Introduction
Malignant melanoma is a malignant tumor with a high
mortality rate that originates from melanocytes (1). In the United States, the incidence of
melanoma makes it the fifth-most-common tumor type, accounting for
5% of all cancers detected in 2013; and the number of newly
diagnosed cases reached 73,870 in the year 2015 (2–4). In
addition, in Queensland, Australia, the area where the highest
incidence of malignant melanoma is seen, the annual incidence rate
of this cancer in the female population was 55.8 per 10 million,
and in the male population was 41.1 per 10 million (5). In China, despite the low incidence of
malignant melanoma, the rate of incidence and death has increased
over the years and this fact has attracted widespread concern
(6). In the early stages of malignant
melanoma, the five-year survival rate of patients is as high as 98%
(7). However, malignant melanoma
easily metastasize to regional lymph nodes, and this is associated
with a poor prognosis due to a lack of effective interventions. The
overall survival of patients thus affected is only 6–9 months, and
the 5-year survival rate is only 16% (8,9). Because
malignant melanoma that has metastasized is not amenable to
conventional radiotherapy and chemotherapy, it is particularly
important to identify relvant molecular markers for early diagnosis
and treatment.
In recent years, researchers have come to realize
the importance of microRNA in the treatment of cancer. MicroRNA
(miRNA) is a short sequence of non-coding RNA that has a length of
21–25 nucleotides. It can regulate the expression of target genes
involved in cell growth, proliferation, apoptosis, metastasis, cell
cycle progression, and differentiation (10,11) by
specific inhibition of translation or degradation of target mRNA
(12). Evidence increasingly suggests
that abnormal regulation of various microRNAs is closely related to
the occurrence and development of tumors (11,13).
miR-150 was reported to be associated with the progression of a
variety of tumors. The reduction of miR-150 expression in
non-small-cell carcinoma can inhibit tumor cell proliferation and
induce cell apoptosis (14). miR-150
can indirectly activate the VEGF signaling pathway, and inhibition
of the expression of miR-150 has an anti-tumor effect (15). miR-150 is highly expressed in gastric
cancer and plays an important role in tumor progression via its
effect on the tumor suppressor gene EGR2 (16).
It has been demonstrated that miR-150 is expressed
at high levels in malignant melanoma and is associated with
decreased long-term survival of metastatic melanoma patients
(17–19). There is a positive association between
miR-150 upregulation and a lowered risk of recurrence, and this
suggests an important role for miR-150 in the treatment of melanoma
(20–22). Although miR-150 is implicated in the
oncogenesis of melanoma (23), the
role of miR-150 in melanoma and the underlying mechanisms are still
unclear.
The programmed cell death protein-4 (PDCD4) is a
novel tumor suppressor protein involved in programmed cell death
(24). Downregulation of PDCD4 is
relevant with invasion, metastasis and poor prognosis of various
types of cancers (24), including
patients with melanoma (25). In this
study, we investigated the functional significance of miR-150 in
melanoma cancer and identified PDCD4 as miR-150-regulated novel
cancer pathway, which could provide new insights into potential
molecular mechanisms of melanoma carcinogenesis.
Materials and methods
Melanoma tissue
Twenty malignant melanoma tissues and adjacent
normal tissues were collected during surgery at Department of
Dermatology, the First Affiliated Hospital of Jinan University
(Guangzhou, China). None of the patients received therapy including
chemotherapy or radiotherapy before surgical resection. The study
and protocol were approved by the Ethics Committee of the First
Affiliated Hospital of Jinan University. A written informed consent
was obtained from participants. All the samples were stored in
liquid nitrogen.
Cell culture and transfection
The human malignant melanoma cell lines including
M14, A357 and WM115 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA). The adult melanocytes were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). Cells were cultured in DMEM (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% FBS
supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin,
and maintained in a humidified incubator containing 5%
CO2 at 37°C.
Subsequent analysis revealed that miR-150 was
differentially expressed among the melanoma cell lines. The tested
cell line which expressed the highest level of miR-150 was used for
loss-of-function analysis by transfection miR-150 inhibitor or
PDCD4 siRNA with Lipofectamine® 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA in cells and tissues were extracted by
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). The RNA
concentration was measured and reverse transcription reaction was
performed according to the manufacturer's instructions (Invitrogen;
Thermo Fisher Scientific, Inc.). PCR analysis was performed on
Applied Biosystems 7500 Sequence Detection system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using SYBR Premix Ex
Taq GC kit (Takara Bio, Inc., Otsu, Japan). The stem-loop primers
used for the PCR amplification were synthesized by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). The relative expression level
of miR-150 was normalized against U6 expression level. The primers
for miR-150: 5′-CTGCTTAGTGGCTCTACTCCTG-3′ (forward) and
5′-TCCCCTCTGGCTTATGTCC-3′ (reverse); for U6:
5′-CTCGCTTCGGCAGCACA-3′ (forward) and 5′-TGGTGTCGTGGAGTCG-3′
(reverse); for the PDCD4: 5′-AAGAAAGGTGGTGCAGGAGG-3′ (forward) and
5′-TGACTAGCCTTCCCCTCCAA-3′ (reverse). Gene expression of PDCD4 was
normalized to the level of β-actin and analyzed by the relative
2−ΔΔCq method. Each experiment was performed in
triplicate.
