Introduction
Legg-Calvé-Perthes disease (LCPD) is a common
disease of femoral head necrosis that mainly affect children's
health at the age of 2–12 years old (1). Study confirmed that boys diagnosed with
LCPD was much more than girls (2).
The clinical presentation of the early stage is the painful
synovitis in the knees, and the follow-up symptoms presented that
the femoral head ossific nuclei of LCPD are much smaller than those
normal children with similar age, which made the traversing blood
vessels are more vulnerable (3). The
prognosis of LCPD in early onset is optimistic, while the treatment
for advanced LCPD had poor outcome. Despite several consensus
asserted that LCPD resulted from the uncoupling of bone metabolism,
the exact pathophysiology remains elusive and the exploration of
its mechanism remains a challenge.
SRY-related high-mobility-group box 9 (SOX9), widely
accepted as a transcription factor, and governed a strong
transactivation domains (4). Various
studies have demonstrated that SOX9 is related to diseases. For
example, downregulated SOX9 inhibited tumorigenesis in prostate;
SOX9 mutations served as a therapeutic target in colorectal
carcinoma (5). It is known that SOX9
is expressed in chondrocytes, and the expression level of SOX9
involved in the differentiation and formation of cartilage
(6,7).
Previous reports have revealed that SOX9 restrained chondrocytes to
osteoblast lineage in the pathogenesis of Campomelic Dysplasia
(8). SOX9 directly administrated
growth plate chondrocytes in intervertebral disc (9). However, whether SOX9 acted as an
important regulators in LCPD was still unclear.
MicroRNA (miRNA or miR) is a class of non-coding RNA
with the length of 18–22 nucleotides, and recognized as the crucial
gene regulators that target mRNAs by binding to its 3′-untranslated
region (3′UTR), and further governed the degradation and
translation processes (10). Its well
known that miRNAs regulates gene expression, cell proliferation and
apoptosis and signaling transduction in both animals and plants and
as well as human. The aberrant expression of miRNA might result in
the disorder of cells and tissues. Some miRNAs served as
carcinogenic genes, while several miRNAs acted as tumor
suppressors. For example, miR-182-5p served as an oncgenic gene in
human bladder cancer by targeting Smad4 and RECK (11). While, miR-34a functioned as tumor
suppressor in neuroblastoma by promoting cell apoptosis (12). Mounting evidences showed that miRNA
played a vital role in the mechanism of diseases, and many miRNAs
were acted as the biomarkers in disease diagnosis and therapy.
miR-206 has been frequently presented in many diseases, and acted
as tumor suppressor or oncogenic gene in different cancers. For
example, miR-206 was downregulated in breast cancer, and suppressed
cell proliferation by targeting cyclinD2 (13). Moreover, miR-206 also mediated cardiac
hypotrophy (14) and oxidative stress
(15). Study has proved that miR-206
mediated in the osteogenic differentiation in steroid-induced
avascular necrosis of femoral head. While, whether it involved in
the LCPD was still unknown.
Thus, in this study, several patients with LCPD were
enrolled, combining with clinical test and in vitro
experiments. Our aims were to explore the potential pathogenesis
mechanism of LCPD, which might important for further LCPD
therapy.
Materials and methods
Patients and ethical approval
Human femoral head cartilage tissues were collected
from patients with LCPD (n=20, age 2–15), normal chondrocytes
species were collected from patients with repair surgery after
fracture (n=20, age 2–15). Cartilage tissues were immediately
stored in −80°C refrigerator and transferred to the laboratory.
Patients with several diseases such as primary osteoarthritis,
ankylosing spondylitis, systemic lupus erythematosus and
inflammatory diseases were excluded.
This study was performed in accordance with The
Third Hospital of Hebei Medical University (Shijiazhuang, China).
All patients were assigned the informed consent.
Chondrocyte isolation and culture
The tissues of femoral head cartilage with LCPD and
its normal control were disserted with trypsin for 30 min. Then
collagenase I was used to digest the tissues overnight. The
chondrocytes were collected using a mesh screen. The single-cell
isolated from the suspension using an centrifuge (Eppendorf)
following the instruction.
