Introduction
Pseudolaric acid B (PAB) is a diterpene acid
isolated from the root and trunk bark of Pseudolarix kaempferi
Gordon (Pinaceae), known as ‘Tu-jin-pi’ in Chinese,
which is used to treat dermatological fungi infections. PAB has
been demonstrated to exert potent cell growth inhibition in
vitro in various tumor cell lines through cell cycle arrest,
apoptosis or autophagy (1–6), although the mechanisms for this effect
have yet to be completely characterized.
Apoptosis has been the focus of a large volume of
research regarding anti-tumor drug development (7,8). The major
mechanisms of apoptosis include the extrinsic or Fas death receptor
pathway, which activates caspase-8 and −10 in response to external
stimuli (9) and the intrinsic or
mitochondrial pathway, which induces the cleavage of pro-caspase-9
in response to internal stimuli (10). In mammalian cells, mitogen-activated
protein kinases, including stress-activated protein kinase,
c-Jun-N-terminal kinase (Jnk), p38 and extracellular
signal-regulated kinase (Erk), are associated with cell death or
proliferation (11). Generally, the
expression of Erk promotes inflammation, apoptosis, cell growth,
differentiation and oncogenic transformation, whereas Jnk and p38
are implicated in cell growth and differentiation, and development
(12,13).
In addition to apoptosis, autophagy has also been
studied as an anti-cancer drug mechanism. Autophagy is the process
by which cellular components are delivered to lysosomes for bulk
degradation (14). In some cases,
autophagy may promote cell death, but autophagy typically promotes
cell survival by enabling cells to adapt to stress conditions
(15). The inhibition of apoptosis by
autophagy has also been demonstrated to decrease the effect of
antitumor drugs (16).
In the present study, it was demonstrated that the
PAB treatment of MRC5 cells induced autophagy, and not apoptosis.
Inhibiting autophagy promoted apoptosis through the upregulation of
phosphorylated (p)-Jnk expression and the downregulation of p-Erk,
whereas inhibiting autophagy had no effect on cell cycle arrest or
microtubule aggregation as induced by PAB. Therefore, inhibiting
autophagy did not affect the role of PAB in microtubule aggregation
and promoted cell apoptosis; this may present a strategy for the
application of PAB against tumors.
Materials and methods
Materials
PAB (National Institute for the Control of
Pharmaceutical and Biological Products, Beijing, China) was
dissolved in dimethyl sulfoxide (DMSO) to produce a stock solution.
DMSO concentration was maintained below 0.01% in all cell culture
to prevent any detectable effect on cell growth or death. Propidium
iodide (PI), phalloidin-tetramethylrhodamine B isothiocyanate,
monodansylcadaverine (MDC), 3-methyladenine (3MA), Hoechst 33258
and RNase A were purchased from Sigma-Alrich (Merck KGaA,
Darmstadt, Germany). TRIzol® reagent was purchased from
Invitrogen and the SuperScript™ III RT-PCR kit was from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The Power SYBR
Green PCR Master mix was acquired from Applied Biosystems (Thermo
Fisher Scientific, Inc). The mouse light chain (LC) 3A/B monoclonal
(cat. no., 66139-1-AP), and rabbit Beclin-1 (cat. no., 11306-1-AP),
Bcl-2 (cat. no., 12789-1-AP), ERK1/2 (cat. no., 16443-1-AP) and Bax
(cat. no., 50599-2-Ig) were purchased from ProteinTech Group, Inc.
(Chicago, IL, USA). The rabbit histone H3 antibody was from
GenScript (cat. no., A01502-40, Piscataway, NJ, USA). JNK1/2 (cat.
no., BA1648, MAPK8/9) antibody and MAPK14 (cat. no., BM4142, p38)
antibody were from Boster Biological Technology (Pleasanton, CA,
USA). Antibodies against caspase-3 (cat. no., SC-373730), caspase-8
(cat. no., SC-6136) and caspase-9 (cat. no., SC-8355), p-p38 (cat.
no., SC-7973, D-8), p-Jnk (SC-6254, G-7) and p-Erk (cat. no.,
SC-9477, T-19), and alkaline phosphatase (AP) labeled-secondary
antibodies (cat. nos., SC-358915 and SC-2057) were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
MRC5 human lung fibroblast cells (cat. no., CCL-171)
were obtained from American Type Culture Collection (Manassas, VA,
USA) and were cultured in DMEM medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal calf serum, 2
mM glutamine (both Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin. The cells were maintained at
37°C with 5% CO2 in a humidified atmosphere.