Western blotting
After transfection for 48 h, cells were treated to
RIPA (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) to extract
the total proteins. And the protein concentrations were determined
using a BCA kit (Beyotime Institute of Biotechnology, Haimen,
China). 30–50 µg of proteins were separated by 10% SDS-PAGE, and
transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA).
Then, membranes were blocked with 5% non-fat milk and incubated
overnight with primary antibody against PDCD4 (1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), followed by an incubation of
horseradish peroxidase-conjugated secondary antibody (1:1,000;
Santa Cruz Biotechnology, Inc.). GAPDH was used as an internal
control.
Cell proliferation
The cell proliferation was detected by Cell Counting
Kit-8 (CCK-8; Beyotime Institute of Biotechnology). The transfected
A357 cells were added into 96-well plates. At 24, 48 and 72 h, the
medium of each well was replaced with 100 µl fresh media contained
10% CCK-8 reaction solution and incubated for 2 h, and then the
absorbance were measured using a microplate reader (Thermo Fisher
Scientific, Inc.) at 450 nm. Also, the cell proliferation were
detected by Edu staining according to the manufacture's instruction
(Beyotime Institute of Biotechnology).
Colony formation assay
The transfected A357 cells were seeded at 4,000
cells/cm2 in 24-well plates in DMEM supplemented with
10% FBS, 100-U/ml PEST and 0.3% agarose with 0.6% agarose underlay.
Dishes were maintained in a humidified incubator containing 5%
CO2 at 37°C. After 10 days, colonies were stained with
crystal violet (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and
counted.
Cell apoptosis and cell cycle
assay
After transfected 48 h, cell apoptosis was detected
by Hoechst 33528 assay. For cell cycle detection, the PI/RNase
staining kits (MultiSciences Biotech Co., Ltd., Hangzhou, China)
was used and detected by a FACScan (BD Biosciences, Franklin Lakes,
NJ, USA). The cells in G0-G1, S, and G2-M phases were counted.
Cell migration and invasion assay
Cell migration and invasion ability of melanoma
cells were evaluated by transwell assay. Transfected A357 cells
were digested and then resuspended in serum-free DMEM. For the
transwell migration assays, cells were planted in the upper
chambers of a 24-well plate and 500 µl DMEM containing 10% FBS was
added into the lower chamber as a chemoattractant. Cells were then
incubated for another 48 h. For the transwell invasion assays,
cells were seeded on the top of the matrigel-coated transwell
chambers (BD Biosciences) and proceeded the same as described
above. Cotton swabs were used to remove the non-invasive cells
after 24 h. The migrated or invaded cells were fixed and stained
with 0.1% crystal violet, and counted using a microscope.
Immunostaining
After transfected for 48 h, cells were fixed with 4%
paraformaldehyde, and permeabilized with 0.1% Triton X-100. After