Human cartilage cell line TC28 were purchased from
American Type Culture Collection. Both the isolated cells and TC28
were maintained in a 96-well plate supplemented with Dulbecco's
modifed Eagle's medium (DMEM) at 37°C with 5% CO2.
Normal chondrocytes were cultured in DMEM pretreated
with 0.01 mM dexamethasone (DEX) for 2 h. The level of miR-206 and
SOX9 were determined to evaluate the effects of DEX.
Cell transfection
TC28 cells were cultured in DMEM for 24 h.
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was utilized to the transient
transfection assay. The miR-206 mimic, miR-206 inhibitor and its
negative control were transfected into cells, individually. The
transfection efficiency was determined 24 h later. The SOX9 was
overexpressed or downregulated in TC28 cells using retrovirus
transduction. Briefly, the SOX9 expression plasmid accompanied with
murine leukemia virus gap and pSV2-β-galactosidase plasmid were
co-transfected into TC28 cells. The cells were harvested after 48
h. And the transfection efficiency was detect using quantitative
polymerase chain reaction (qPCR). The empty plasmid served as
control. The sequence of si-SOX9: 5′-ACAGAAUUGUGUUAUGUGATT-3′.
qPCR
Total genome RNA was isolated from cells by using of
TRIzol reagent kit (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instruction. The RNA quality was
determined by 1% agarose gel. Then 1 µg of RNA was taken out for
the synthesize of cDNA using a Reverse Transcription kit (Takara
Biotechnology Co., Ltd., Dalian, China). qPCR was carried out on an
ABI 7900 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The relative expression of mRNA was normalized
by GAPDH. The primers' sequences were showing as following: SOX9:
forward 5′-ATGAATCTCCTGGACCCCTT-3′, reverse
3′-AACTTTGCCAGCTTGCACGT-5′; GAPDH: Forward
5′-TGAACGGGAAGCTCACTGG-3′, reverse 3′-TCCACCACCCTGTTGCTGTA-5′;
miR-206: Forward 5′-CAAGATGGCGACTTACGGATG-3′, reverse
3′-GTGCAAACAGGATGGACGTC-5′.
Western blot analysis
Cells were digested by lysis buffer, the protein was
extracted using s supercentrifuge (Eppendorf). The protein
concentrations were determined using a bicinchoninic acid assay kit
(BCA; Beyotime Institute of Biotechnology, Haimen, China). SDS-PAGE
were performed to separate protein extracts with equal amounts.
Then the protein extracts were transferred to polyvinylidene
fluoride (PVDF) membranes, and incubated with the primary
antibodies (anti-SOX9, 1:500, Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany; anti-β-actin, 1;500, R&D Systems, Inc., Minneapolis,
MN, USAD) at 4°C for 24 h. the PVDF membranes were washed by PBS
for twice and incubated for secondary antibodies for another 2 h at
room temperature. The proteins were visualized using an enhanced
chemiluminescence method. The quantity of protein was normalized by
β-actin.
Apoptosis analysis
Cell apoptosis was evaluated by using a Annexin
V-fluorescein isothiocyanate (FITC) cell apoptosis detection kit
(BD Pharmingen, San Diego, CA, USA) according to the manufacturer's
instruction. Briefly, chondrocytes cells were maintained in the
DMEM, then the cells were washed by PBS and resuspended with
Annexin V-FITC and PI. The rate of cell apoptosis was evaluated by
flow cytometry.
Luciferase reporter assay
The sequence of SOX9 was searched from Genbank and
http://www.microrna.org/microrna/home.do. For
luciferase reporter assay, cells were co-transfected with specific
mutant-type or wild-type vector, and cotransfected with miR-206
inhibitor, mimics and their negative control (NC), respectively,
using Lipofectamine® 2000 transfection kit (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells were collected after
transfection for 24 h. relative luciferase activity was analyzed
using dual-luciferase reporter assay (Promega Corporation, Madison,
WI, USA).