Observation of morphological changes
by light microscopy
MRC5 cells (5×105 cells/well) were
cultured in 6 well plates for 24 h. Then 4 µM PAB and/or 2 mM 3MA
were added, and the cells were incubated for a further 36 h. Cell
morphology was observed with phase contrast microscopy (Leica
Microsystems GmbH, Wetzlar, Germany).
Determination of DNA fragmentation by
agarose gel electrophoresis
Cells were trypsinized; adherent and floating cells
were collected by centrifugation at 1,000 × g at 4°C for 5 min.
Further procedures were performed as described in a previous study
(5).
Fluorescence staining of microtubule
aggregation
MRC5 cells (5×105) were placed on cover
slips in a 6-well plate. Following a 24-h incubation, they were
treated with 4 µM PAB and/or 2 mM 3MA for 36 h, washed with PBS,
fixed in 3.7% formaldehyde, then rinsed three times in PBS.
TritonX-100 (0.8%) was added for 15 min, then cells were stained
with 5 mg/ml phalloidin-tetramethylrhodamine B isothiocyanate for
40 min, rinsed once in PBS and stained with 5 mg/l Hoechst 33258
for 30 min. The intensity of red staining was measured by
fluorescence microscopy with an excitation wavelength of 584 nm and
an emission filter of 607 nm (Leica Microsystems GmbH). Changes in
nuclear morphology were observed by fluorescence microscopy at the
excitation wavelength of 350 nm and an emission filter of 460 nm
(Leica Microsystems GmbH).
Observation of MDC staining by
fluorescence microscopy
MDC is a fluorescent compound that stains autophagic
vacuoles. MRC5 cells were treated with 4 µM PAB and/or 2 mM 3MA for
36 h, then were incubated with 0.05 mM MDC at 37°C for 1 h.
Following the incubation, cells were washed in PBS. Intracellular
MDC was measured by fluorescence microscopy at an excitation
wavelength of 380 nm and an emission filter of 525 nm (Leica
Microsystems GmbH).
Cell counting
Trypan Blue (Sigma-Aldrich; Merck KGaA) was used as
a vital stain. Live cells appeared colorless and bright
(refractile) under phase contrast and dead cells were stained blue
and were non-refractile. Following staining with a final
concentration of 0.2% trypan blue for 3 min at room temperature,
live cells were visualized in four quadrants and counted using a
hemocytometer with phase contrast microscopy (Leica Microsystems
GmbH, Wetzlar, Germany).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from control and drug-treated
cells using TRIzol as specified by the manufacturer's protocol. The
RNA was treated with DNase (DNase I-RNase-Free; Ambion; Thermo
Fisher Scientific, Inc.) to remove any contaminating DNA; 200 ng of
total RNA was reverse-transcribed with oligo dT primers using the
SuperScript™ III RT-PCR kit (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in a 20 µl reaction, as specified by the
manufacturer's protocol. For quantitative PCR, the template cDNA
was added to a 20 µl reaction with Power SYBR-Green PCR Master mix
and 0.2 µM of primers for the target genes and GAPDH.
The primer sequences were as follows: LC3A forwards,
GCC TTT CAA GCA GCG GCG GAG C, and reverse, TTG GTC TTG TCC AGG ACG
GGC A; LC3B forwards, CAG CGT CTC CAC ACC AAT CTC A, and reverse,
AAT TTC ATC CCG AAC GTC TCC T; Beclin-1 forwards, CTC CAT TAC TTA
CCA CAG CCC A, and reverse, GGA TGA ATC TGC GAG AGA CAC C; GAPDH
forwards, GCA AAT TCC ATG GCA CCG T, and reverse, TCG CCC CAC TTG
ATT TTG G.
Amplification was performed with a Prism 7000
machine (Applied Biosystems; Thermo Fisher Scientific, Inc.), with
the following conditions: Initial denaturation at 95°C for 10 min,
then 40 cycles of 95°C for 15 sec followed by 60°C for 1 min. The
fold changes relative to GAPDH were calculated using the
2−∆∆Cq method (17).
Cell cycle analysis by flow
cytometry
Nuclear DNA content was measured using PI staining
and fluorescence-activated cell sorting (FACS). Adherent cells were
collected by treatment with trypsin and washed with PBS. Cells were
fixed in 1 ml of 70% ethanol overnight at 4°C and resuspended in
staining buffer (50 µg/ml PI and 20 µg/ml RNase, in PBS) for 2 h at
4°C. The PI-stained cells were then analyzed using FACS (FACScan;
BD Biosciences, Franklin Lakes, NJ, USA); ≤104 cells
were counted for each sample. Data analysis was performed using
ModFit LT version 2.0 (Verity Software House, Inc., Topsham, ME,
USA) (18,19).