blocked by 2% serum goat for 30 min, the cells were incubated with
the primary antibody against PDCD4 (1:200; Santa Cruz
Biotechnology, Inc.) overnight and subsequent incubation with AF488
secondary antibody for 1 h. Cell nucleus were stained by DAPI.
Then, images were acquired by confocal microscopy (Zeiss 510; Carl
Zeiss, Jena, Germany).
Luciferase report assay
The 3′UTR fragment of PDCD4 containing the putative
binding sequences of miR-150 was cloned into pMIR-REPORT vectors,
and a mutated plasmid was used as a control. The H293T cells were
co-transfected with a miR-150 mimics and related reporter plasmids.
The luciferase activities were measured using a Dual Luciferase
Reporter Assay System (Promega Corporation, Madison, WI, USA) after
transfection 48 h according to the manufacturer's instructions.
Statistically analysis
Data was presented as mean ± standard deviation and
analyzed by SPSS 15.0 (SPSS, Inc., Chicago, IL, USA) by using
student's t-test or one-way ANOVO. For detection the relationship
between miR-150 and PDCD4 mRNA, the Pearson correlation analysis
was performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
The upregulation of miR-150 is closely
related to the downregulation of PDCD4 in melanoma
To investigate the relationship between miR-150 and
PDCD4 in melanoma patients, cancer tissues and the adjacent normal
tissues were collected from 20 melanoma patients. qPCR assay was
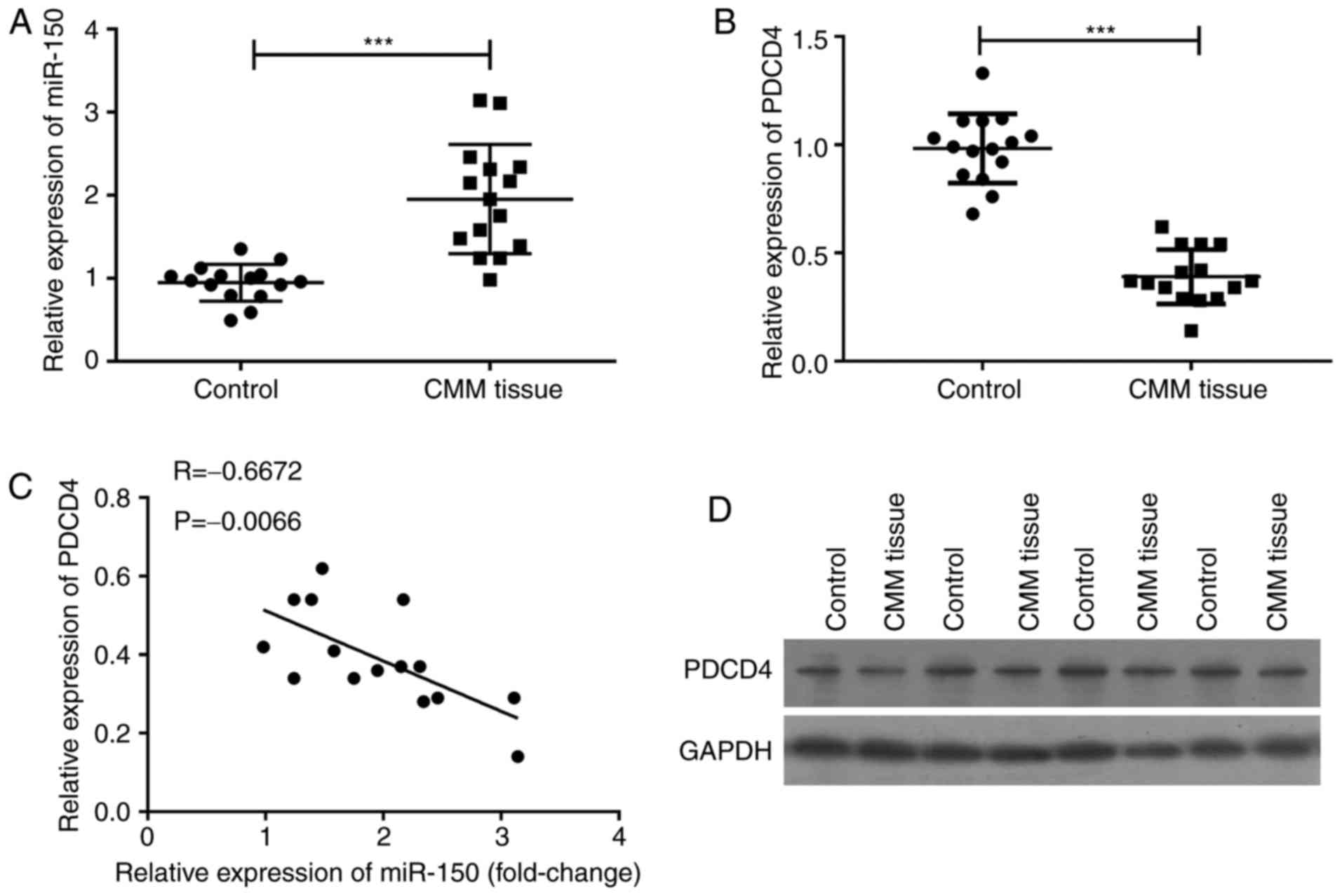
used to detect the expression of miR-150 and PDCD4, and the results
revealed that miR-150 was significantly upregulated (P<0.001)
(Fig. 1A), and that PDCD4 mRNA was
significantly downregulated (P<0.001) (Fig. 1B). The level of PDCD4 mRNA was
inversely related to the expression of miR-150 (P=0.0066) (Fig. 1C). The levels of PDCD4 in cancer
tissue and in its adjacent normal tissues were confirmed by western
blot assay (Fig. 1D). PDCD4 protein
expression in melanoma tissues was lower than that in adjacent
normal tissues, suggesting that the expression of miR-150 was
inversely related to the level of PDCD4 in melanoma.
Knockdown of miR-150 in melanoma cells
by miR-150 inhibitors
To detect the role of miR-150 in melanoma, the
levels of miR-150 in three melanoma cells, including M14, A357, and
WM115-and in NHEM cells, were detected (Fig. 2A). The levels of miR-150 in all the
melanoma cells, including M14, A357, and WM115, were significantly
upregulated as compared with levels in NHEM cells. As the levels in
the A357 cells were the highest among those expressed within
melanoma cells, we used A357 cells to detect the role of miR-150 in
the subsequent experiments. A357 cells were transfected with
miR-150 inhibitors, and our results showed that the level of
miR-150 was significantly downregulated by miR-150 inhibitors
transfection (Fig. 2B), and that the
expression of PDCD4 was significantly upregulated (Fig. 2C), indicating that the PDCD4 was a
target gene of miR-150.