Statistical analysis
Data were presented as means ± standrad deviation
for three individual experiments. Data analysis were processed
using SPSS 18.0. Statistical differences were analyzed by Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
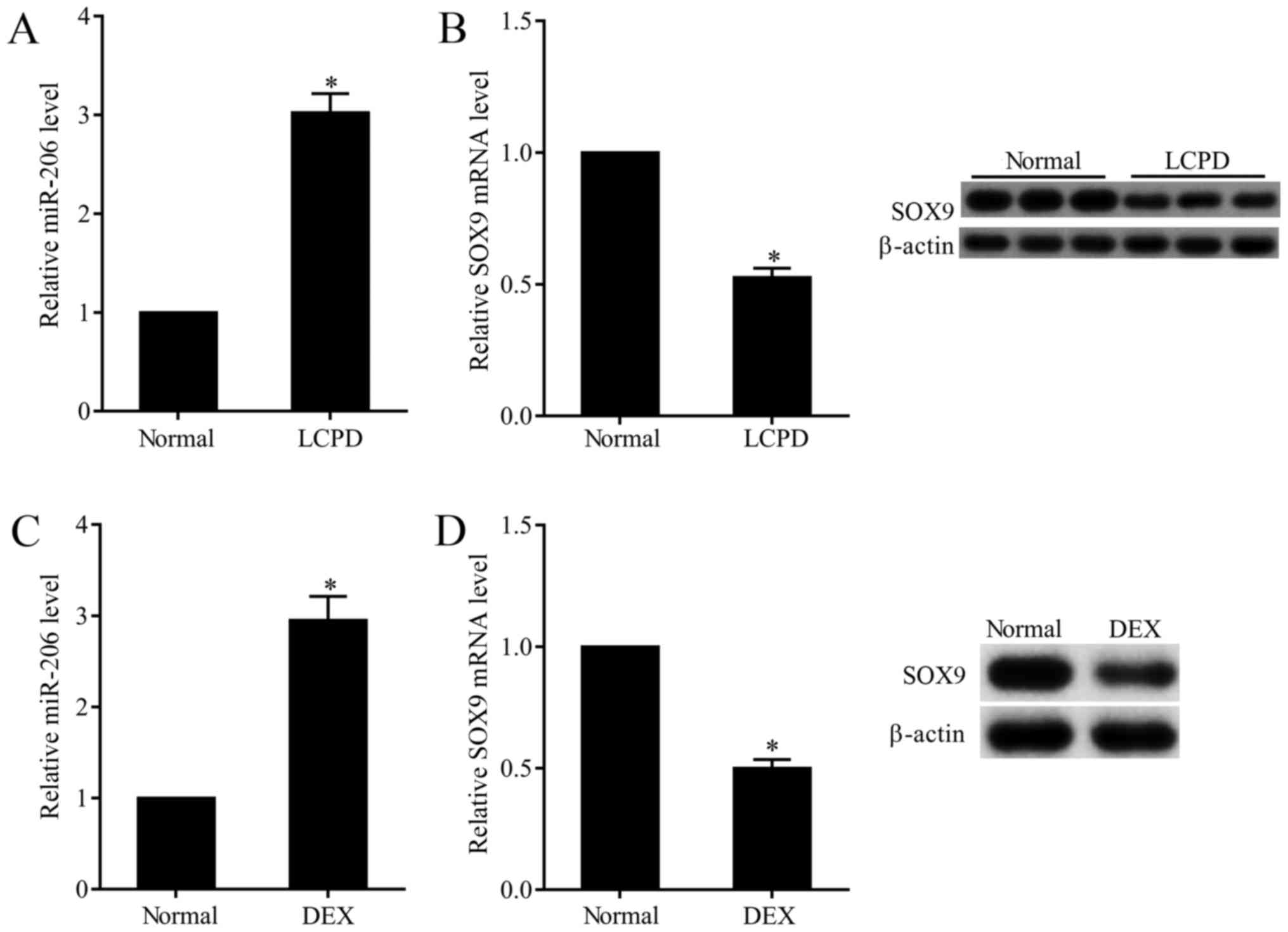
Overexpressed miR-206 and
downregulated SOX9 in chondrocytes of LCPD patients
In this study, we observed that the expression of
miR-206 in chondrocytes that isolated from LCPD patients was
3-folds that of normal chondrocytes (Fig.