Western blot analysis of protein
expression
MRC5 cells (106) were cultured in in a
25-ml culture flask for 24 h, then treated with 4 µM PAB and/or 2
mM 3MA for 36 h. Adherent and floating cells were collected and
frozen at −80°C. Western blot analysis was performed as described
in previous reports (5,20–23).
Protein expression was detected using the aforementioned primary
antibodies (dilution, 1:1,000) followed by a corresponding
AP-conjugated secondary antibody (dilution, 1:1,000). Proteins were
visualized using nitro-blue tetrazolium and
5-bromo-4-chloro-3-indolyl phosphate.
Statistical analysis
All data represent ≥3 independent experiments, and
are expressed as the mean ± standard deviation. Statistical
comparisons were made using Student's t-test in Microsoft Excel for
the data in Fig. 2, and using a
one-way analysis of variance with Dunnett's post-hoc test for
Figs. 3 and 5 with SPSS 10.0 (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
PAB does not induce apoptosis in MRC5
cells
In order to identify whether apoptosis was induced
in MRC5 cells by treatment with PAB, the cellular and nuclear
morphology was observed. At 36 h, no characteristics of apoptosis,
including the appearance of apoptotic bodies or cell condensation,
were observed in PAB-treated cells (Fig.
1A). Following 36 h of PAB treatment, cell nuclei did not
appear brighter, indicating that there was no induction of
apoptosis, although multinuclear cells were observed (arrow,
Fig. 1B). In the DNA ladder test, no
DNA ladder was visible following 36 h of PAB treatment (Fig. 1C). Therefore, it was concluded that
PAB treatment did not induce the apoptosis of MRC5 cells.
PAB treatment induces autophagy in
MRC5 cells
It was identified in our previous study that
autophagy was induced by PAB treatment in the cells where apoptosis
did not occur (4), so the rate of
autophagy was assessed. It was observed that treatment with 4 µM
PAB increased the number of MDC positive points, indicative of
autophagy (Fig. 2A). It was also
identified that at 36 h, LC3I and Beclin-1 protein expression were
increased, and LC3I was converted into LC3II, which were also
indications of autophagy (Fig. 2B).
The mRNA levels of LC3A, LC3B and Beclin-1 were also quantified to
ascertain whether they corresponded with the increase in protein
expression; it was demonstrated that treatment with PAB did not
significantly increase the mRNA levels for these genes relative to
the control treatment group. The relative mRNA level of the PAB
treatment group was 0.80±0.05 for LC3A, 0.62±0.21 for LC3B and
0.84±0.09 for Beclin-1 compared with the control group (Fig. 2C). Therefore, PAB induced autophagy
through upregulating autophagy-associated protein expression,
whereas it did not affect the transcription of autophagy-associated
genes.
Inhibiting autophagy promotes
apoptosis in MRC5 cells treated with PAB
It was identified in our previous study that
inhibiting autophagy subsequent to PAB treatment promoted apoptosis
(4); it was assessed in the present
study whether this also occurred in MRC5 cells. It was identified
that PAB treatment increased the intensity of MDC staining; when
combined with 3MA, an autophagy inhibitor, the intensity of MDC
staining was decreased compared with PAB treatment alone (Fig. 3A). It was therefore demonstrated that
3MA inhibited the induction of autophagy by PAB treatment.
It was determined by morphological analysis that
there was no induction of apoptosis subsequent to PAB treatment
alone, whereas following treatment with a combination of 3MA and
PAB, cells detaching from the base could be observed (black arrow,
Fig. 3B). At 36 h, the total number
of viable cells was 54.75±1.71 in the PAB-treated group and
102.50±2.08 for the control group (Fig.
3C; P<0.001). In the PAB and 3MA combination group, the
number of viable cells was 36.00±2.58, which was significantly
lower than in the PAB treatment group (Fig. 3C; P<0.001). Therefore, it was
concluded that inhibiting autophagy may have promoted cell death.
It was finally determined whether inhibiting autophagy promoted
apoptosis with a DNA ladder test; it was confirmed that following
PAB treatment, there was no DNA ladder, indicating no apoptosis,
whereas when combined with 3MA, PAB treatment induced the
appearance of a DNA ladder (Fig. 3D).
Therefore, inhibiting autophagy promoted apoptosis in the MRC5
cells treated with PAB.