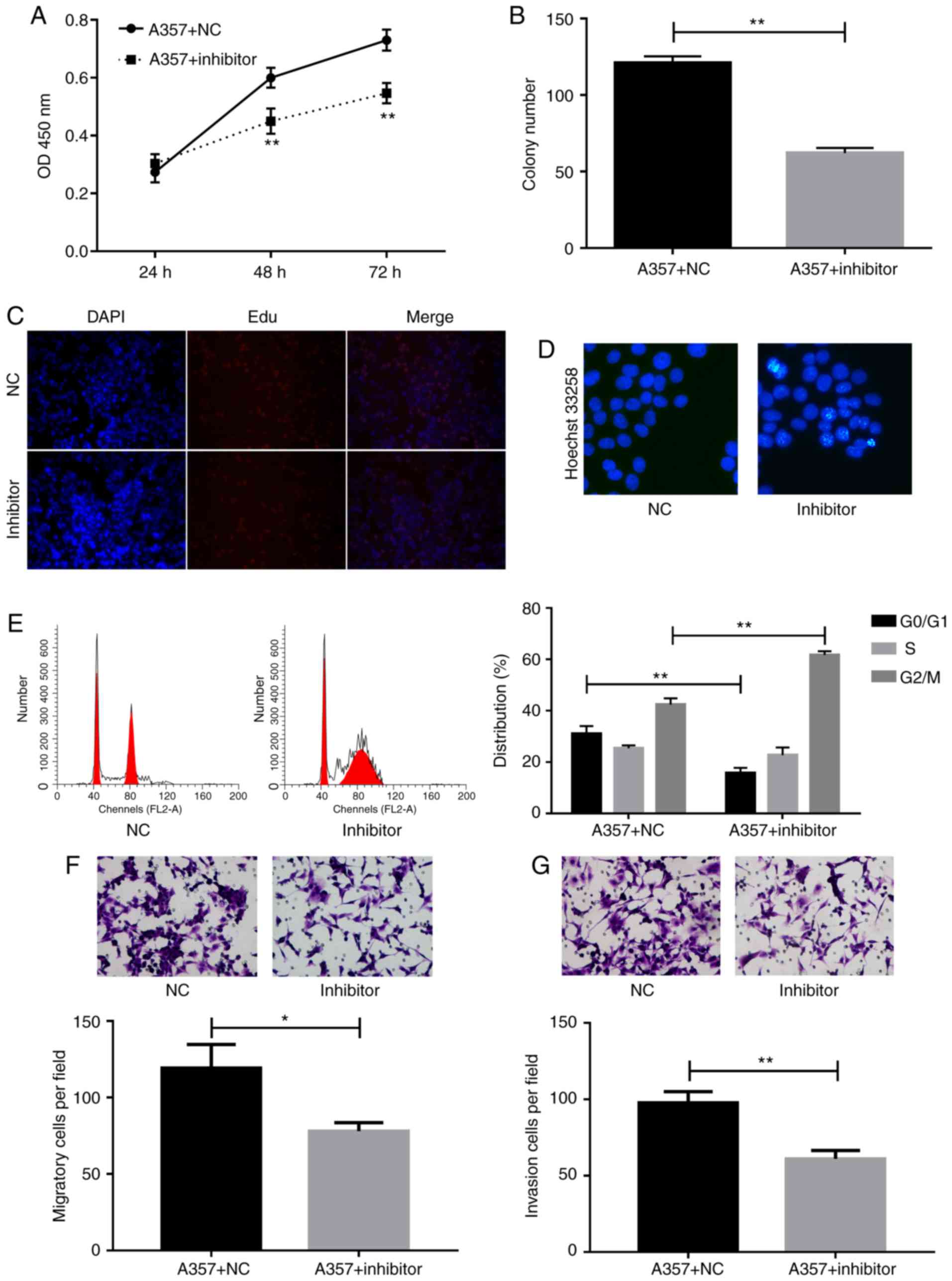
miR-150 inhibitor suppressed cell proliferation,
migration, and invasion, whereas it enhanced cell aptoptosis and
induced G2/M cell arrest. To detect the role of miR-150 in
melanoma, cells were transfected with miR-150 inhibitors and then
the cell proliferation were detected by using CCK-8, Edu, and colon
formation assays. The CCK-8 assay showed that miR-150 inhibitors
significantly suppressed cell proliferation at 48 h as compared
with the NC group (Fig. 3A).
Decreased colon formation was also observed after miR-150
inhibitors transfection in A357 cells (Fig. 3B). The inhibition of cell
proliferation by miR-150 inhibitors was further confirmed by the
Edu assay (Fig. 3C).
To investigate the effect of miR-150 on cell
apoptosis, the hoechst 33258 method was used to perform apoptosis
assays. The data demonstrated that cell apoptosis rate was
increased after the inhibition of miR-150 (Fig. 3D). Cell cycle assay provided more
detailed information. As shown in Fig.
3E, accumulation of cells at G2/M phase and loss of cells at
G0/G1 phase were detected after transfection of A357 cells with
miR-150 inhibitors.
Moreover, migration Transwell assay indicated
significant inhibition of cell migration in A357 cells transfected
with miR-150 inhibitors compared to control group (Fig. 3F). Invasion capability of A357 cells
transfected with NC or miR-150 inhibitors were evaluated by
Matrigel Invasion assay. Indeed, the number of invading cells was
decreased markedly in A357 cells as a result of knockdown of
miR-150 (Fig. 3G).