1A), while the mRNA and protein expression of SOX9 was 50.9%
that of normal chondrocytes (Fig.
1B). To further explore the effect of DEX on the expression of
miR-206 and SOX9, normal chondrocytes (TC28) were subjected to 0.01
mM DEX. Results presented that DEX stimulation dramatically
increased the expression of miR-206 (Fig.
1C) and decreased the mRNA and protein expression of SOX9
(Fig. 1D).
Overexpressed miR-206 promoted cell
apoptosis
To evaluate the effect of miR-206 overexpression on
cell apoptosis of chondrocytes, cells of chondrocytes that isolated
from patients with repair surgery after fracture were transfected
with miR-206 mimic, and 48 h later, cell apoptosis were determined
by flow cytometry. As is presented in Fig. 2, miR-206 overexpression significantly
promoted cell apoptosis.
miR-206 targeted SOX9 to regulate its
expression
Online prediction found that miR-206 bound with the
3′UTR of SOX9 mRNA (Fig. 3A). To test
whether miR-206 targeted SOX9 by binding 3′UTR, we constructed
dual-luciferase reporter plasmid and transfected into TC28 cells.
Our results revealed that miR-206 mimic significantly inhibited the
relative luciferase activity of SOX9-3′UTR-WT. Moreover, miR-206
overexpression significantly decreased the expression of SOX9
(Fig. 3B). In addition, miR-206
inhibitor dramatically increased the relative luciferase activity
of SOX9-3′UTR-WT, and miR-206 downregulation promoted the
expression of SOX9 (Fig. 3C).
Interaction effects of miR-206 and
SOX9 on cell apoptosis
To investigate the effects of miR-206 and SOX9,
cells of TC28 were co-transfected with miR-206 mimic and
pcDNA-SOX9. As is shown in Fig. 4A,
overexpressed miR-206 obviously promoted cell apoptosis, while the
effect was abolished by overexpressed SOX9. Then to explore the
effects of miR-206 inhibotor, si-SOX9 and DEX on the expression of
SOX9 and cell apoptosis. Cells were pretreated with DEX and then
co-transfected with miR-206 inhibitor and si-SOX9. The results
demonstrated that DEX supplementation promoted cell apoptosis,
miR-206 inhibitor transfection reversed the increased apoptosis,
while si-SOX9 abolished the effects of miR-206 down-regulation
(Fig. 4B). On the other hand, DEX
supplementation dramatically suppressed the expression of SOX9,
miR-206 inhibitor reversed the effect of DEX, while si-SOX9
abolished the effect of miR-206 inhibitor (Fig. 4C).
Discussion
Recent years, the excessive usage of glucocorticoids
has resulted in increasingly incidence of femoral head necrosis.
Previous studies have tried to prevent the generation of
steroid-induced femoral head osteonecrosis (SANFH), for example,
enoxaparin and nitrate patch could use to prevent the occurrence of
SANFH (16,17), while the potential mechanism was not
fully understand. Liu et al found that miR-206 regulated
connexin43 that involved in the osteogenic differentiation in SANFH
(13). DEX was an anti-inflammatory
drug, and was widely used in various serious disease (18). Study has reported that DEX could
inhibit osteoblast differentiation (19,20). In
this study, we aims to evaluate whether DEX supplementation could
induce the similar effects in normal chondrocytes as LCPD
chondrocytes.
There has been many publications reported that
miRNAs exhibited a specific expression pattern in different tissues
and development stages as well as lesion tissues. miR-206 was
widely expressed in osteoblasts, and its expression was related to
the differentiation of osteoblasts (21). Previous studies have demonstrated that
overexpressed miR-206 suppressed the differentiation of osteoblast
(13). While in this study, we found
that comparing with the normal control, the expression of miR-206
was significantly increased in LCPD patients. In addition, 0.01 mM
DEX subjected to normal chondrocytes significantly promoted the
expression of miR-206. All those results suggested that miR-206 was
overexpressed in both LCPD and DEX-pretreated chondrocytes. SOX9 is
an important family member of SOX that mediated cell proliferation
process (22). SOX9 is crucial on
bone development, and is expressed in proliferating chondrocytes.
