | 100 |
| 3 | 82 | 211 | 89 | 70 | 200 |
| 4 | 83 | 222 | 127 | 57 | 71 |
| 5 | 81 | 200 | 120 | 73 | 134 |
| 6 | 75 | 320 | 172 | 74 | 373 |
| 7 | 95 | 188 | 100 | 50 | 190 |
| 8 | 80 | 265 | 153 | 91 | 106 |
| 9 | 85 | 200 | 110 | 70 | 135 |
| Mean ± SD |
83.4±13.03 |
220.7±42.9 |
122.1±26.3 |
68.4±11.03 |
154.4±87.9 |
|
| C, Normal range
of parameters |
|
| Parameter,
mg/dl | FG | TC | LDL | HDL | TG |
|
| Normal range | <100 | <240 | <160 | >60 | <200 |
At the beginning of the study, the classification of
nutritional status in the KD group according to body mass index
(BMI) demonstrated that there was a predominance of overweight
patients (n=4; 44%), followed by patients in the normal range (n=3;
33%) and patients with grade I obesity (n=2; 22%), both at baseline
and at the end of the study. One patient exhibited a worsening of
nutritional status, evolving from overweight to obesity grade I
following 90 days of KD, and one patient had an improvement in
nutritional status, from grade I obesity to overweight (Table IV).
 | Table IV.Anthropometric parameters and
nutritional status of the KD treatment group. |
Table IV.
Anthropometric parameters and
nutritional status of the KD treatment group.
|
|
|
| Before KD | After KD |
|---|
|
|
|
|
|
|
|---|
| PI | Height, cm | Sex | BM, kg | BMI,
kg/m2 | Rating | BM, kg | BMI,
kg/m2 | Rating |
|---|
| 1 | 1.68 | M |
79.8 | 28.29 | Overweight |
82.3 | 29.18 | Overweight |
| 2 | 1.61 | M |
86.6 | 33.43 | Obese |
81.0 | 31.24 | Obese |
| 3 | 1.66 | M |
81.8 | 29.74 | Overweight |
80.0 | 29.03 | Overweight |
| 4 | 1.79 | M |
79.8 | 24.93 | Normal range |
76.4 | 23.87 | Normal range |
| 5 | 1.68 | M |
79.8 | 28.29 | Overweight |
82.3 | 29.18 | Overweight |
| 6 | 1.70 | F |
68.7 | 23.77 | Normal range |
65.7 | 22.73 | Normal range |
| 7 | 1.79 | F |
79.8 | 24.93 | Normal range |
76.4 | 23.87 | Normal range |
| 8 | 1.87 | F | 100.9 | 28.91 | Overweight | 113.4 | 32.49 | Obese |
| 9 | 1.78 | F | 102.0 | 32.27 | Obese |
94.5 | 29.81 | Overweight |
| Median | 1.70 | – |
81.8 | 29.69 | – |
81.0 | 29.16 | – |
Discussion
The lack of effective treatments for patients with
malignant glioma requires the design of novel therapeutic
strategies that take advantage of common glioma phenotypes,
including the altered metabolism of glioma cells (23). It has been postulated that a ketogenic
diet, i.e., a diet consisting of high fat, low carbohydrates and
adequate protein, may be useful in the treatment of brain tumors
(25–31). The present study was conducted to
assess the therapeutic efficacy and safety of a KD concomitant with
intranasal perillyl alcohol as a novel therapeutic strategy for
recurrent GBM. Following three months of adherence to the
treatment, the following were observed: i) The PR rate was 77.8%
(7/9 patients), including the reduction of the MRI tumor image and
peritumoral edema; ii) reduced serum lipid levels; and v) decreased
LDL-C levels.
Kambach et al (40) demonstrated that glioma cells are
sensitive to cholesterol synthesis inhibition downstream of the
mevalonate pathway, suggesting that specifically targeting
cholesterol synthesis could be an adjuvant treatment for GBM.
Another study (41) proposed a
potential molecular mechanism by which cholesterol reduction may be
effective for preventing and treating the progression of malignant
tumors; it suggested that upregulation of the cholesterol pathway
may be associated with a poor prognosis in patients with GBM.
Consistent with this model, the data of the present study indicated
that there is a statistically significant difference in total
cholesterol levels prior to subsequent to treatment with a KD with
concomitant intranasal POH, with lower values at the end of
treatment (Fig. 3). However, no such
difference was observed in the standard diet group. Furthermore,
there was a statistically significant difference in LDL-C and
triglyceride levels prior to subsequent to treatment in the KD
group, with higher values at the onset (Table II).