Knockdown of miR-150 increased the
expression of PDCD4
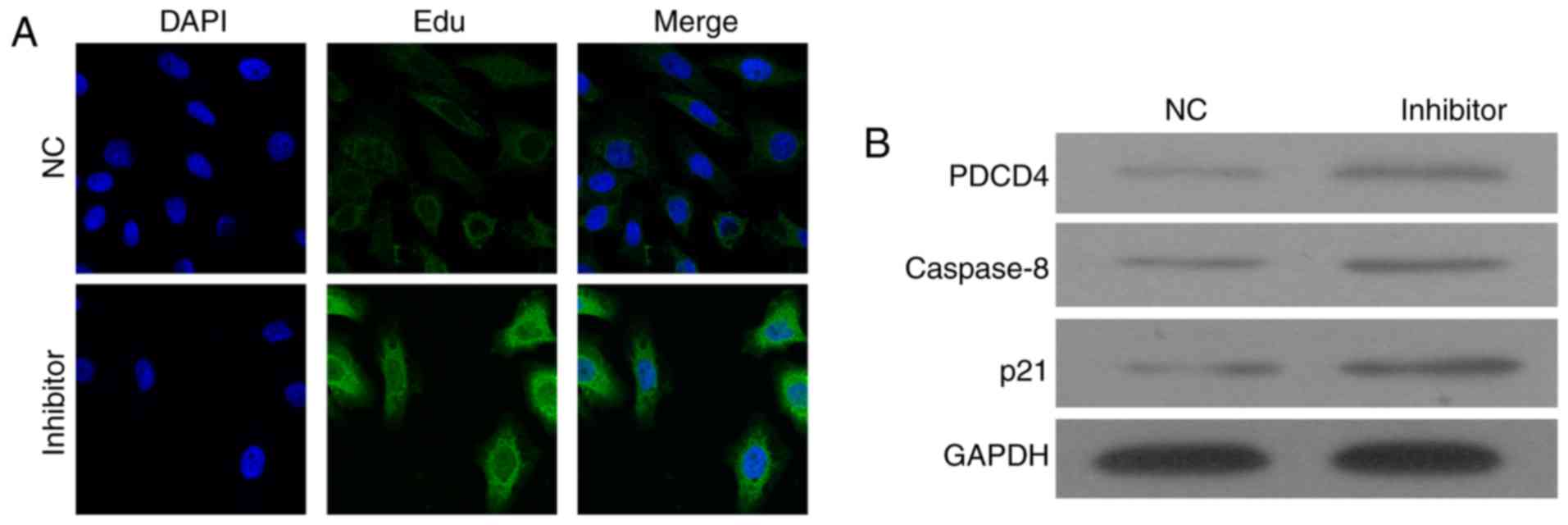
To detect the molecular mechanism underlying the
miR-150-inhibitor-induced cell apoptosis, the expression of PDCD4
was detected by immunostaining and western blot assay (Fig. 4). The immunostaining results showed
that miR-150 inhibitors increased the expression of PDCD4 in cell
plasma (Fig. 4A). Western blotting
confirmed the upregulation of PDCD4 in cells transfected with
miR-150 inhibitors (Fig. 4B).
The expressions of caspase-8 and p21 were also
detected, and results showed that caspase-8 and p21 were
upregulated by the miR-150 inhibitors. It indicated that knockdown
of miR-150 might enhance cell apoptosis via upregulation of
PDCD4-mediated activation of caspase-8 and p21.
Knockdown of miR-150-enhanced cell
apoptosis via direct targeting of PDCD4
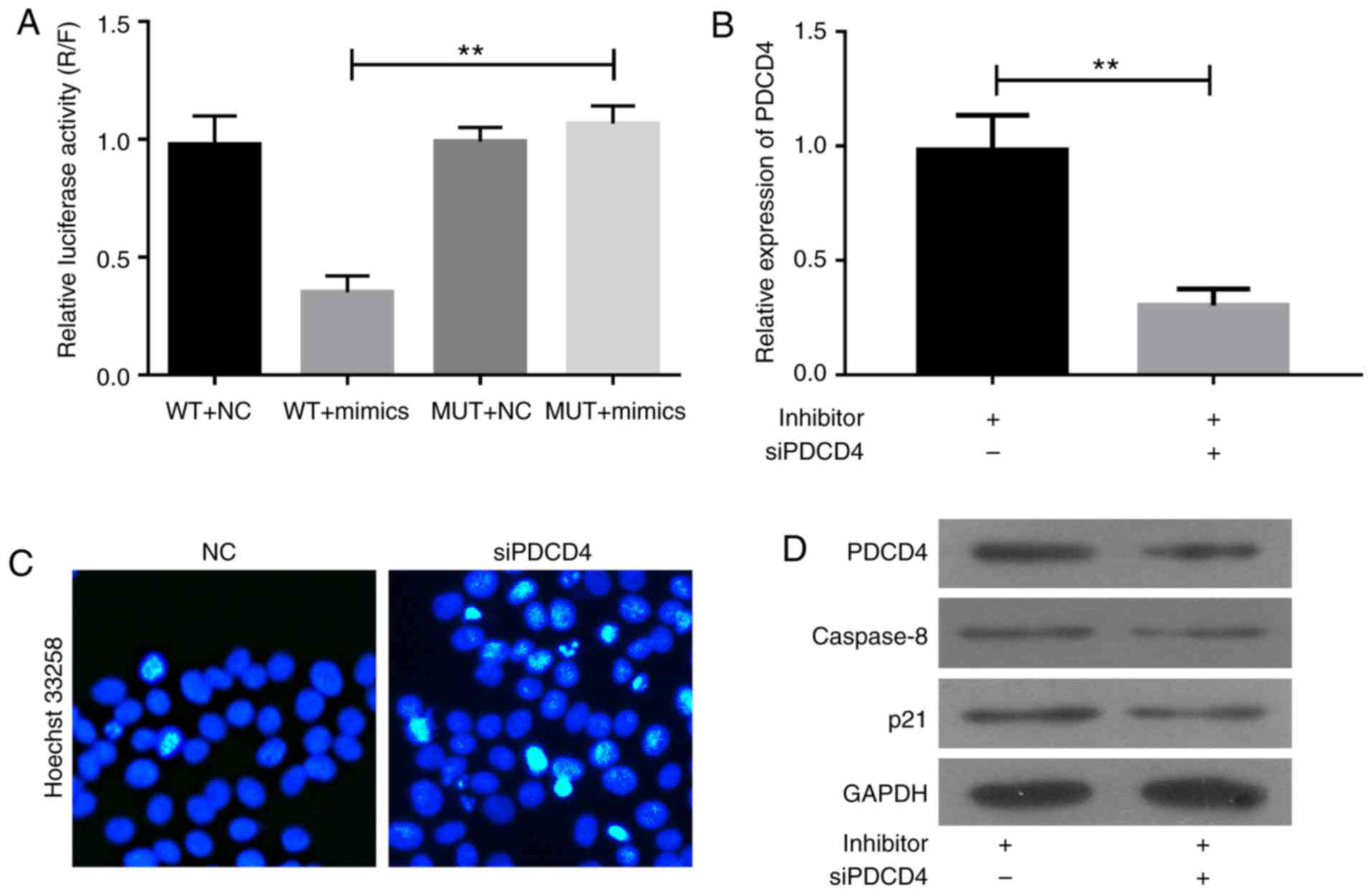
MiRNA mediates post-transcriptional regulation by
binding to the 3′UTR of the downstream genes. To verify whether
PDCD4 is a direct target of miR-150, the wild type or mutation of
3′UTR of PDCD4 was inserted into the downstream of luciferase
reporter vector and transfected into H293T cells, together with the
miR-150 mimics. Overexpression of miR-150 significantly suppressed
the luciferase activity of reporter genes containing 3′UTR of PDCD4
compared with control group but partially rescued when the binding
site was mutated (Fig. 5A). Thus,
miR-150 directly targets PDCD4.
The role of PDCD4 in miR-150-inhibitor-induced cell
apoptosis was confirmed by siPDCD4 transfection (Fig. 5B-D). The A357 cells were
co-transfected with NC or miR-150 inhibitors together with siPDCD4
for 48 h. As shown in Fig. 5B, the
upregulation of PDCD4 induced by miR-150 inhibitors in A357 cells
was abolished by siPDCD4 transfection. Hoechst 33258 assay revealed
that cell apoptosis induced by miR-150 inhibitors was significantly
inhibited by siPDCD4 transfection (Fig.
5C). The knockdown of PDCD4 significantly inhibited the
expression of caspase-8 and p21 induced by miR-150 inhibitors
(Fig. 5D). Thus, miR-150 inhibitors
enhance cell apoptosis via upregulation of PDCD4-mediated
activation of caspase-8 and p21.
Discussion
Melanoma is one of the most aggressive type of
malignant skin cancers (26). In
recent years, given the increase of incidence rate, it has become a
common malignant tumor. Melanoma poses serious risks to human
health, due to its rapid progression rate, ease of migration, and