Thus, in this study, we evaluated the expression of SOX9 to
analysis the proliferation and apoptosis of chondrocytes in LCPD.
Our study revealed that, the expression of SOX9 in chondrocytes of
LCPD was decreased, DEX supplementation significantly decreased its
expression. To further confirm the potential relationship between
miR-206 and SOX9, both online TargetScan and http://www.microrna.org/microrna/home.dowere used
to selected the sequences, results found that miR-206 could bind
SOX9 in the site of 3′UTR. Then luciferase reporter assay revealed
that miR-206 targeted SOX9 to regulate it expression. Thus we
inferred that both miR-206 and SOX9 played an vital role in LCPD.
Next, to verify the relationship of miR-206 and SOX9, TC28 cells
were transfected with miR-206 mimic, and we found that
overexpressed miR-206 significantly promoted cell apoptosis, while
the effect was abolished by overexpressed SOX9.
To better understand the role of miR-206 and SOX9 in
chondrocytes, the possible molecular mechanism was also
investigated. We found that DEX stimulation significantly increased
the expression of SOX9 and promoted cell apoptosis, while miR-206
inhibitor reversed it, however, si-SOX9 abolished the effects of
downregulated miR-206. However, our study was accordance with the
previous study of Liu et al, who also found that miR-206 was
higher in SANFH (13), indicating the
reliability of our results. Taken together, all those results
indicated that miR-206 and SOX9 was mediated the mechanism of
DEX-induced LCPD, which was important for the further clinical
therapy of glucicorticoid- induced LCPD.
In summary, this study revealed that miR-206 was
highly expressed while SOX9 was low expressed in chondrocytes in
LCPD. SOX9 mediated by miR-206 was probably mediated the pathogenic
mechanism of LCPD. Further clinical therapy of LCPD based on the
regulation of miR-206 and SOX9 expression is important. The present
study provided firm foundation for exploration of the mechanism of
LCPD.
References
|
1
|
Pathak-Bhatt K, Couterier R and Tobis JS:
Poster 176: Legg-calve-perthes disease in a 9-Year-Old Girl: A
clinical case report. Pm R. 2 Suppl:S812010. View Article : Google Scholar
|
|
2
|
Zhang C, Li Y, Cornelia R, Swisher S and
Kim H: Regulation of VEGF expression by HIF-1α in the femoral head
cartilage following ischemia osteonecrosis. Sci Rep. 2:6502012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murphey MD, Foreman KL, Klassen-Fischer
MK, Fox MG, Chung EM and Kransdorf MJ: From the radiologic
pathology archives imaging of osteonecrosis: Radiologic-pathologic
correlation. Radiographics. 34:1003–1028. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mata-Rocha M, Hernández-Sánchez J,
Guarneros G, de la Chesnaye E, Sánchez-Tusié AA, Treviño CL, Felix
R and Oviedo N: The transcription factors Sox5 and Sox9 regulate
Catsper1 gene expression. FEBS Lett. 588:3352–3360. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Javier BM, Yaeger R, Wang L, Sanchez-Vega
F, Zehir A, Middha S, Sadowska J, Vakiani E, Shia J, Klimstra D, et
al: Recurrent, truncating SOX9 mutations are associated with SOX9
overexpression, KRAS mutation, and TP53 wild type status in
colorectal carcinoma. Oncotarget. 7:50875–50882. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tamamura Y, Katsube K, Mera H, Itokazu M
and Wakitani S: Irx3 and Bmp2 regulate mouse mesenchymal cell
chondrogenic differentiation in both a Sox9-dependent
and-independent manner. J Cell Physiol. 232:3317–3336. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cucchiarini M, Orth P and Madry H: Direct
rAAV SOX9 administration for durable articular cartilage repair
with delayed terminal differentiation and hypertrophy in vivo. J
Mol Med (Berl). 91:625–636. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheah KSE, Au TYK, Wynn S, Tan TY and Yip
RKH: Sox9 restrains chondrocyte to osteoblast lineage
progression-implications for the pathogenesis of Campomelic
Dysplasia. Proceedings of The 13th Annual Meeting of the
International Society For Stem Cell Research (ISSCR 2015).