Apoptosis is induced in MRC5 cells
treated with PAB and 3MA via the upregulation of p-Jnk and the
downregulation of p-Erk
As it had been demonstrated that 3MA inhibited
autophagy and promoted apoptosis in MRC5 cells co-treated with PAB,
the mechanism by which 3MA prevented autophagy and induced
apoptosis was investigated. PAB treatment upregulated the
expression of the autophagy-associated proteins Beclin-1 and LC
3A/B. Following treatment with 3MA, the upregulation of Beclin-1
and LC 3A/B expression by PAB treatment was inhibited (Fig. 4A).
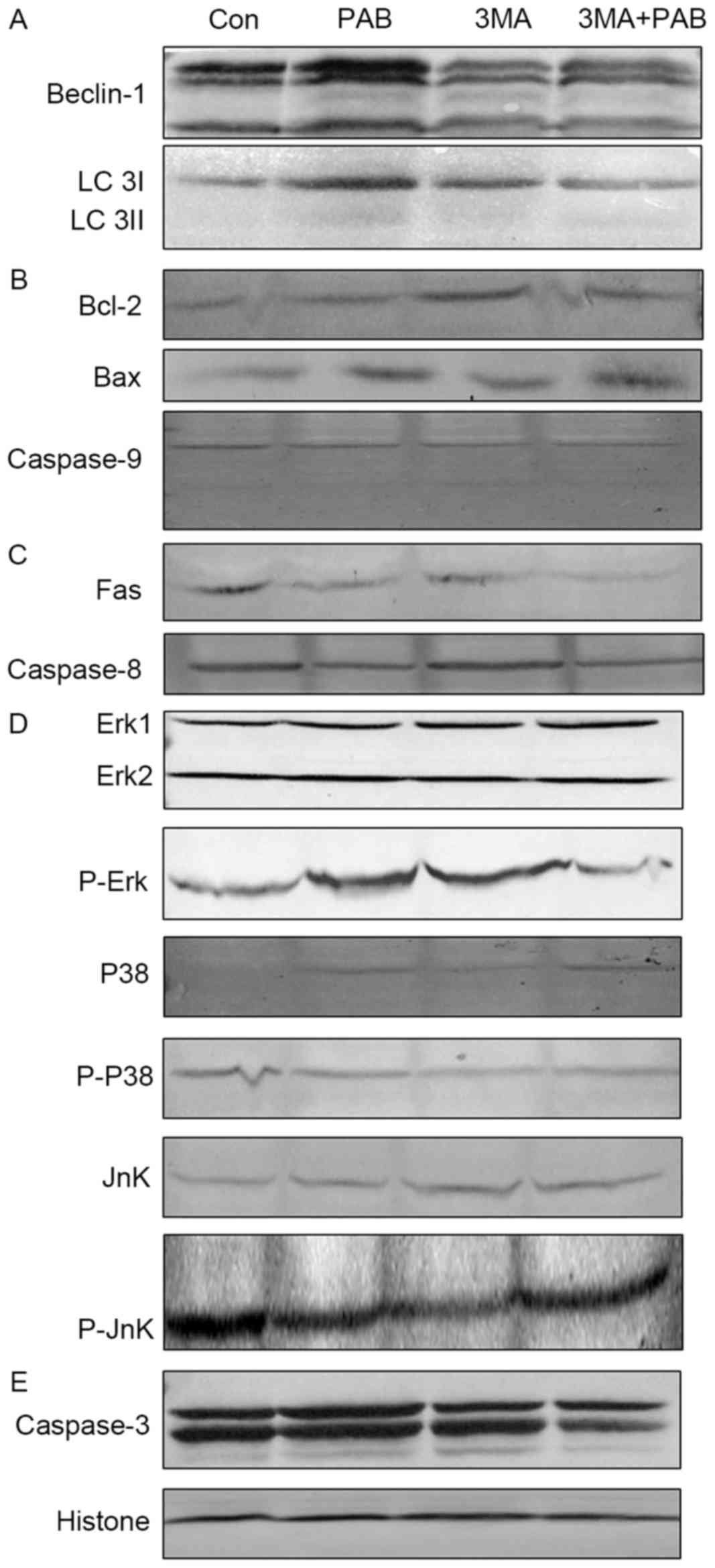 | Figure 4.Expression of apoptosis-associated
proteins following treatment with PAB and/or 3MA. (A) Beclin-1 and
LC 3 expression. (B) Bcl-2, Bax and pro-caspase-9 expression. (C)
Fas and pro-caspase-8 expression. (D) p38, p-p38, Jnk, p-Jnk, Erk
and p-Erk expression. (E) Pro-caspase-3 expression, and histone H3
as a loading control. PAB, pseudolaric acid B; 3MA,
3-methyladenine; LC 3, light chain 3; Bax, Bcl-2 associated X; p,
phosphorylated; Jnk, Jun N-terminal kinase; Erk, extracellular
signal-related kinase; Con, control. |
The role of the mitochondrial pathway in PAB and/or
3MA treated cells was assessed; compared with PAB treatment, PAB
and 3MA co-treatment did not affect the expression of Bcl-2, Bax or
pro-caspase-9 (Fig. 4B); it was
therefore concluded that 3MA promoted the apoptosis of PAB-treated
cells dependent of the mitochondrial pathway.
