Tumorigenesis is a chronic process involving
numerous factors, including genetic, environmental, life-style and
psychological factors. Prophylaxis and etiological treatments are
important methods used to control cancer progression. In the early
1930s, Marble (1) first demonstrated
the association between diabetes and cancer. Over the past 15
years, an increasing number of studies have demonstrated that the
incidence of tumor development is higher in diabetic patients than
in healthy controls (2–4), and that cancer patients with diabetes
mellitus (DM) are less sensitive to chemotherapy and exhibit a
higher risk of mortality (5–10). Epidemiological studies revealed that
the incidence of tumorigenesis and the mortality rate of patients
with diabetes were significantly reduced in the metformin-treated
group compared with that in patients treated with insulin or
sulfonylureas (4,10–12).
Further studies have demonstrated that metformin has a direct
antitumor effect in vivo and in vitro, which may
repress the proliferation of tumor cells, and induce apoptosis,
autophagy and cell cycle arrest (13–15). Taken
together, these results suggest that metformin may become an
alternative adjuvant therapy for the treatment of cancer.
Controversy remains regarding the association
between diabetes and prostate cancer. A meta-analysis demonstrated
that the risk of prostate cancer in patients with diabetes was
lower than that in those without diabetes (19). Gong et al (20) also discovered that the risk of
prostate cancer was lower in patients with diabetes, when compared
with that in those without diabetes. Subsequently, a prospective
study of 328,316 males who were followed up for 5 years observed
that a history of diabetes is associated with a lower incidence of
prostate cancer (21). The biological
mechanisms of this association remain unknown, but may be
associated with increased physical activity resulting in lower
circulating levels of insulin and testosterone, or with changes in
the transcription factor 2 hepatic gene (21–24). A
multi-ethnic cohort-based prospective study revealed that patients
with diabetes were at a lower risk of developing prostate cancer
and that this was not associated with their ethnicity (25). However, whether or not the mortality
of men with prostate cancer is associated with diabetes remains
unknown. It has previously been demonstrated that pre-existing
diabetes affects the mortality rate of patients with prostate
cancer (25), however this hypothesis
is contested by a different study observing no significant
association between these two factors (26).
Epidemiological evidence demonstrates an association
between diabetes and hematological malignancies (27,28). Using
a random-effects model, a meta-analysis of observational studies
revealed that T2DM is associated with an increased risk of
developing non-Hodgkin's lymphoma, leukemia and myeloma (28). Table I
summarizes the meta-analyses of the associations between diabetes
and different types of cancer over a number of years (29–41).
The mechanisms behind the fact that patients with
T2DM are more likely to develop tumors have not yet been fully
elucidated. Previous studies have suggested that there are three
factors serving important functions in this process.
T2DM, which is characterized by insulin resistance
and hyperinsulinemia, causes an increased level of insulin and
insulin-like growth factor (I/IGF), which could bind to receptors
and activate the downstream phosphatidylinositol 3-kinase
(PI3K)/Akt and mitogen-activated protein kinase (MAPK) signaling
pathways, ultimately leading to the proliferation of cells
(11,37,42–45). It
has been confirmed that I/IGF and its downstream signaling pathway
have an important function in tumor development, and thus, may
serve as targets for tumor therapy (46,47). I/IGF
signaling pathways are also recognized as playing an important role
in the relationship between diabetes and cancer (48).
Previously, certain researchers believed diabetes to
be an inflammatory disease (49–51).
Metabolic disturbances and enhanced oxidative stress in patients
with diabetes promote a continuous pro-inflammatory state,
resulting in the decreased antioxidant capacity of cells. The
insulin resistance that characterizes T2DM may produce large
numbers of cytokines, including tumor necrosis factor α (TNF-α),
interleukin (IL)-6 and IL-1β (49,52). TNF-α
and IL-6 may activate nuclear factor-κB and Janus kinase/signal
transducer and activator of transcription 3 pathways, which are
important signaling pathways in tumorigenesis (53,54).
Patients with DM are more likely to have persistent
infections, suggesting that these patients may be immunodeficient
and therefore more susceptible to opportunistic infections
(55).
In 1957, the FDA approved the use of metformin for
the treatment of T2DM. Following this, metformin became recognized
as the first-line treatment for diabetes due to its excellent
hypoglycemic and cardiovascular protective effects. In 2005, Evans
et al (56), in a
case-controlled study that included 11,876 T2DM patients,
identified for the first time that metformin may reduce the risk of
cancer in patients with diabetes (unadjusted odds ratio, 0.79; 95%
CI, 0.67–0.93), and that this effect was positively correlated with
the dosage of metformin. In 2006, a population-based retrospective
cohort study by Bowker et al (57) revealed that the metformin treatment
group had a lower cancer-associated mortality rate compared with
that of the sulfonylurea group and the insulin treatment group
consisting of other patients with cancer and DM. In 2009, Evans and
colleagues re-highlighted the association between metformin
treatment and tumorigenesis in patients with T2DM. The tumor
incidence in 4,085 patients with diabetes treated with metformin
was lower than that in the control group (7.3 vs. 11.6%), and the
adjusted odds ratio was 0.63 (95% CI, 0.53–0.75), further
demonstrating that metformin may reduce the risk of tumorigenesis
(58).
In 2010, a prospectively-followed cohort study
assessed the association between the use of metformin and cancer
mortality in 1,353 patients with T2DM (63). Metformin-treated patients were
revealed to exhibit a reduced cancer mortality time compared with
that of the controls with a median of 9.6 years and an adjusted HR
of 0.43 (95% CI, 0.23–0.80) (63).
Diabetic patients taking metformin had a 31% reduced tumor risk
compared with those taking any other antidiabetic drug,
particularly for pancreatic cancer and HCC, but not for colon,
breast or prostate cancer (64).
