Introduction
Hepatocellular carcinoma (HCC) is a malignancy with
one of the highest mortality rates, and it has been ranked as the
fifth most common malignancy globally (1). Although substantial progress has been
made in the diagnosis and treatment of HCC, it remains the third
leading cause of cancer-associated mortality globally (2). Therefore, there is an urgent requirement
to investigate the molecular mechanisms by which HCC progresses and
to find novel diagnostic factors and effective therapeutic
strategies to improve patient survival rates.
The epithelial-mesenchymal transition (EMT)
comprises a complex series of reversible events that may lead to
the loss of epithelial cell adhesion and the indication of a
mesenchymal phenotype (3). EMT serves
crucial roles during embryonic development, tumor metastasis and
invasion, and is one of the major molecular mechanisms through
which invasion and metastasis are promoted during the oncogenic
process (4,5). Previous studies demonstrated that EMT
activation in cancer cells contributed to tumor invasion and
metastasis in various types of cancer, including HCC, resulting in
aggressive cancer progression (6,7). Taken
together, these findings indicate that EMT is associated with
metastasis.
AMPK-related protein kinase 5 (ARK5) is a member of
the human AMP-activated protein kinase family. ARK5 was identified
as a key molecule in mediating the migration of cancer cells in
human pancreatic cancer cells, and its activation was induced by
Akt-dependent Ser600 phosphorylation (8). It has been reported that the
overexpression of ARK5 was involved in tumor progression and
metastatic activity in colorectal cancer (9). Tumor malignancy, including invasion and
metastasis, is accelerated by the activation of Akt, a process that
has been demonstrated for breast, ovarian, colorectal and
pancreatic cancer, and squamous cell carcinoma (10–13). ARK5
was reported to promote invasion and metastasis in cancer cells,
including breast cancer, colorectal cancer and glioma (9,14,15). However, the mechanism by which ARK5
alters invasion and metastasis has not been fully identified in HCC
cells.
In the present study, the authors hypothesized that
ARK5 may be involved in HCC cell invasion and metastasis through
EMT. Suppression of ARK5 may be used as a potential target for the
treatment of HCC. To investigate this hypothesis, the role of ARK5
in invasion and metastasis of cancer was investigated using HCC
cells.
Materials and methods
Cell culture
The human HCC Huh7 and SNU387 cells were purchased
from the American Type Culture Collection (Manassas, VA, USA). Huh7
cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM;
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.)
and 1% penicillin/streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). SNU387 cells were maintained in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS and 1% penicillin/streptomycin. All cells were grown in a
humidified incubator at 37°C and with 5% CO2.
Short interfering RNA (siRNA)
transfection
HCC Huh7 and SNU387 cells were transfected with 100
nmol/l ARK5 siRNA (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
or negative siRNA control (Invitrogen; Thermo Fisher Scientific,
Inc.) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) following the manufacturer's protocol. The
transfection medium Opti-MEM (Gibco; Thermo Fisher Scientific,
Inc.) was used. After 6 h, the transfection medium was removed, and
the cells were maintained in DMEM and RPMI-1640 medium for an
additional 24 h, at which point all subsequent experiments were
performed.
Western blot analysis
HCC Huh7 and SNU387 cells were washed with cold
phosphate-buffered saline (PBS) and treated with cell lysis buffer
(Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C or on
ice for 2 h. The concentration of the proteins was measured using
BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). The
protein (40 µg per lane) were separated by 10% SDS-PAGE and
transferred onto polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were then blocked
with 5% bovine serum albumin (Sangon Biotech Co., Ltd., Shanghai,
China) in 0.1% Tween-20 (TBS/T) and incubated with primary
antibodies against ARK5 (cat no. ab71814), E-cadherin (cat no.
ab76055) and vimentin (cat no. ab8978) (all 1:1,000; Abcam,
Cambridge, MA, USA) at 4°C overnight. The membranes were then
washed three times with TBST and then incubated with the
appropriate secondary antibody (1:2,000, Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) at 37°C for 2 h. The protein bands were
visualized by chemiluminesence using an enhanced chemiluminesence
kit (GE Healthcare, Piscataway, NJ, USA) and were quantified by
densitometry using Image Lab 5.0 software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). GAPDH (1:2,000; Cell Signaling
Technology, Inc.; cat no. 5174) was used as an internal
control.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from HCC Huh7 and SNU387
cells with TRIzol (Invitrogen, Carlsbad, CA, USA). cDNA was
synthesized using M-MLV Reverse Transcript reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. qPCR was performed using SYBR Green PCR kit (Takara Bio,
Inc., Otsu, Japan) and analyzed using an ABI Prism 7500 Real-time
PCR system (Applied Biosystems, CA, USA) under the following
reaction conditions: 50°C for 2 min; 95°C for 30 sec and followed
by 40 cycles of 60°C for 30 sec. All reactions were performed in
triplicate. ARK5 mRNA expression was quantified and reported as
2−ΔΔCq (16). The
expression level of each gene was normalized to the expression
level of GAPDH. The primers were as follows: ARK forward,
5′-GAGTCCACTCTATGCATC-3′ and reverse, 5′-GGCCACTATTGAGGACA-3′.