The expression of proteins from the death
receptor-dependent pathway in PAB and/or 3MA treated cells was also
considered, and it was confirmed that compared with the PAB
treatment group, the PAB and 3MA combination group exhibited
similar expression of Fas and pro-caspase-8 (Fig. 4C); it was therefore concluded that 3MA
promoted the apoptosis of PAB-treated cells dependent of the death
receptor pathway.
Finally, the effects on the MAPK pathway of PAB
and/or 3MA treatment were investigated; it was observed that
compared with PAB treatment alone, PAB combined with 3MA did not
alter the expression of p38, p-p38, Jnk or Erk, whereas the
expression of p-Jnk and p-Erk was altered (Fig. 4D); therefore, it was concluded that
3MA treatment upregulated the phosphorylation of Jnk and
downregulated the phosphorylation of Erk to promote apoptosis in
PAB-treated cells. It was noted that the expression of
pro-caspase-3 was reduced in the combination treatment group
compared with PAB alone (Fig. 4E);
therefore, 3MA promoted caspase-3 activation independent of
caspase-8 and −9.
Inhibiting autophagy does not affect
the aggregation of microtubule fibers induced by PAB
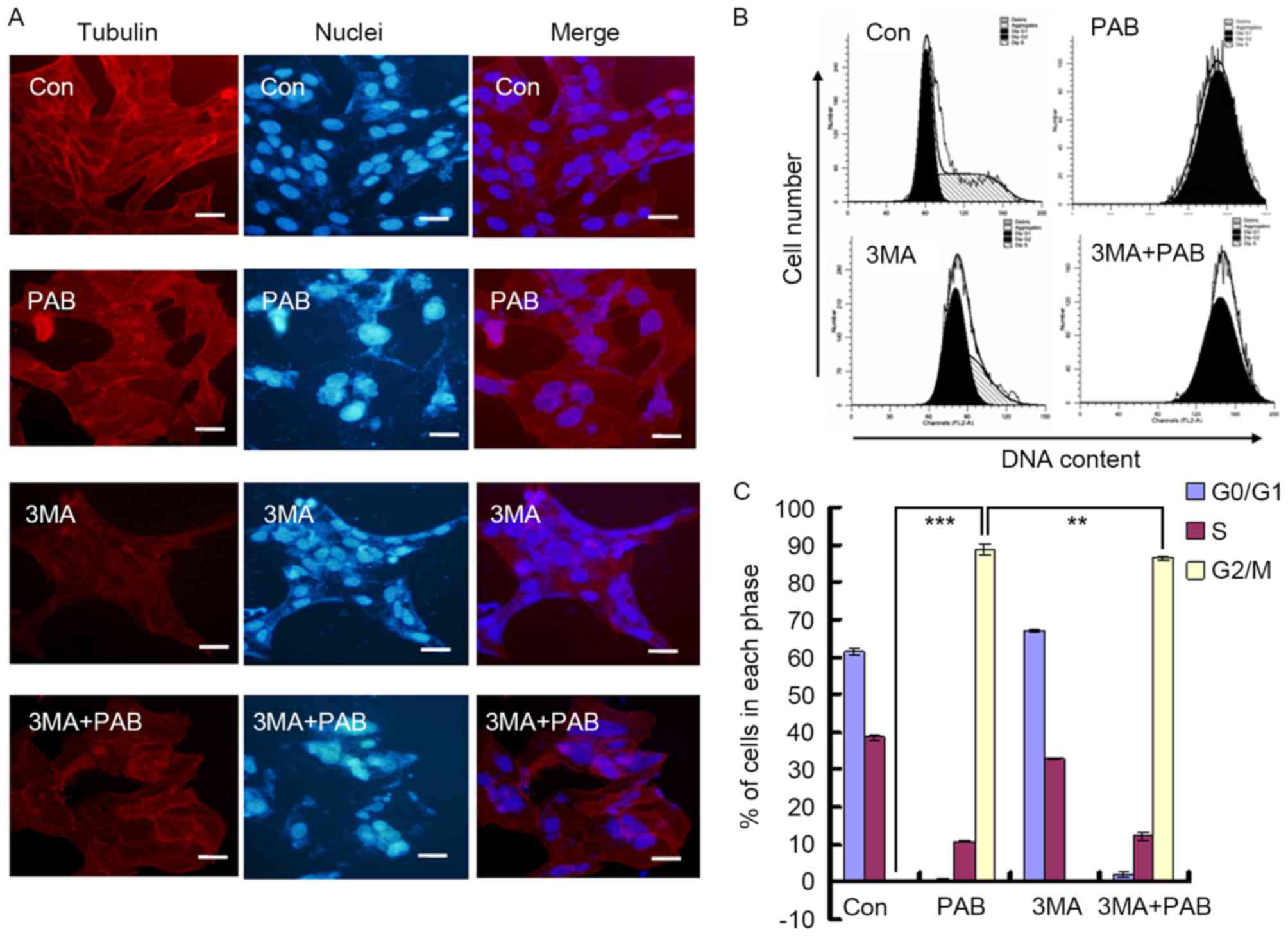
In a previous study, it was identified that the
function of PAB was mediated through affecting the aggregation of
microtubule fibers (24). In the
present study, it was observed that PAB treatment promoted the
aggregation of microtubule fibers; combination treatment with 3MA
did not prevent this effect (Fig.