Jiralerspong et al (65)
observed that diabetic patients with breast cancer treated with
metformin and neoadjuvant chemotherapy acquired a higher
pathological complete response rate than those not being treated
with metformin. A nested case-controlled analysis including 22,621
female patients with T2DM demonstrated that patients with diabetes
who used metformin for ≥5 years had a decreased risk of developing
breast cancer (adjusted odds ratio, 0.44; 95% CI, 0.24–0.82)
(66).
Certain studies have also revealed that diabetic
patients with thyroid cancer treated with metformin exhibit a
higher rate of remission. Tumor size in the metformin-treated group
is significantly smaller than that in the control groups. An in
vitro study demonstrated that metformin may activate
AMP-inducible protein kinase (AMPK) and downregulate p70S6K/pS6
protein to inhibit the growth of tumor cells (67). Kumar et al (12) revealed that metformin treatment was
associated with an improved survival time in patients with ovarian
cancer. The progression-free survival time of patients with ovarian
cancer and T2DM was longer in the metformin group (68).
A large volume of epidemiological data has suggested
that metformin may benefit cancer patients. In vitro and
in vivo experiments have confirmed that metformin may
inhibit the proliferation of a variety of tumor cells, but the
mechanism underpinning this has not yet been fully elucidated. At
present, two major pathways are recognized as the main ways in
which metformin exerts its antitumor effect. The first pathway, the
I/IGF pathway, may reduce the level of I/IGF-1 in the blood
circulation, thereby inactivating its downstream PI3K/Akt/mTOR
signaling pathways to inhibit tumor cell proliferation. The second
pathway, the AMPK signaling pathway, may facilitate metformin to
directly act on tumor cells, upregulate AMPK and inhibit downstream
mTOR (42).
I/IGF promote cell mitosis, stimulate cell growth
and inhibit cell apoptosis, all of which serve important functions
in tumor genesis and development (47). Studies in which glyconeogenesis in the
liver was reduced have indicated that metformin may effectively
reduce blood insulin levels by increasing the sensitivity of
surrounding tissue to insulin and inhibiting the intestinal cells
from absorbing glucose (76,77). This suggests that metformin may reduce
blood insulin levels and may inactivate the I/IGF signaling pathway
to exert its antitumor effect. This hypothesis was confirmed by a
number of other studies. Goodwin et al (78) demonstrated that, in breast cancer
patients without overt DM, the use of metformin may significantly
decrease insulin levels and improve insulin resistance. Memmott
et al (79) observed that when
mice were exposed to the tobacco carcinogen,
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, the use of
metformin reduced lung tumor burden by up to 53%. This mechanistic
study revealed that metformin may directly inhibit mTOR by
activating AMPK in liver tissue and may indirectly inhibit mTOR by
decreasing activation of insulin receptor/IGF-1 receptor and Akt in
lung tissue (79). Algire et
al (80) observed that, when
colon carcinoma MC38 cells or Lewis lung carcinoma 1 (LLC1) cells
were transfected with short hairpin RNA (shRNA) against liver
kinase B1 (LKB1), the growth of MC38 and LLC1 cells was
significantly inhibited and the phosphorylation of AMPK was
markedly increased by metformin in vitro. Additionally,
under low-glucose conditions in vitro, MC38 and LLC1 cells
transfected with shRNA against LKB1 were more sensitive to
metformin. LKB1+ and LKB1− MC38 cells were
subcutaneously transplanted in the high-fat diet and normal diet
mice respectively. The results showed that regardless of the
expression of LKB, mice with a high-fat diet were more likely to
have tumors, and metformin was able to significantly inhibit
insulin levels in high-fat diet mice (80). Pollak (81) demonstrated that, while metformin
reduced the insulin level, no effect on the IGF-I/IGF-II level was
observed (47,77,81).
Karnevi et al (82)
demonstrated that metformin may inhibit IGF-IR and activate AMPK in
pancreatic cancer cells.
AMPK, which is a cellular energy sensor, may be
activated by an increased AMP/ATP ratio. Metformin may inhibit the
effect of respiratory complex I, leading to reduced oxidative
phosphorylation and reduced ATP production, resulting in a
reduction in cellular ATP and activation of AMPK (47). In 2006, Zakikhani et al
(83) demonstrated that metformin
inhibited the proliferation of breast cancer cells through
activation of AMPK, leading to the inhibition of mTOR. This growth
inhibition was AMPK-dependent and was blocked by small interfering
RNA against AMPK (83). There are two
pathways known to inhibit mTOR following activation of AMPK.
Firstly, AMPK may directly phosphorylate tuberous sclerosis complex
2 (TSC2) on T1227 and S1345, and activate the TSC1/TSC2 compounds,
which inhibit the activity of Ras homolog enriched in brain and
mTOR (84). Secondly, AMPK directly
phosphorylates the mTOR binding partner raptor on 722 and 792
serine residues, which inactivates raptor and mTOR (85,86).
Dowling et al (87) revealed
that metformin may activate the expression of AMPK, which inhibits
phosphorylation of mTOR and its downstream ribosome S6 protein
kinase (p70S6K) and eIF4E-binding proteins (4E-BP1). Similarly,
other studies observed that the proliferation of cells in leukemia,
lymphoma, and prostate, ovarian, colon, endometrial and liver
cancer was inhibited by metformin through the AMPK/mTOR pathway
(88–93).
Metformin also exerts its antitumor effect in an
AMPK-independent manner. Ben Sahra et al (94) reported that metformin may inhibit the
cell proliferation and induce the cell-cycle arrest of prostate
cancer cell lines by increasing regulated in development and DNA
damage response 1 (REDD1) expression in a p53-dependent manner in
the absence of AMPK. Inhibition of REDD1 reverses metformin-induced
cell-cycle arrest (94). Kalender
et al (95) demonstrated that
metformin may inhibit mTORC1 in a rag GTPase-dependent manner in
the absence of AMPK and TSC1/2. Certain studies have also revealed
that metformin may induce the apoptosis and cell-cycle arrest of
melanoma cells (96) and epithelial
ovarian cancer cell lines (OVCAR-3 and OVCAR-4) (97) in an AMPK-independent manner. Zi et
al (98) revealed that metformin
may inhibit myeloma cell proliferation through the PI3K/Akt/mTOR
signaling pathway, but not through the AMPK/mTOR pathway (98). Taken together, these results
demonstrate that metformin exerts an anti-proliferation function
through a range of mechanisms.