Stockholm, Sweden. International Society For Stem Cell Research,
Skokie, IL. pp. 2812015;
|
|
9
|
Oh CD, Yasuda H, Zhao W, Henry SP, Zhang
Z, Xue M, de Crombrugghe B and Chen D: SOX9 directly regulates
CTGF/CCN2 transcription in growth plate chondrocytes and in nucleus
pulposus cells of intervertebral disc. Sci Rep. 6:299162016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Godfrey AC, Xu Z, Weinberg CR, Getts RC,
Wade PA, DeRoo LA, Sandler DP and Taylor JA: Serum microRNA
expression as an early marker for breast cancer risk in
prospectively collected samples from the Sister Study cohort.
Breast Cancer Res. 15:R422013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirata H, Ueno K, Shahryari V, Tanaka Y,
Tabatabai ZL, Hinoda Y and Dahiya R: Oncogenic miRNA-182-5p targets
Smad4 and RECK in human bladder cancer. PLoS One. 7:e510562012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boominathan L: The tumor suppressors p53,
p63, and p73 are regulators of microRNA processing complex. PLoS
One. 5:e106152012. View Article : Google Scholar
|
|
13
|
Liu G, Luo G, Bo Z, Liang X, Huang J and
Li D: Impaired osteogenic differentiation associated with
connexin43/microRNA-206 in steroid-induced avascular necrosis of
the femoral head. Exp Mol Pathol. 101:89–99. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou J, Tian Y, Li J, Lu B, Sun M, Zou Y,
Kong R, Luo Y, Shi Y, Wang K and Ji G: miR-206 is down-regulated in
breast cancer and inhibits cell proliferation through the
up-regulation of cyclinD2. Biochem Biophys Res Commun. 433:207–212.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ciesla M, Marona P, Kozakowska M, Jez M,
Seczynska M, Loboda A, Bukowska-Strakova K, Szade A, Walawender M,
Kusior M, et al: Heme Oxygenase-1 Controls an HDAC4-miR-206 pathway
of oxidative stress in rhabdomyosarcoma. Cancer Res. 76:57072016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beckmann R, Shaheen H, Kweider N, Ghassemi
A, Fragoulis A, Hermanns-Sachweh B, Pufe T, Kadyrov M and Drescher
W: Enoxaparin prevents steroid-related avascular necrosis of the
femoral head. ScientificWorldJournal. 2014:3478132014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drescher W, Beckmann R, Kasch R, Pufe M,
Knobe M, Kweider N, Hassenpflug J, Tingart M, Pufe T and Kadyrov M:
Nitrate patch prevents steroid-related bone necrosis. J Orthop Res.
29:1517–1520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeng M, Li ZY, Ma J, Cao PP, Wang H, Cui
YH and Liu Z: Clarithromycin and dexamethasone show similar
anti-inflammatory effects on distinct phenotypic chronic
rhinosinusitis: An explant model study. BMC Immunol. 16:372015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Li T, Fan J, Li T, Fan L, Wang S,
Weng X, Han Q and Zhao RC: miR-216a rescues dexamethasone
suppression of osteogenesis, promotes osteoblast differentiation
and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT
pathway. Cell Death Differ. 22:1935–1945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghali O, Broux O, Falgayrac G, Haren N,
van Leeuwen JP, Penel G, Hardouin P and Chauveau C: Dexamethasone
in osteogenic medium strongly induces adipocyte differentiation of
mouse bone marrow stromal cells and increases osteoblast
differentiation. BMC Cell Biol. 16:92015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Inose H, Ochi H, Kimura A, Fujita K, Xu R,
Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, et al: A
microRNA regulatory mechanism of osteoblast differentiation. Proc
Natl Acad Sci USA. 106:pp. 20794–20799. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Wang L, Liu Z, Zu C, Xing F, Yang P,
Yang Y, Dang X and Wang K: MicroRNA-494 inhibits cell proliferation
and invasion of chondrosarcoma cells in vivo and in vitro by
directly targeting SOX9. Oncotarget. 6:26216–26229. 2015.
View Article : Google Scholar : PubMed/NCBI
|