poor clinical prognosis. Although surgical resection is greatly
facilitated the early treatment of melanoma, there is still no
effective therapy for advanced melanoma. At present, it is very
important to unveil the mechanisms of human melanoma cancer, and
find effective therapeutic targets to improve the prognosis of
melanoma cancer patients.
miRNA is a class of endogenous non-coding small RNA
molecules that are involved in post-transcriptional regulation of
single-stranded and conserved genes (12,13).
Increasing evidence demonstrate that miRNA is involved in the
development of many types of cancer, including melanoma cancer
(27). Recent genome-wide miRNA
expression technologies have clarified the alternation of miR-150
in several types of human cancers (28). Interestingly, divergent expression
patterns of miR-150 among human cancers have been reported. miR-150
down-regulation was described in malignant lymphoma (29), osteosarcoma (30), and colorectal cancer (31), whereas its up-regulation was shown in
lung cancer (28) and breast cancer
(32). These controversial results
suggest that the role of miR-150 is possibly tumor specific and
highly dependent on its targets in different cancers. Since the
role of miR-150 as a tumor suppressor or as an oncogene of tumor
cell growth and metastasis in various cancers has been extensively
investigated, we focused on its potential role in melanoma cancer.
In this study, we observed that the expression level of miR-150 was
increased in melanoma cancer specimens. In agreement with our
results, Friedman et al have demonstrated that miR-150 is
up-regulated in metastatic melanoma specimens and the patients with
higher circulating expression of miR-150 have a high recurrence
risk (33). In the in vitro
study, we found that the loss of miR-150 suppresses proliferation,
migration and invasion of melanoma cancer cell line, and we also
showed that knockdown of miR-150 induces cell cycle arrest and
apoptosis in A357 cells. Given that invasion and metastasis are two
leading attributes of malignant cancer, these results suggest that
miR-150 is a potent tumor suppressor in melanoma cancer.
Additionally, it has been reported that the inhibition of miR-150
accelerates apoptosis in cancer cells and renders them more
sensitive to various chemotherapy drugs, including gemcitabine and
5-fluorouracil, suggesting the association between miR-150 and
apoptosis-related proteins during tumorigenesis.