Whether or not AMPK is an oncogene or a tumor
suppressor gene remains to be fully elucidated (99,100).
Studies have revealed that AMPK is overactivated in multiple
myeloma cells and prostate cancer cells, which may result in cell
apoptosis (101,102). At present, metformin is considered
to be an AMPK activator by the majority of researchers. However, it
is difficult to confirm whether or not metformin is more effective
in the treatment of tumors without the functional LKB1-AMPK pathway
(80,103). Liu et al (104) observed that, compared with that in
normal tissue, AMPK is constitutively activated in human and mouse
gliomas (104). However, using an
AMPK direct activator, A769662, did not induce glioma cell
apoptosis, suggesting that metformin may not exert its antitumor
effect through the AMPK pathway (104). Metformin may increase glucose
consumption and inhibit the production of ATP in cells. As an
‘energy sensor’, the activation of AMPK may be a result of cells
adapting under survival pressure (80). The authors of the present review
hypothesize that when the time or concentration of metformin
exposure are increased, cells may have been unable to adapt to
compensate for energy deprivation, AMPK phosphorylation may have
been suppressed and tumor cell apoptosis may eventually have been
induced. Furthermore, it was observed that when AMPK was knocked
down, the tolerance of myeloma cells for nutritional deficiency was
decreased when compared with that of the control group. Therefore,
the effects of metformin on AMPK require further investigation.
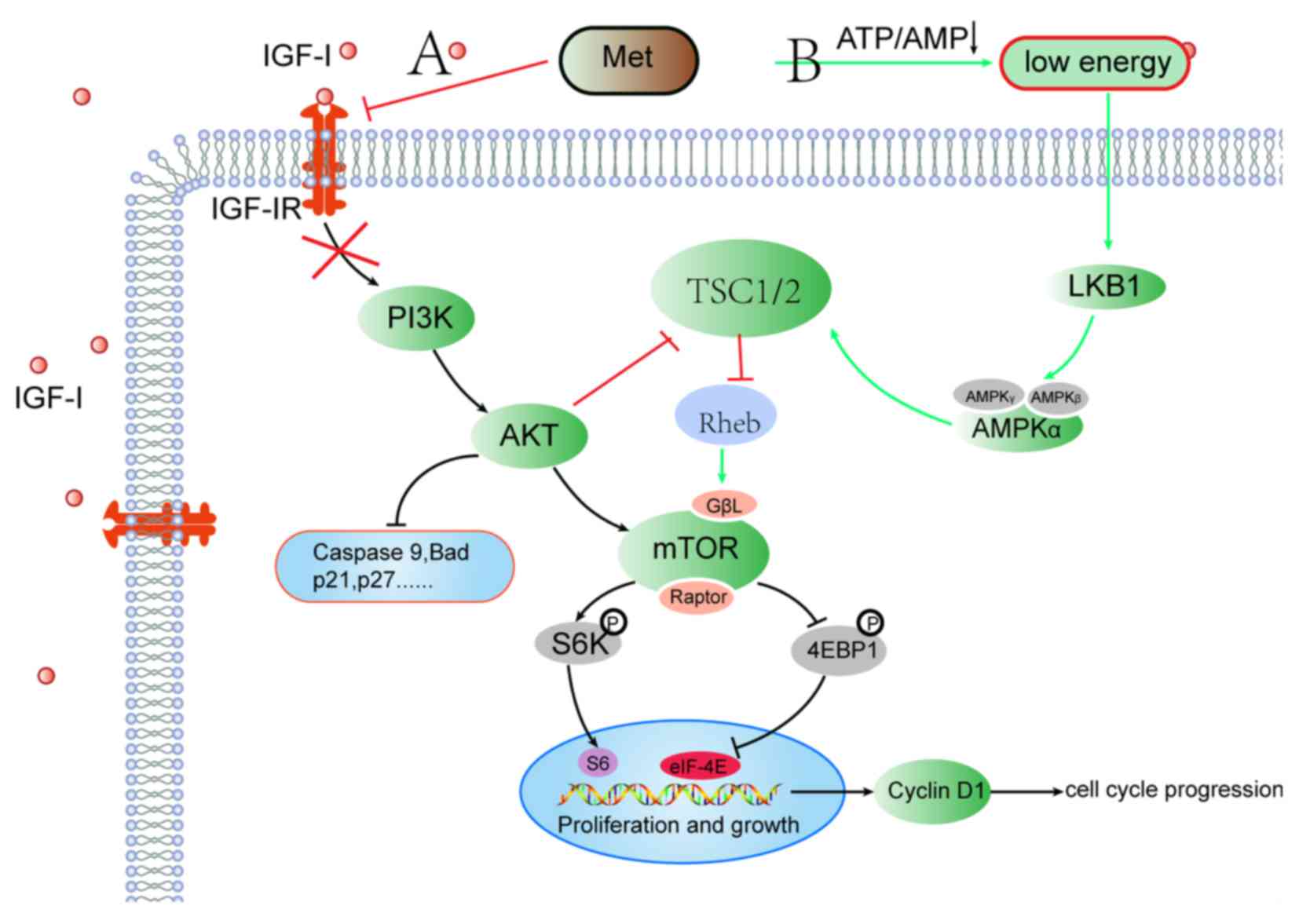
Fig. 1 summarizes the mechanisms
through which metformin exerts its antitumor effect.
Combined treatment with metformin may enhance the
curative effect of and reduce the adverse reactions associated with
chemotherapy. Therefore, combining metformin with traditional
chemotherapy drugs has become a novel aspect of cancer therapy.