5A). PAB treatment induced G2/M cell cycle arrest in
MRC5 cells (G2/M proportion, 88.78±1.34%), whereas 3MA
decreased the proportion of G2/M phase cells following
treatment with PAB (86.44±0.66%; P<0.01; Fig. 5B and C). Therefore, the inhibition of
autophagy had no effect on the aggregation of microtubule fibers,
whereas the extent of cell cycle arrest induced by PAB was
reduced.
Discussion
Autophagy is an intracellular pathway for the bulk
degradation of damaged proteins and organelles in the
lysosome/vacuole that recycles material for biosynthesis and
cellular energy production in stress conditions (25). Its role in cancer is complex and
controversial; it may act as a tumor-promoting or a
tumor-suppressive mechanism depending on the cellular context and
genetic background (26). Autophagy
and apoptosis may interact with each other, and a number of the
molecular constituents in this interplay have been identified
(27,28).
In previous studies, it was demonstrated that PAB
induced the apoptosis of A375-S2 human melanoma (2), MCF-7 human breast cancer (5) and HeLa human cervical carcinoma
(1) cells. However, in L929 murine
fibrosarcoma (4) and SW579 human
thyroid squamous cell carcinoma (22)
cells, PAB induced autophagy without apoptosis. The cells in which
autophagy was induced without apoptosis were considered as
potential models for the study of the association of apoptosis and
autophagy. As L929 cells are murine (4), they were considered imperfect for the
study of the mechanisms in human cells; as SW579 cells are cultured
without CO2 (22), they
were considered relatively inconvenient. Therefore, MRC5 cells were
selected for use as a model in the present study to study the
association between autophagy and apoptosis following PAB
treatment, as the MRC5 cell line is human and it has a
proliferative ability, which mimics human cancer cell behavior.
In the present study, it was confirmed through cell
and nuclear morphology analysis, and a DNA ladder test, that PAB
did not induce apoptosis in MRC5 cells. It was also confirmed,
through MDC staining, and the determination of autophagy-associated
protein and mRNA expression, that PAB induced autophagy. It was
therefore concluded that in MRC5 cells, PAB only induced autophagy,
and not apoptosis. To the best of our knowledge, the mechanism by
which PAB did not induce apoptosis in SW579, L929 and MRC5 cells
was never previously identified. We hypothesized that the cell
lines lacked the expression of a key factor in the initiation of
apoptosis; therefore, when PAB treatment activated an upstream
signal, autophagy occurred without apoptosis.
Autophagy typically delays or prevents apoptosis,
although it may also promote apoptosis (29). In addition to apoptosis, autophagy may
affect tumor immunity; it has been identified that cancer cell
autophagy serves a critical function in subverting anti-tumor
immunity (30). To further establish
a relationship between apoptosis and autophagy, 3MA was used to
inhibit the autophagy induced by PAB in the present study. It was
revealed that inhibiting autophagy promoted apoptotic cell death.
Therefore, it was demonstrated that in MRC5 cells treated with PAB,
autophagy inhibited apoptosis. It has thus been identified that
L929, SW579 and MRC5 cells undergo autophagy without apoptosis as a
response to PAB treatment; however, the common characteristics of
these cells that may underlie this effect have yet to be identified
and require further study. As autophagy inhibits anti-tumor
immunity (30) in addition to
apoptosis, in the development of anti-tumor drugs, including PAB,
inhibiting autophagy may be important.