PDCD4 is a key protein involved in programmed cell
death (24). It has been reported
that PDCD4 inhibits the translation of proteins and accelerates
apoptosis by binding to the translation initiation factor eIF4A
(34). In addition, by activating and
regulating the transcription activator protein AP-1 and matrix
metalloproteinase 2 (MMP-2), PDCD4 also inhibits tumor growth,
invasion, and metastasis (35).
Downexpression of PDCD4 is significantly associated with short
overall survival of various types of cancer patients (24), including those suffering from melanoma
(25). POLINA N found that PDCD4 loss
is not a common event in melanoma progression, and PDCD4 can be
used for molecular typing of melanoma (36). Loss of PDCD4 increases the
proliferative activity, promotes tumor cell invasion, and
contributes to apoptosis resistance of cancer cells, revealing the
significance of PDCD4 loss in tumorigenesis. In the present study,
we showed that miR-150 enhanced the cell apoptosis and elicited
cell cycle arrest at G2/M phases in A357 cells. However,
miR-150-inhibitor-induced cell apoptosis was reversed by knockdown
of PDCD4 gene, suggesting that PDCD4 is an important mediator of
cell apoptosis regulation by miR-150 in A357 cells. Our results
also show that the increased levels of caspase-8 and p21, which
were hallmarks of apoptosis induction and cell cycle arrest, was
observed after miR-150 inhibitors transfection. Moreover, the
induction of apoptosis by miR-150 inhibitors was attenuated by
PDCD4 knockdown in A357 cells. Collectively, our results suggest
that miR-150 induces cell proliferation and invasion via a
mechanism dependent on PDCD4. Also, clinical melanoma cancer
samples were used to confirm the relationship between the
endogenous expression levels of PDCD4 and miR-150. We further
confirmed through luciferase reporter gene assays that miR-150
directly targets PDCD4 by binding the 3′-UTR of PDCD4 mRNA, which
is consistent with Lei et al (37). In essence, this provided the evidence
that the loss of miR-150 may lead to PDCD4-mediated cell apoptosis
and cell cycle in melanoma cancer, which would constitute a
promising target for melanoma cancer therapy.
In conclusion, our study suggests that miR-150 is an
anti-apoptotic factor in melanoma cancer that maintains tumor cell
growth via regulation of PDCD4, and thus miR-150 may play an
important role in melanoma carcinogenesis. The newly identified
miR-150/PDCD4 link provides novel insights into the metastasis of
melanoma cancer, especially with respect to cell apoptosis and cell
cycle in vitro; and sheds new lights on therapeutic strategy
for melanoma cancer.
References
|
1
|
Kosary CL, Altekruse SF, Ruhl J, Lee R and
Dickie L: Clinical and prognostic factors for melanoma of the skin
using SEER registries: Collaborative stage data collection system,
version 1 and version 2. Cancer. 23 Suppl 120:S3807–S3814. 2014.
View Article : Google Scholar
|
|
2
|
Coit DG, Thompson JA, Andtbacka R, Anker
CJ, Bichakjian CK, Carson WE III, Daniels GA, Daud A, Dimaio D,
Fleming MD, et al: Melanoma, version 4.2014. J Natl Compr Canc
Netw. 12:621–629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rastrelli M, Tropea S, Rossi CR and
Alaibac M: Melanoma: Epidemiology, risk factors, pathogenesis,
diagnosis and classification. In Vivo. 28:1005–1011.
2014.PubMed/NCBI
|
|
6
|
Hao M, Zhao G, Du X, Yang Y and Yang J:
Clinical characteristics and prognostic indicators for metastatic
melanoma: Data from 446 patients in north China. Tumour Biol.
37:10339–10348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song X, Zhao Z, Barber B, Farr AM, Ivanov
B and Novich M: Overall survival in patients with metastatic
melanoma. Curr Med Res Opin. 31:987–991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eriksson H, Frohm-Nilsson M, Järås J,
Kanter-Lewensohn L, Kjellman P, Månsson-Brahme E, Vassilaki I and
Hansson J: Prognostic factors in localized invasive primary
cutaneous malignant melanoma: Results of a large population-based
study. Br J Dermatol. 172:175–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan Y, Haydon AM, McLean CA, McDonald PB
and Kelly JW: Prognosis associated with cutaneous melanoma
metastases. Australas J Dermatol. 56:25–28. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin X, Khalid S, Qureshi MZ, Attar R,
Yaylim I, Ucak I, Yaqub A, Fayyaz S, Farooqi AA and Ismail M: VEGF
mediated signaling in oral cancer. Cell Mol Biol (Noisy-le-grand).
62:64–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oliveto S, Mancino M, Manfrini N and Biffo
S: Role of microRNAs in translation regulation and cancer. World J
Biol Chem. 8:45–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stein M, Ruggiero P, Rappuoli R and
Bagnoli F: Helicobacter pylori CagA: From pathogenic mechanisms to
its use as an anti-cancer vaccine. Front Immunol. 4:3282013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bentwich I, Avniel A, Karov Y, Aharonov R,
Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, et al:
Identification of hundreds of conserved and nonconserved human
microRNAs. Nat Genet. 37:766–770. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang R, Peng Y, Wang W and Su B: Rapid
evolution of an X-linked microRNA cluster in primates. Genome Res.