Jiralerspong et al (65)
observed that breast cancer patients with DM treated with metformin
and neoadjuvant chemotherapy had a higher complete pathological
response rate when compared with the control group (65). Another study reported that this
enhanced antitumor effect of chemotherapy may have been due to the
inactivation of vitamin B12 induced by metformin through the
N2O pathway (105). Ben
Sahra et al (106) discovered
that metformin combined with 2-deoxyglucose (2-DG) may inhibit
mitochondrial respiration and glycolysis through tumor protein p53
(p53)-dependent apoptosis via the AMPK pathway. Furthermore, the
re-expression of a functional p53 in p53-deficient prostate cancer
cells may restore caspase-3 activity (106). A later study observed that combined
therapy with metformin and 2-DG may inhibit autophagy and induce
AMPK-dependent apoptosis in prostate cancer cells (107). Colquhoun et al (108) revealed that bicalutamide combined
with metformin may significantly reduce prostate cancer cell growth
compared with single-agent monotherapy. In androgen receptor
(AR)-positive cells, this effect appeared to be mediated by
enhanced antiproliferation, while the same effect appeared to be
mediated by enhanced apoptosis in AR-negative cells (108).
Metformin is the first-line drug for the treatment
of T2DM. Epidemiological and basic studies have demonstrated that
it may also inhibit the growth of a variety of tumor cells, and an
increasing number of ongoing clinical trials on the antitumor
activity of metformin are being processed for the treatment of
cancer. Metformin has been proven to be safe as a treatment drug
for T2DM and has subsequently been used clinically for a number of
years. If large-scale clinical trials are able to attest to the
antitumor effects of metformin, this drug may become an alternative
cancer adjuvant therapy, providing a novel approach for cancer
prevention and treatment.
The present study was supported by the National
Natural Science Foundation of China (grant no. 81560030), the
Natural Science Foundation of Jiangxi Province (grant no.
20151BAB205021), the Natural Science Foundation of Social
Development Projects of Jiangxi Province (grant no. 20151BBG70170)
and the Health and Family Planning Commission Projects of Jiangxi
Province (grant no. 20161066).
|
1
|
Marble A: Diabetes and cancer. N Engl J
Med. 211:339–349. 1934. View Article : Google Scholar
|
|
2
|
Simon D: I.15 Diabetes and cancer.
Diabetes Res Clin Pract. 103:S52014. View Article : Google Scholar
|
|
3
|
Pandey A, Forte V, Abdallah M, Alickaj A,
Mahmud S, Asad S and McFarlane SI: Diabetes mellitus and the risk
of cancer. Minerva Endocrinol. 36:187–209. 2011.PubMed/NCBI
|
|
4
|
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN,
Chang YH and Huang YC: Type 2 diabetes increases and metformin
reduces total, colorectal, liver and pancreatic cancer incidences
in Taiwanese: A representative population prospective cohort study
of 800,000 individuals. BMC Cancer. 11:202011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meyerhardt JA, Catalano PJ, Haller DG,
Mayer RJ, Macdonald JS, Benson AB III and Fuchs CS: Impact of
diabetes mellitus on outcomes in patients with colon cancer. J Clin
Oncol. 21:433–440. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coughlin SS, Calle EE, Teras LR, Petrelli
J and Thun MJ: Diabetes mellitus as a predictor of cancer mortality
in a large cohort of US adults. Am J Epidemiol. 159:1160–1167.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barone BB, Yeh HC, Snyder CF, Peairs KS,
Stein KB, Derr RL, Wolff AC and Brancati FL: Long-term all-cause
mortality in cancer patients with preexisting diabetes mellitus: A
systematic review and meta-analysis. JAMA. 300:2754–2764. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rao Kondapally, Seshasai S, Kaptoge S,
Thompson A, Thompson A, Di Angelantonio E, Gao P, Sarwar N, Whincup
PH, Mukamal KJ, Gillum RF, et al: Diabetes mellitus, fasting
glucose, and risk of cause-specific death. N Engl J Med.
364:829–841. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Ji J, Sundquist K, Sundquist J and
Hemminki K: The impact of type 2 diabetes mellitus on
cancer-specific survival: A follow-up study in Sweden. Cancer.
118:1353–1361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Currie CJ, Poole CD, Jenkins-Jones S, Gale
EA, Johnson JA and Morgan CL: Mortality after incident cancer in
people with and without type 2 diabetes: Impact of metformin on
survival. Diabetes Care. 35:299–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vigneri P, Frasca F, Sciacca L, Pandini G
and Vigneri R: Diabetes and cancer. Endocr Relat Cancer.
16:1103–1123. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar S, Meuter A, Thapa P, Langstraat C,
Giri S, Chien J, Rattan R, Cliby W and Shridhar V: Metformin intake
is associated with better survival in ovarian cancer: A
case-control study. Cancer. 119:555–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Emami Riedmaier A, Fisel P, Nies AT,
Schaeffeler E and Schwab M: Metformin and cancer: From the old
medicine cabinet to pharmacological pitfalls and prospects. Trends
Pharmacol Sci. 34:126–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rizos CV and Elisaf MS: Metformin and
cancer. Eur J Pharmacol. 705:96–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bost F, Sahra IB, Le Marchand-Brustel Y
and Tanti JF: Metformin and cancer therapy. Curr Opin Oncol.
24:103–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang YX, Hennessy S and Lewis JD: Type 2
diabetes mellitus and the risk of colorectal cancer. Clin
Gastroenterol Hepatol. 3:587–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee MY, Lin KD, Hsiao PJ and Shin SJ: The
association of diabetes mellitus with liver, colon, lung, and
prostate cancer is independent of hypertension, hyperlipidemia, and
gout in Taiwanese patients. Metabolism. 61:242–249. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Goodwin P: Meta-analysis: Diabetes appears
to increase risk, mortality of breast and colon cancers. Oncol
Times. 35:332013. View Article : Google Scholar
|
|
19
|
Kasper JS and Giovannucci E: A
meta-analysis of diabetes mellitus and the risk of prostate cancer.