In our previous study, it was demonstrated that
although the consistent use of intranasal POH in recurrent GBM
patients induced clinical responses, MRI data presented no evidence
for the regression in the extent of peritumoral edema (42). In the present study, the extent of
peritumoral edema was notably reduced, along with a decrease of the
tumor area (Figs. 1 and 2). These results are consistent with another
study (43) that investigated the
effects of the KD on tumor growth and progression in animal models.
The results revealed that peritumoral edema was significantly
reduced in animals fed the KD, and protein analyses showed altered
expression of zona occludens-1 and aquaporin-4. The authors
concluded that the KD directly or indirectly altered the expression
of several proteins involved in malignant progression and may be a
useful tool for the treatment of gliomas. In addition to the
MRI-based observation of decreased cerebral edema and tumor volume,
a significant decrease in cholesterol and cholesterol esters in
particles of LDL was identified in the present study. This
observation is in line with an earlier study that demonstrated that
proliferating tumor cells utilize serum-derived cholesterol esters,
which are presumably carried by LDL particles (44). The decrease of cholesterol in the
present study can be explained as there was attention to the
quality of the lipids offered (monounsaturated fatty acid >
polyunsaturated > saturated fatty acid) and the amount of
cholesterol was limited to 200 mg/day. These results are not
consistent with other studies (44–46), which
concluded that KD increased the serum levels of total lipids and
LDL-C. However, Sharma et al (47) did not observe significant alterations
to the lipid profile of children with refractory epilepsy treated
with KD, which remained within normal limits throughout the
study.
In the present study, a moderate decrease in fasting
glucose levels was documented, although without statistical
significance (Table IV). The reason
for this discrepancy is unclear and further investigation of this
aspect will be required. It is important to note that the results
of the present study were obtained with a 90-day period of dietary
intervention. It is conceivable that a longer duration of the
concomitant regimen may provide additional insight into the
benefits of this regimen. However, shorter interventions may prove
beneficial as well; for example, using a type 2 diabetic mouse
model, Zhang et al (45)
demonstrated that just 8 weeks of KD was sufficient to improve
glucose and insulin tolerance, although hepatic lipid accumulation
and hepatic steatosis were observed. Future studies investigating
the KD as an adjuvant strategy to combat malignant gliomas should
explore the optimal duration of this dietary intervention to
support superior therapeutic outcomes.
In summary, the results of the present study are
encouraging and suggest that KD associated with intranasal POH may
represent a viable option as an adjunct therapy for recurrent GBM.
Currently established therapeutic approaches for this patient
cohort are lacking comprehensive efficacy, and the vast majority of
patients succumb to the disease within a few years. Clearly, more
effective treatments are urgently required. This small study will
hopefully fuel further discussion and stimulate additional
investigations to explore the potential benefit of adding a KD to
existing regimens for patients with GBM.
Acknowledgements
The authors would like to thank Professor Licinio da
Silva of the Mathematical Institute of the Fluminense Federal
University for his technical assistance and statistical
analysis.
References
|
1
|
Stupp R, Mason WP, van der Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hegi ME, Diserens AC, Gorlia T, Hamou MF,
De Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani
L, et al: MGMT gene silencing and benefit from temozolomide in
glioblastoma. N Engl J Med. 352:997–1003. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hegi ME, Liu L, Herman JG, Stupp R, Wick
W, Weller M, Mehta MP and Gilbert MR: Correlation of
O6-methylguanine methyltransferase (MGMT) promoter methylation with
clinical outcomes in glioblastoma and clinical strategies to
modulate MGMT activity. J Clin Oncol. 26:4189–4199. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schuster J, Lai RK, Recht LD, Reardon DA,
Paleologos NA, Groves MD, Mrugala MM, Jensen R, Baehring JM, Sloan
A, et al: A phase II, multicenter trial of rindopepimut (CDX-110)
in newly diagnosed glioblastoma: The ACT III study. Neuro Oncol.
17:854–861. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narita Y: Bevacizumab for glioblastoma.
Ther Clin Risk Manag. 11:1759–1765. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reardon DA, Herndon JE II, Peters KB,
Desjardins A, Coan A, Lou E, Sumrall AL, Turner S, Lipp ES,
Sathornsumetee S, et al: Bevacizumab continuation beyond initial
bevacizumab progression among recurrent glioblastoma patients. Br J
Cancer. 107:1481–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Abrams DA, Hanson JA, Brown JM, Hsu FP,
Delashaw JB Jr and Bota DA: Timing of surgery and bevacizumab
therapy in neurosurgical patients with recurrent high grade glioma.