The mechanisms for the inhibition of autophagy and
the promotion of apoptosis were investigated in the present study
by examining the expression of proteins from the major pathways of
apoptosis. Subsequent to the inhibition of autophagy with 3MA in
PAB-treated cells, the expression of proteins from the
mitochondrial and death receptor pathways was not altered compared
with treatment with PAB alone, whereas the expression of caspase-3
was activated. It was therefore concluded that caspase-3 activation
was not dependent on the mitochondrial or death receptor pathways
of apoptosis. It was identified that the expression of p38 was
unaffected, whereas Jnk was activated and Erk was inactivated,
following 3MA treatment in PAB treated cells compared with PAB
alone, therefore 3MA may have inhibited autophagy and promoted
apoptosis through the upregulation of p-Jnk expression and the
downregulation of p-Erk expression, and caspase-3 activation might
be the downstream of Jnk and Erk protein.
The effect of PAB on microtubules and the induction
of cell cycle arrest was previously identified in other cell lines
(24,31). In the present study, the aggregation
of microtubules and cell cycle arrest were also identified as a
response to PAB treatment, but following the inhibition of
autophagy by 3MA, the aggregation of microtubules was unaffected,
and the proportion of cells in G2/M was only decreased
from 88.78 to 86.44%. Therefore, autophagy may be downstream of the
aggregation of microtubules and cell cycle arrest. In conclusion,
inhibiting autophagy did not affect the microtubule aggregation
role of PAB and promoted cell apoptosis. Inhibiting autophagy
should be considered as a means to enhance the anticancer effect of
PAB treatment.
Acknowledgements
The present study was supported by funding from the
National Natural Science Foundation of China (grant no., 81301416),
the Postdoctoral Science Foundation of China (grant nos,
2014M561302 and 2015T80299), the Norman Bethune Program of Jilin
University (grant no, 2015202), the Jilin Provincial Science and
Technology Department (grant nos, 20140204004YY and 20160414025GH),
and the Department of Human Resources and Social Security of Jilin
Province (grant no., 2016014).
References
|
1
|
Gong X, Wang M, Tashiro S, Onodera S and
Ikejima T: Involvement of JNK-initiated p53 accumulation and
phosphorylation of p53 in pseudolaric acid B induced cell death.
Exp Mol Med. 38:428–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong XF, Wang MW, Tashiro S, Onodera S and
Ikejima T: Pseudolaric acid B induces apoptosis through p53 and
Bax/Bcl-2 pathways in human melanoma A375-S2 cells. Arch Pharm Res.
28:68–72. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pan DJ, Li ZL, Hu CQ, Chen K, Chang JJ and
Lee KH: The cytotoxic principles of Pseudolarix kaempferi:
Pseudolaric acid-A and -B and related derivatives. Planta Med.
56:383–385. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu J, Li X, Tashiro S, Onodera S and
Ikejima T: Bcl-2 family proteins were involved in pseudolaric acid
B-induced autophagy in murine fibrosarcoma L929 cells. J Pharmacol
Sci. 107:295–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yu JH, Cui Q, Jiang YY, Yang W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces apoptosis,
senescence, and mitotic arrest in human breast cancer MCF-7. Acta
Pharmacol Sin. 28:1975–1983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu JH, Wang HJ, Li XR, Tashiro S, Onodera
S and Ikejima T: Protein tyrosine kinase, JNK, and ERK involvement
in pseudolaric acid B-induced apoptosis of human breast cancer
MCF-7 cells. Acta Pharmacol Sin. 29:1069–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li FF, Yi S, Wen L, He J, Yang LJ, Zhao J,
Zhang BP, Cui GH and Chen Y: Oridonin induces NPM mutant protein
translocation and apoptosis in NPM1c+ acute myeloid leukemia cells
in vitro. Acta Pharmacol Sin. 35:806–813. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qi M, Yao G, Fan S, Cheng W, Tashiro S,
Onodera S and Ikejima T: Pseudolaric acid B induces mitotic
catastrophe followed by apoptotic cell death in murine fibrosarcoma
L929 cells. Eur J Pharmacol. 683:16–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Adams JM: Ways of dying: Multiple pathways
to apoptosis. Genes Dev. 17:2481–2495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagata S and Golstein P: The Fas death
factor. Science. 267:1449–1456. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Klekotka PA, Santoro SA, Wang H and Zutter
MM: Specific residues within the alpha 2 integrin subunit
cytoplasmic domain regulate migration and cell cycle progression
via distinct MAPK pathways. J Biol Chem. 276:32353–32361. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoon S and Seger R: The extracellular
signal-regulated kinase: Multiple substrates regulate diverse
cellular functions. Growth Factors. 24:21–44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee YJ, Won AJ, Lee J, Jung JH, Yoon S,
Lee BM and Kim HS: Molecular mechanism of SAHA on regulation of
autophagic cell death in tamoxifen-resistant MCF-7 breast cancer
cells. Int J Med Sci. 9:881–893. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YZ, Yang CW, Chang HY, Hsu HY, Chen
IS, Chang HS, Lee CH, Lee JC, Kumar CR, Qiu YQ, et al: Discovery of
selective inhibitors of Glutaminase-2, which inhibit mTORC1,
activate autophagy and inhibit proliferation in cancer cells.