Cancer Epidemiol Biomarkers Prev. 15:2056–2062. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong Z, Neuhouser ML, Goodman PJ, Albanes
D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM
and Kristal AR: Obesity, diabetes, and risk of prostate cancer:
Results from the prostate cancer prevention trial. Cancer Epidemiol
Biomarkers Prev. 15:1977–1983. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Calton BA, Chang SC, Wright ME, Kipnis V,
Lawson K, Thompson FE, Subar AF, Mouw T, Campbell DS, Hurwitz P, et
al: History of diabetes mellitus and subsequent prostate cancer
risk in the NIH-AARP Diet and Health Study. Cancer Causes Control.
18:493–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gudmundsson J, Sulem P, Steinthorsdottir
V, Bergthorsson JT, Thorleifsson G, Manolescu A, Rafnar T,
Gudbjartsson D, Agnarsson BA, Baker A, et al: Two variants on
chromosome 17 confer prostate cancer risk, and the one in TCF2
protects against type 2 diabetes. Nat Genet. 39:977–983. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Frayling TM, Colhoun H and Florez JC: A
genetic link between type 2 diabetes and prostate cancer.
Diabetologia. 51:1757–1760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stevens VL, Ahn J, Sun J, Jacobs EJ, Moore
SC, Patel AV, Berndt SI, Albanes D and Hayes RB: HNF1B and JAZF1
genes, diabetes, and prostate cancer risk. Prostate. 70:601–607.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waters KM, Henderson BE, Stram DO, Wan P,
Kolonel LN and Haiman CA: Association of diabetes with prostate
cancer risk in the multiethnic cohort. Am J Epidemiol. 169:937–945.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Snyder CF, Stein KB, Barone BB, Peairs KS,
Yeh HC, Derr RL, Wolff AC, Carducci MA and Brancati FL: Does
pre-existing diabetes affect prostate cancer prognosis? A
systematic review. Prostate Cancer Prostatic Dis. 13:58–64. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Issa ZA, Zantout MS and Azar ST: Multiple
myeloma and diabetes. ISRN Endocrinol. 2011:8150132011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Castillo JJ, Mull N, Reagan JL, Nemr S and
Mitri J: Increased incidence of non-Hodgkin lymphoma, leukemia, and
myeloma in patients with diabetes mellitus type 2: A meta-analysis
of observational studies. Blood. 119:4845–4850. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Noto H, Tsujimoto T, Sasazuki T and Noda
M: Significantly increased risk of cancer in patients with diabetes
mellitus: A systematic review and meta-analysis. Endocr Pract.
17:616–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Serag HB, Hampel H and Javadi F: The
association between diabetes and hepatocellular carcinoma: A
systematic review of epidemiologic evidence. Clin Gastroenterol
Hepatol. 4:369–380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen
Y, Li G and Wang L: Increased risk of hepatocellular carcinoma in
patients with diabetes mellitus: A systematic review and
meta-analysis of cohort studies. Int J Cancer. 130:1639–1648. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Friberg E, Orsini N, Mantzoros CS and Wolk
A: Diabetes mellitus and risk of endometrial cancer: A
meta-analysis. Diabetologia. 50:1365–1374. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Larsson SC, Orsini N and Wolk A: Diabetes
mellitus and risk of colorectal cancer: A meta-analysis. J Natl
Cancer Inst. 97:1679–1687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huxley R, Ansary-Moghaddam A, Berrington
de González A, Barzi F and Woodward M: Type-II diabetes and
pancreatic cancer: A meta-analysis of 36 studies. Br J Cancer.
92:2076–2083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Everhart J and Wright D: Diabetes mellitus
as a risk factor for pancreatic cancer: A meta-analysis. JAMA.
273:1605–1609. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Larsson SC, Mantzoros CS and Wolk A:
Diabetes mellitus and risk of breast cancer: A meta-analysis. Int J
Cancer. 121:856–862. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wolf I, Sadetzki S, Catane R, Karasik A
and Kaufman B: Diabetes mellitus and breast cancer. Lancet Oncol.
6:103–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bonovas S, Filioussi K and Tsantes A:
Diabetes mellitus and risk of prostate cancer: A meta-analysis.
Diabetologia. 47:1071–1078. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Larsson SC, Orsini N, Brismar K and Wolk
A: Diabetes mellitus and risk of bladder cancer: A meta-analysis.
Diabetologia. 49:2819–2823. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chao C and Page JH: Type 2 diabetes
mellitus and risk of non-Hodgkin lymphoma: A systematic review and
meta-analysis. Am J Epidemiol. 168:471–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mitri J, Castillo J and Pittas AG:
Diabetes and Risk of Non-Hodgkin's Lymphoma A meta-analysis of
observational studies. Diabetes Care. 31:2391–2397. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gallagher EJ and LeRoith D: The
proliferating role of insulin and insulin-like growth factors in
cancer. Trends Endocrinol Metab. 21:610–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chowdhury T: Diabetes and cancer. QJM.
103:905–915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Djiogue S, Nwabo Kamdje AH, Vecchio L,
Kipanyula MJ, Farahna M, Aldebasi Y and Seke Etet PF: Insulin
resistance and cancer: The role of insulin and IGFs. Endocr Relat
Cancer. 20:R1–R17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Del Barco S, Vazquez-Martin A, Cufi S,
Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B
and Menendez JA: Metformin: Multi-faceted protection against
cancer. Oncotarget. 2:896–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Clayton PE, Banerjee I, Murray PG and
Renehan AG: Growth hormone, the insulin-like growth factor axis,
insulin and cancer risk. Nat Rev Endocrinol. 7:11–24. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pollak M: The insulin and insulin-like
growth factor receptor family in neoplasia: An update. Nat Rev
Cancer. 12:159–169. 2012.PubMed/NCBI
|
|
48
|
Cohen DH and LeRoith D: Obesity, type 2
diabetes, and cancer: The insulin and IGF connection. Endocr Relat
Cancer. 19:F27–F45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Donath MY and Shoelson SE: Type 2 diabetes
as an inflammatory disease. Nat Rev Immunol. 11:98–107. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xie W and Du L: Diabetes is an
inflammatory disease: Evidence from traditional Chinese medicines.