J Clin Neurosc. 22:35–39. 2015. View Article : Google Scholar
|
|
9
|
Swanson KD, Lok E and Wong ET: An overview
of alternating electric fields therapy (NovoTTF Therapy) for the
treatment of malignant Glioma. Curr Neurol Neurosci Rep. 16:82016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stupp R, Taillibert S, Kanner AA, Kesari
S, Steinberg DM, Toms SA, Taylor LP, Lieberman F, Silvani A, Fink
KL, et al: Maintenance therapy with tumor-treating fields plus
temozolomide vs temozolomide alone for glioblastoma: A randomized
clinical trial. JAMA. 314:2535–2543. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen TC, Da Fonseca CO and Schönthal AH:
Preclinical development and clinical use of perillyl alcohol for
chemoprevention and cancer therapy. Am J Cancer Res. 5:1580–1593.
2015.PubMed/NCBI
|
|
12
|
da Fonseca CO, Schwartsmann G, Fischer J,
Nagel J, Futuro D, Quirico-Santos T and Gattass CR: Preliminary
results from a phase I/II study of perillyl alcohol intranasal
administration in adults with recurrent malignant gliomas. Surg
Neurol. 70:259–267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
da Fonseca CO, Simão M, Lins IR, Caetano
RO, Futuro D and Quirico-Santos T: Efficacy of monoterpene perillyl
alcohol upon survival rate of patients with recurrent glioblastoma.
J Cancer Res Clin Oncol. 137:287–293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Da Fonseca CO, Teixeira RM, Silva JC, DE
Saldanha DA, Fischer J Gama, Meirelles OC, Landeiro JA and
Quirico-Santos T: Long-term outcome in patients with recurrent
malignant glioma treated with Perillyl alcohol inhalation.
Anticancer Res. 33:5625–5631. 2013.PubMed/NCBI
|
|
15
|
Cross H: Epilepsy: Behavioural,
psychological, and ketogenic diet treatments. BMJ Clin Evid.
2015:pii: 12142015.
|
|
16
|
Rho JM: How does the ketogenic diet induce
anti-seizure effects? Neurosci Lett. 637:4–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruna J, Miró J and Velasco R: Epilepsy in
glioblastoma patients: Basic mechanisms and current problems in
treatment. Expert Rev Clin Pharmacol. 6:333–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kargiotis O, Markoula S and Kyritsis AP:
Epilepsy in the cancer patient. Cancer Chemother Pharmacol.
67:489–501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Armstrong TS, Grant R, Gilbert MR, Lee JW
and Norden AD: Epilepsy in glioma patients: Mechanisms, management,
and impact of anticonvulsant therapy. Neuro Oncol. 18:779–789.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liberti MV and Locasale JW: The warburg
effect: How does it benefit cancer cells? Trends Biochem Sci.
41:211–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kroemer G and Pouyssegur J: Tumor cell
metabolism: Cancer's Achilles' heel. Cancer Cell. 13:472–482. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu J, Tan M and Cai Q: The Warbu'rg effect
in tumor progression: Mitochondrial oxidative metabolism as an
anti-metastasis mechanism. Cancer Lett. 356:156–164. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Seyfried TN, Flores R, Poff AM, D'Agostino
DP and Mukherjee P: Metabolic therapy: A new paradigm for managing
malignant brain cancer. Cancer Lett. 356:289–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhattacharya B, Omar MF Mohd and Soong R:
The Warburg effect and drug resistance. Br J Pharmacol.
173:970–979. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Woolf EC, Syed N and Scheck AC: Tumor
metabolism, the ketogenic diet and -hydroxybutyrate: Novel
approaches to adjuvant brain tumor therapy. Front Mol Neurosci.
9:1222016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Klement RJ, Champ CE, Otto C and Kämmerer
U: Anti-tumor effects of ketogenic diets in mice: A meta-analyais.