Oncotarget. 5:6087–6101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He H, Feng YS, Zang LH, Liu WW, Ding LQ,
Chen LX, Kang N, Hayashi T, Tashiro S, Onodera S, et al: Nitric
oxide induces apoptosis and autophagy; autophagy down-regulates NO
synthesis in physalin A-treated A375-S2 human melanoma cells. Food
Chem Toxicol. 71:128–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang ZY, Zhong T, Wang Y, Song FM and Yu
XF, Xing LP, Zhang WY, Yu JH, Hua SC and Yu XF: Human enterovirus
68 interferes with the host cell cycle to facilitate viral
production. Front Cell Infect Microbiol. 7:292017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhong T, Zhang LY, Wang ZY, Wang Y, Song
FM, Zhang YH and Yu JH: Rheum emodin inhibits enterovirus 71 viral
replication and affects the host cell cycle environment. Acta
Pharmacol Sin. 38:392–401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu YQ, Han XF, Bo JX and Ma HP:
Wedelolactone enhances osteoblastogenesis but inhibits
osteoclastogenesis through Sema3A/NRP1/PlexinA1 pathway. Front
Pharmacol. 7:3752016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu J, Chen C, Xu T, Yan M, Xue B, Wang Y,
Liu C, Zhong T, Wang Z, Meng X, et al: Pseudolaric acid B activates
autophagy in MCF-7 human breast cancer cells to prevent cell death.
Oncol Lett. 11:1731–1737. 2016.PubMed/NCBI
|
|
22
|
Yu J, Ren P, Zhong T, Wang Y, Yan M, Xue
B, Li R, Dai C, Liu C, Chen G and Yu XF: Pseudolaric acid B
inhibits proliferation in SW579 human thyroid squamous cell
carcinoma. Mol Med Rep. 12:7195–7202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Wang Z, Ren P, Zhong T, Wang Y, Song
F, Hou J, Yu X and Hua S: Pseudolaric acid B inhibits the secretion
of hepatitis B virus. Oncol Rep. 37:519–525. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong VK, Chiu P, Chung SS, Chow LM, Zhao
YZ, Yang BB and Ko BC: Pseudolaric acid B, a novel
microtubule-destabilizing agent that circumvents multidrug
resistance phenotype and exhibits antitumor activity in vivo. Clin
Cancer Res. 11:6002–6011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Feng Y, He D, Yao Z and Klionsky DJ: The
machinery of macroautophagy. Cell Res. 24:24–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galluzzi L, Pietrocola F, Bravo-San Pedro
JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J,
Gewirtz DA, Karantza V, et al: Autophagy in malignant
transformation and cancer progression. EMBO J. 34:856–880. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crighton D, Wilkinson S, O'Prey J, Syed N,
Smith P, Harrison PR, Gasco M, Garrone O, Crook T and Ryan KM:
DRAM, a p53-induced modulator of autophagy, is critical for
apoptosis. Cell. 126:121–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Behrends C, Sowa ME, Gygi SP and Harper
JW: Network organization of the human autophagy system. Nature.
466:68–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mariño G, Niso-Santano M, Baehrecke EH and
Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salah FS, Ebbinghaus M, Muley VY, Zhou Z,
Al-Saadi KR, Pacyna-Gengelbach M, O'Sullivan GA, Betz H, König R,
Wang ZQ, et al: Tumor suppression in mice lacking GABARAP, an
Atg8/LC3 family member implicated in autophagy, is associated with
alterations in cytokine secretion and cell death. Cell Death Dis.
7:e22052016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tong YG, Zhang XW, Geng MY, Yue JM, Xin
XL, Tian F, Shen X, Tong LJ, Li MH, Zhang C, et al: Pseudolarix
acid B, a new tubulin-binding agent, inhibits angiogenesis by
interacting with a novel binding site on tubulin. Mol Pharmacol.
69:1226–1233. 2006. View Article : Google Scholar : PubMed/NCBI
|