Diabetes Obes Metab. 13:289–301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cosentino F and Assenza GE: Diabetes and
Inflammation. Herz. 29:749–759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kern PA, Ranganathan S, Li C, Wood L and
Ranganathan G: Adipose tissue tumor necrosis factor and
interleukin-6 expression in human obesity and insulin resistance.
Am J Physiol Endocrinol Metab. 280:E745–751. 2001.PubMed/NCBI
|
|
53
|
Neurath MF and Finotto S: IL-6 signaling
in autoimmunity, chronic inflammation and inflammation-associated
cancer. Cytokine Growth Factor Rev. 22:83–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Waters JP, Pober JS and Bradley JR: Tumour
necrosis factor and cancer. J Pathol. 230:241–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Geerlings SE and Hoepelman AI: Immune
dysfunction in patients with diabetes mellitus (DM). FEMS Immunol
Med Microbiol. 26:259–265. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Evans JM, Donnelly LA, Emslie-Smith AM,
Alessi DR and Morris AD: Metformin and reduced risk of cancer in
diabetic patients. BMJ. 330:1304–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin. Diabetes Care.
29:254–258. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Libby G, Donnelly LA, Donnan PT, Alessi
DR, Morris AD and Evans JM: New users of metformin are at low risk
of incident cancer: A cohort study among people with type 2
diabetes. Diabetes Care. 32:1620–1625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li D, Yeung SC, Hassan MM, Konopleva M and
Abbruzzese JL: Antidiabetic therapies affect risk of pancreatic
cancer. Gastroenterology. 137:482–488. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Currie CJ, Poole CD and Gale EA: The
influence of glucose-lowering therapies on cancer risk in type 2
diabetes. Diabetologia. 52:1766–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Donadon V, Balbi M, Valent F and Avogaro
A: Glycated hemoglobin and antidiabetic strategies as risk factors
for hepatocellular carcinoma. World J Gastroenterol. 16:3025–3032.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Donadon V, Balbi M, Mas MD, Casarin P and
Zanette G: Metformin and reduced risk of hepatocellular carcinoma
in diabetic patients with chronic liver disease. Liver Int.
30:750–758. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Landman GW, Kleefstra N, van Hateren KJ,
Groenier KH, Gans RO and Bilo HJ: Metformin associated with lower
cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care.
33:322–326. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Decensi A, Puntoni M, Goodwin P, Cazzaniga
M, Gennari A, Bonanni B and Gandini S: Metformin and cancer risk in
diabetic patients: A systematic review and meta-analysis. Cancer
Prev Res (Phila). 3:1451–1461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jiralerspong S, Palla SL, Giordano SH,
Meric-Bernstam F, Liedtke C, Barnett CM, Hsu L, Hung MC, Hortobagyi
GN and Gonzalez-Angulo AM: Metformin and pathologic complete
responses to neoadjuvant chemotherapy in diabetic patients with
breast cancer. J Clin Oncol. 27:3297–3302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bodmer M, Meier C, Krähenbühl S, Jick SS
and Meier CR: Long-term metformin use is associated with decreased
risk of breast cancer. Diabetes Care. 33:1304–1308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Klubo-Gwiezdzinska J, Costello J Jr, Patel
A, Bauer A, Jensen K, Mete M, Burman KD, Wartofsky L and Vasko V:
Treatment with metformin is associated with higher remission rate
in diabetic patients with thyroid cancer. J Clin Endocrinol Metab.
98:3269–3279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Romero IL, McCormick A, McEwen KA, Park S,
Karrison T, Yamada SD, Pannain S and Lengyel E: Relationship of
type II diabetes and metformin use to ovarian cancer progression,
survival, and chemosensitivity. Obstet Gynecol. 119:61–67. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Patel T, Hruby G, Badani K, Abate-Shen C
and McKiernan JM: Clinical outcomes after radical prostatectomy in
diabetic patients treated with metformin. Urology. 76:1240–1244.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Azoulay L, Dell'Aniello S, Gagnon B,
Pollak M and Suissa S: Metformin and the incidence of prostate
cancer in patients with type 2 diabetes. Cancer Epidemiol
Biomarkers Prev. 20:337–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wright JL and Stanford JL: Metformin use
and prostate cancer in Caucasian men: Results from a
population-based case-control study. Cancer Causes Control.
20:1617–1622. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
He XX, Tu SM, Lee MH and Yeung SC:
Thiazolidinediones and metformin associated with improved survival
of diabetic prostate cancer patients. Ann Oncol. 22:2640–2645.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Sadeghi N, Abbruzzese JL, Yeung SC, Hassan
M and Li D: Metformin use is associated with better survival of
diabetic patients with pancreatic cancer. Clin Cancer Res.
18:2905–2912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Noto H, Goto A, Tsujimoto T and Noda M:
Cancer risk in diabetic patients treated with metformin: A
systematic review and meta-analysis. PLoS One. 7:e334112012.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hosono K, Endo H, Takahashi H, Sugiyama M,
Sakai E, Uchiyama T, Suzuki K, Iida H, Sakamoto Y, Yoneda K, et al:
Metformin suppresses colorectal aberrant crypt foci in a short-term
clinical trial. Cancer Prev Res (Phila). 3:1077–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Freemark M and Bursey D: The effects of
metformin on body mass index and glucose tolerance in obese
adolescents with fasting hyperinsulinemia and a family history of
type 2 diabetes. Pediatrics. 107:E552001. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Pollak M: Insulin and insulin-like growth
factor signalling in neoplasia. Nat Rev Cancer. 8:915–928. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Goodwin PJ, Pritchard KI, Ennis M, Clemons
M, Graham M and Fantus IG: Insulin-lowering effects of metformin in
women with early breast cancer. Clin Breast Cancer. 8:501–505.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Memmott RM, Mercado JR, Maier CR, Kawabata
S, Fox SD and Dennis PA: Metformin prevents tobacco
carcinogen-induced lung tumorigenesis. Cancer Prev Res (Phila).