PLoS One. 11:e01550502016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Abdelwahab MG, Fenton KE, Preul MC, Rho
JM, Lynch A, Stafford P and Scheck AC: The ketogenic diet is an
effective adjuvant to radiation therapy for the treatment of
malignant glioma. PLoS One. 7:e361972012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maroon JC, Seyfried TN, Donohue JP and
Bost J: The role of metabolic therapy in treating glioblastoma
multiforme. Surg Neurol Int. 6:612015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zuccoli G, Marcello N, Pisanello A,
Servadei F, Vaccaro S, Mukherjee P and Seyfried TN: Metabolic
management of glioblastoma multiforme using standard therapy
together with a restricted ketogenic diet: Case report. Nutr Metab
(Lond). 7:332010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Champ CE, Palmer JD, Volek JS,
Werner-Wasik M, Andrews DW, Evans JJ, Glass J, Kim L and Shi W:
Targeting metabolism with a ketogenic diet during the treatment of
glioblastoma multiforme. J Neurooncol. 117:125–131. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schwartz K, Chang HT, Nikolai M, Pernicone
J, Rhee S, Olson K, Kurniali PC, Hord NG and Noel M: Treatment of
glioma patients with ketogenic diets: Report of two cases treated
with an IRB-approved energy-restricted ketogenic diet protocol and
review of the literature. Cancer Metab. 3:32015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wagner M and Moore DD: Endoplasmic
reticulum stress and glucose metabolism. Curr Opin Clin Nutr Metab
Care. 14:367–373. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schönthal AH, Chen TC, Hofman FM, Louie SG
and Petasis NA: Preclinical development of novel anti-glioma drugs
targeting the endoplasmatic reticulum stress response. Curr Pharm
Des. 17:2428–2438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kato H and Nishitoh H: Stress responses
from the endoplasmic reticulum in cancer. Front Oncol. 5:932015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peñaranda Fajardo NM, Meijer C and Kruyt
FA: The endoplasmatic reticulum stress/unfolded protein responde in
gliomagenesis, tumor progression and as a therapeutic target in
glioblastoma. Biochem Pharmacol. 118:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Johnson GG, White MC and Grimaldi M:
Stressed to death: Targeting endoplasmic reticulum stress response
induced apoptosis in gliomas. Curr Pharm Des. 17:284–292. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cho HY, Wang W, Jhaveri N, Torres S, Tseng
J, Leong MN, Lee DJ, Goldkorn A, Xu T, Petasis NA, et al: Perillyl
alcohol for the treatment of temozolomide-resistant gliomas. Mol
Cancer Ther. 11:2462–2472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schonthal AH: Targeting endoplasmic
reticulum stress for cancer therapy. Front Biosci (Schol Ed).
4:412–431. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Strowd RE III and Grossman SA: The role of
glucose modulation and dietary supplementation in patients with
central nervous system tumors. Curr Treat Options Oncol. 16:362015.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kambach DM, Halim AS, Cauer AG, Sun Q,
Tristan CA, Celiku O, Kesarwala AH, Shankavaram U, Batchelor E and
Stommel JM: Disabled cell density sensing leads to dysregulated
cholesterol synthesis in glioblastoma. Oncotarget. 8:14860–14875.
2017.PubMed/NCBI
|
|
41
|
Murai T, Maruyama Y, Mio K, Nishiyama H,
Suga M and Sato C: Low cholesterol triggers membrane
microdomain-dependent CD44 shedding and suppresses tumor cell
migration. J Biol Chem. 286:1999–2007. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Da Fonseca CO, Silva JT, Lins IR, Simão M,
Arnobio A, Futuro D and Quirico-Santos T: Correlation of tumor
topography and peritumoral edema of recurrent malignant gliomas
with therapeutic response to intranasal administration of perillyl
alcohol. Invest New Drugs. 27:557–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Woolf EC, Curley KL, Liu Q, Turner GH,
Charlton JA, Preul MC and Scheck AC: The ketogenic diet alters the
hypoxic response and affects expression of proteins associated with
angiogenesis, invasive potential and vascular permeability in a
mouse glioma model. PLoS One. 10:e01303572015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nygren C, von Holst H, Månsson JE and
Fredman P: Increased levels of cholesterol esters in glioma tissue
and surrounding areas of human brain. Br J Neurosurg. 11:216–220.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang X, Qin J, Zhao Y, Shi J, Lan R, Gan
Y, Ren H, Zhu B, Qian M and Du B: Long-term ketogenic diet
contributes to glycemic control but promotes lipid accumulation and
hepatic steatosis in type 2 diabetic mice. Nutr Res. 36:349–358.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zamani GR, Mohammadi M, Ashrafi MR, Karimi
P, Mahmoudi M, Badv RS, Tavassoli AR and Malamiri R Azizi: The
effects of classic ketogenic diet on serum lipid profile in
children with refractory seizures. Acta Neurol Belg. 116:529–534.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sharma S, Gulati S, Kalra V, Agarwala A
and Kabra M: Seizure control and biochemical profile on the
ketogenic diet in young children with refractory epilepsy-Indian
experience. Seizure. 18:446–449. 2009. View Article : Google Scholar : PubMed/NCBI
|