17:612–617. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kunz M: MicroRNAs in melanoma biology. Adv
Exp Med Biol. 774:103–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shiiyama R, Fukushima S, Jinnin M,
Yamashita J, Miyashita A, Nakahara S, Kogi A, Aoi J, Masuguchi S,
Inoue Y and Ihn H: Sensitive detection of melanoma metastasis using
circulating microRNA expression profiles. Melanoma Res. 23:366–372.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tembe V, Schramm SJ, Stark MS, Patrick E,
Jayaswal V, Tang YH, Barbour A, Hayward NK, Thompson JF, Scolyer
RA, et al: MicroRNA and mRNA expression profiling in metastatic
melanoma reveal associations with BRAF mutation and patient
prognosis. Pigment Cell Melanoma Res. 28:254–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fleming NH, Zhong J, da Silva IP, de Miera
Vega-Saenz E, Brady B, Han SW, Hanniford D, Wang J, Shapiro RL,
Hernando E and Osman I: Serum-based miRNAs in the prediction and
detection of recurrence in melanoma patients. Cancer. 121:51–59.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thanarajasingam U, Sanz L, Diaz R, Qiao J,
Sanchez-Perez L, Kottke T, Thompson J, Chester J and Vile RG:
Delivery of CCL21 to metastatic disease improves the efficacy of
adoptive T-cell therapy. Cancer Res. 67:300–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mullins IM, Slingluff CL, Lee JK, Garbee
CF, Shu J, Anderson SG, Mayer ME, Knaus WA and Mullins DW: CXC
chemokine receptor 3 expression by activated CD8+ T cells is
associated with survival in melanoma patients with stage III
disease. Cancer Res. 64:7697–7701. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Latchana N, Ganju A, Howard JH and Carson
WE III: MicroRNA dysregulation in melanoma. Surg Oncol. 25:184–189.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li JZ, Gao W, Ho WK, Lei WB, Wei WI, Chan
JY and Wong TS: The clinical association of programmed cell death
protein 4 (PDCD4) with solid tumors and its prognostic
significance: A meta-analysis. Chin J Cancer. 35:952016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mao XH, Chen M, Wang Y, Cui PG, Liu SB and
Xu ZY: MicroRNA-21 regulates the ERK/NF-κB signaling pathway to
affect the proliferation, migration, and apoptosis of human
melanoma A375 cells by targeting SPRY1, PDCD4, and PTEN. Mol
Carcinog. 56:886–894. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Naves LB, Dhand C, Venugopal JR, Rajamani
L, Ramakrishna S and Almeida L: Nanotechnology for the treatment of
melanoma skin cancer. Prog Biomater. 6:13–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mueller DW and Bosserhoff AK: Role of
miRNAs in the progression of malignant melanoma. Br J Cancer.
101:551–556. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang N, Wei X and Xu L: miR-150 promotes
the proliferation of lung cancer cells by targeting P53. FEBS Lett.
587:2346–2351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tagawa H, Watanabe A and Sawada K:
Abstract 146: The role of Mir-150 as a tumor suppressor in
malignant lymphoma. Cancer Res. 71 8 Suppl:S1462011. View Article : Google Scholar
|
|
30
|
Li CH, Yu TB, Qiu HW, Zhao X, Zhou CL and
Qi C: miR-150 is downregulated in osteosarcoma and suppresses cell
proliferation, migration and invasion by targeting ROCK1. Oncol
Lett. 13:2191–2197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ma Y, Zhang P, Wang F, Zhang H, Yang J,
Peng J, Liu W and Qin H: miR-150 as a potential biomarker
associated with prognosis and therapeutic outcome in colorectal
cancer. Gut. 61:1447–1453. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang S, Chen Y, Wu W, Ouyang N, Chen J,
Li H, Liu X, Su F, Lin L and Yao Y: miR-150 promotes human breast
cancer growth and malignant behavior by targeting the pro-apoptotic
purinergic P2X7 receptor. PLoS One. 8:e807072013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Friedman EB, Shang S, de Miera EV, Fog JU,
Teilum MW, Ma MW, Berman RS, Shapiro RL, Pavlick AC, Hernando E, et
al: Serum microRNAs as biomarkers for recurrence in melanoma. J
Transl Med. 10:1552012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Colburn NH, Yang HS and Jansen A: Pdcd4
targets eIF4A to inhibit translation, transcription, tumorigenesis
and invasion. EJC Suppl. 4:232006. View Article : Google Scholar
|
|
35
|
Li JZ, Gao W, Lei WB, Zhao J, Chan JY, Wei
WI, Ho WK and Wong TS: MicroRNA 744-3p promotes MMP-9-mediated
metastasis by simultaneously suppressing PDCD4 and PTEN in
laryngeal squamous cell carcinoma. Oncotarget. 7:58218–58233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vikhreva PN and Korobko IV: Expression of
Pdcd4 tumor suppressor in human melanoma cells. Anticancer Res.
34:2315–2318. 2014.PubMed/NCBI
|
|
37
|
Lei Y, Hu X, Li B, Peng M, Tong S, Zu X,
Wang Z, Qi L and Chen M: miR-150 modulates cisplatin
chemosensitivity and invasiveness of muscle-invasive bladder cancer
cells via targeting PDCD4 in vitro. Med Sci Monit. 20:1850–1857.
2014. View Article : Google Scholar : PubMed/NCBI
|