3:1066–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Algire C, Amrein L, Bazile M, David S,
Zakikhani M and Pollak M: Diet and tumor LKB1 expression interact
to determine sensitivity to anti-neoplastic effects of metformin in
vivo. Oncogene. 30:1174–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pollak M: Metformin and other biguanides
in oncology: Advancing the research agenda. Cancer Prev Res
(Phila). 3:1060–1065. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Karnevi E, Said K, Andersson R and
Rosendahl AH: Metformin-mediated growth inhibition involves
suppression of the IGF-I receptor signalling pathway in human
pancreatic cancer cells. BMC Cancer. 13:2352013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zakikhani M, Dowling R, Fantus IG,
Sonenberg N and Pollak M: Metformin is an AMP kinase-dependent
growth inhibitor for breast cancer cells. Cancer Res.
66:10269–10273. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Inoki K, Zhu T and Guan KL: TSC2 mediates
cellular energy response to control cell growth and survival. Cell.
115:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gwinn DM, Shackelford DB, Egan DF,
Mihaylova MM, Mery A, Vasquez DS, Turk BE and Shaw RJ: AMPK
phosphorylation of raptor mediates a metabolic checkpoint. Mol
Cell. 30:214–226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Efeyan A and Sabatini DM: mTOR and cancer:
Many loops in one pathway. Curr Opin Cell Biol. 22:169–176. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Dowling RJ, Zakikhani M, Fantus IG, Pollak
M and Sonenberg N: Metformin inhibits mammalian target of
rapamycin-dependent translation initiation in breast cancer cells.
Cancer Res. 67:10804–10812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zakikhani M, Dowling RJ, Sonenberg N and
Pollak MN: The effects of adiponectin and metformin on prostate and
colon neoplasia involve activation of AMP-activated protein kinase.
Cancer Prev Res (Phila). 1:369–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gotlieb WH, Saumet J, Beauchamp MC, Gu J,
Lau S, Pollak MN and Bruchim I: In vitro metformin anti-neoplastic
activity in epithelial ovarian cancer. Gynecol Oncol. 110:246–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Cantrell LA, Zhou C, Mendivil A, Malloy
KM, Gehrig PA and Bae-Jump VL: Metformin is a potent inhibitor of
endometrial cancer cell proliferation-implications for a novel
treatment strategy. Gynecol Oncol. 116:92–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Green AS, Chapuis N, Maciel TT, Willems L,
Lambert M, Arnoult C, Boyer O, Bardet V, Park S, Foretz M, et al:
The LKB1/AMPK signaling pathway has tumor suppressor activity in
acute myeloid leukemia through the repression of mTOR-dependent
oncogenic mRNA translation. Blood. 116:4262–4273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zheng L, Yang W, Wu F, Wang C, Yu L, Tang
L, Qiu B, Li Y, Guo L, Wu M, et al: Prognostic significance of AMPK
activation and therapeutic effects of metformin in hepatocellular
carcinoma. Clin Cancer Res. 19:5372–5380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Shi WY, Xiao D, Wang L, Dong LH, Yan ZX,
Shen ZX, Chen SJ, Chen Y and Zhao WL: Therapeutic metformin/AMPK
activation blocked lymphoma cell growth via inhibition of mTOR
pathway and induction of autophagy. Cell Death Dis. 3:e2752012.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ben Sahra I, Regazzetti C, Robert G, et
al: Metformin, independent of AMPK, induces mTOR inhibition and
cell-cycle arrest through REDD1. Cancer Res. 71:4366–4372. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Kalender A, Selvaraj A, Kim SY, Gulati P,
Brûlé S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et
al: Metformin, independent of AMPK, inhibits mTORC1 in a rag
GTPase-dependent manner. Cell Metab. 11:390–401. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Janjetovic K, Harhaji-Trajkovic L,
Misirkic-Marjanovic M, Vucicevic L, Stevanovic D, Zogovic N,
Sumarac-Dumanovic M, Micic D and Trajkovic V: In vitro and in vivo
anti-melanoma action of metformin. Eur J Pharmacol. 668:373–382.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yasmeen A, Beauchamp MC, Piura E, Segal E,
Pollak M and Gotlieb WH: Induction of apoptosis by metformin in
epithelial ovarian cancer: Involvement of the Bcl-2 family
proteins. Gynecol Oncol. 121:492–498. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zi FM, He JS, Li Y, Wu C, Yang L, Yang Y,
Wang LJ, He DH, Zhao Y, Wu WJ, et al: Metformin displays
anti-myeloma activity and synergistic effect with dexamethasone in
in vitro and in vivo xenograft models. Cancer Lett. 356:443–453.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Liang J and Mills GB: AMPK: A contextual
oncogene or tumor suppressor? Cancer Res. 73:2929–2935. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Hardie DG: The LKB1-AMPK pathway-friend or
foe in cancer? Cancer Cell. 23:131–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Park HU, Suy S, Danner M, Dailey V, Zhang
Y, Li H, Hyduke DR, Collins BT, Gagnon G, Kallakury B, et al:
AMP-activated protein kinase promotes human prostate cancer cell
growth and survival. Mol Cancer Ther. 8:733–741. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Baumann P, Mandl-Weber S, Emmerich B,
Straka C and Schmidmaier R: Inhibition of adenosine
monophosphate-activated protein kinase induces apoptosis in
multiple myeloma cells. Anticancer Drugs. 18:405–410. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Shackelford DB, Abt E, Gerken L, Vasquez
DS, Seki A, Leblanc M, Wei L, Fishbein MC, Czernin J, Mischel PS
and Shaw RJ: LKB1 inactivation dictates therapeutic response of
non-small cell lung cancer to the metabolism drug phenformin.
Cancer Cell. 23:143–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Liu X, Chhipa RR, Pooya S, Wortman M,
Yachyshin S, Chow LM, Kumar A, Zhou X, Sun Y, Quinn B, et al:
Discrete mechanisms of mTOR and cell cycle regulation by AMPK
agonists independent of AMPK. Proc Natl Acad Sci USA. 111:pp.
E435–E444. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Garcia A and Tisman G: Metformin, B(12),
and enhanced breast cancer response to chemotherapy. J Clin Oncol.
28:e19–e20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Ben Sahra I, Laurent K, Giuliano S,
Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y,
Giorgetti-Peraldi S, Cormont M, Bertolotto C, et al: Targeting
cancer cell metabolism: The combination of metformin and
2-deoxyglucose induces p53-dependent apoptosis in prostate cancer
cells. Cancer Res. 70:2465–2475. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Ben Sahra I, Tanti JF and Bost F: The
combination of metformin and 2 deoxyglucose inhibits autophagy and
induces AMPK-dependent apoptosis in prostate cancer cells.
Autophagy. 6:670–671. 2010. View Article : Google Scholar
|
|
108
|
Colquhoun AJ, Venier NA, Vandersluis AD,
Besla R, Sugar LM, Kiss A, Fleshner NE, Pollak M, Klotz LH and
Venkateswaran V: Metformin enhances the antiproliferative and
apoptotic effect of bicalutamide in prostate cancer. Prostate
Cancer Prostatic Dis. 15:346–352. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Rocha GZ, Dias MM, Ropelle ER,
Osório-Costa F, Rossato FA, Vercesi AE, Saad MJ and Carvalheira JB:
Metformin amplifies chemotherapy-induced AMPK activation and
antitumoral growth. Clin Cancer Res. 17:3993–4005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Rattan R, Graham RP, Maguire JL, Giri S
and Shridhar V: Metformin suppresses ovarian cancer growth and
metastasis with enhancement of cisplatin cytotoxicity in vivo.
Neoplasia. 13:483–491. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Iliopoulos D, Hirsch HA and Struhl K:
Metformin decreases the dose of chemotherapy for prolonging tumor
remission in mouse xenografts involving multiple cancer cell types.
Cancer Res. 71:3196–3201. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Hanna RK, Zhou C, Malloy KM, Sun L, Zhong
Y, Gehrig PA and Bae-Jump VL: Metformin potentiates the effects of
paclitaxel in endometrial cancer cells through inhibition of cell
proliferation and modulation of the mTOR pathway. Gynecol Oncol.
125:458–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Janjetovic K, Vucicevic L, Misirkic M,
Vilimanovich U, Tovilovic G, Zogovic N, Nikolic Z, Jovanovic S,
Bumbasirevic V, Trajkovic V and Harhaji-Trajkovic L: Metformin
reduces cisplatin-mediated apoptotic death of cancer cells through
AMPK-independent activation of Akt. Eur J Pharmacol. 651:41–50.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Donnenberg VS and Donnenberg AD: Multiple
drug resistance in cancer revisited: The cancer stem cell
hypothesis. J Clin Pharmacol. 45:872–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Eyler CE and Rich JN: Survival of the
fittest: Cancer stem cells in therapeutic resistance and
angiogenesis. J Clin Oncol. 26:2839–2845. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hirsch HA, Iliopoulos D, Tsichlis PN and
Struhl K: Metformin selectively targets cancer stem cells, and acts
together with chemotherapy to block tumor growth and prolong
remission. Cancer Res. 69:7507–7511. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Cufi S, Corominas-Faja B, Vazquez-Martin
A, Oliveras-Ferraros C, Dorca J, Bosch-Barrera J, Martin-Castillo B
and Menendez JA: Metformin-induced preferential killing of breast
cancer initiating CD44+CD24-/low cells is sufficient to overcome
primary resistance to trastuzumab in HER2+ human breast cancer
xenografts. Oncotarget. 3:395–398. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Song CW, Lee H, Dings RP, Williams B,
Powers J, Santos TD, Choi BH and Park HJ: Metformin kills and
radiosensitizes cancer cells and preferentially kills cancer stem
cells. Sci Rep. 2:3622012. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shank JJ, Yang K, Ghannam J, Cabrera L,
Johnston CJ, Reynolds RK and Buckanovich RJ: Metformin targets
ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol.
127:390–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Bao B, Wang Z, Ali S, Ahmad A, Azmi AS,
Sarkar SH, Banerjee S, Kong D, Li Y, Thakur S and Sarkar FH:
Metformin inhibits cell proliferation, migration and invasion by
attenuating CSC function mediated by deregulating miRNAs in
pancreatic cancer cells. Cancer Prev Res (Phila). 5:355–364. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Mayer MJ, Klotz LH and Venkateswaran V:
Metformin and prostate cancer stem cells: A novel therapeutic
target. Prostate Cancer Prostatic Dis. 18:303–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Di Francesco AM, Toesca A, Cenciarelli C,
Giordano A, Gasbarrini A and Puglisi MA: Metabolic modification in
gastrointestinal cancer stem cells: Characteristics and therapeutic
approaches. J Cell Physiol. 231:2081–2087. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Florio T: Antitumoral effects of metformin
on cancer stem cells. Ann d'Endocrinologie. 76:2962015. View Article : Google Scholar
|
|
125
|
Rattan R, Ali Fehmi R and Munkarah A:
Metformin: An emerging new therapeutic option for targeting cancer
stem cells and metastasis. J Oncol. 2012:9281272012. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Bednar F and Simeone DM: Metformin and
cancer stem cells: Old drug, new targets. Cancer Prev Res (Phila).
5:351–354. 2012. View Article : Google Scholar : PubMed/NCBI
|