Wound-healing assay
HCC Huh7 and SNU387 cells were seeded into 6-well
plates at a density of 3×105 cells/well and cultured in
the appropriate medium (DMEM used for Huh7 cells, and RPMI-1640
used for SNU387 cells) containing 10% FBS, 1%
penicillin/streptomycin at 37°C with 5% CO2 for 24 h
until 90% confluence, and then the medium was changed to the
corresponding medium (DMEM used for Huh7 cells, and RPMI-1640 used
for SNU387 cells) containing 0.05% FBS, 1% penicillin/streptomycin
overnight to synchronize the cells. The cells were wounded with 100
µl pipette tips, and the cell debris was washed away with PBS. The
wound scars were photographed with an inverted light microscope at
0 and 24 h after the scratch was made. The ratio of the remaining
wound area relative to the initial wound area was calculated, and
the wound area was quantified using Image-Pro Plus software
(version 6.0; Media Cybernetics, Inc., Rockville, MD, USA).
Transwell Matrigel invasion assay
The Huh7 and SNU387 cells were seeded at a density
of 5×104 cells/well in the upper chamber of a Transwell
24-insert plate with corresponding medium (DMEM used for Huh7
cells, and RPMI-1640 used for SNU387 cells). Following transfection
with ARK5 siRNA (100 nmol/ml) for 6 h, the cells were treated with
TGF-β1 (10 ng/ml) for 48 h. The upper chambers were coated with
Matrigel (BD Biosciences, San Jose, CA, USA), and the lower chamber
contained the same medium supplemented with 10% FBS. After 24 h,
the bottom of the inserts were fixed in methanol for 10 min and
stained with H&E for 3 min at 37°C. The cells that had invaded
to the lower surface were counted using an inverted phase-contrast
microscope (magnification, 40x) and images were captured.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance (ANOVA) followed by Tukey's post hoc test.
Student's t-test was also used. GraphPad Prism (version 5.0;
GraphPad Software, Inc., La Jolla, CA, USA) was used to perform all
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed at least three times as independent experiments.
Results
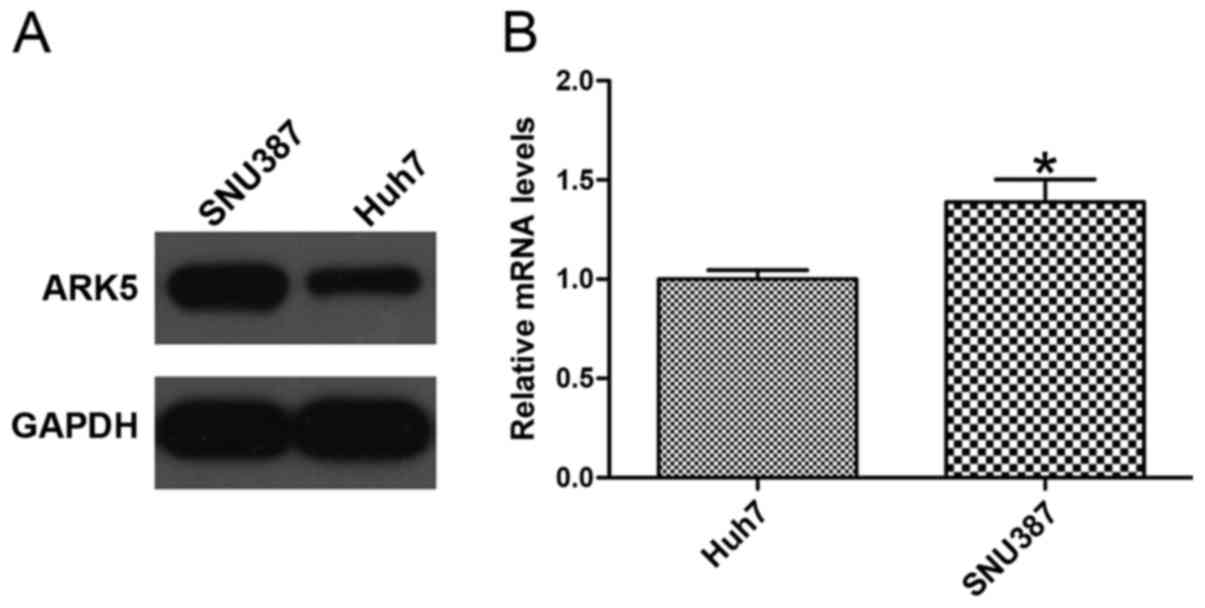
ARK5 expression level is different
between Huh7 and SNU387 cell lines
To confirm whether the expression level of ARK5 was
associated with HCC cell lines, western blotting and RT-qPCR were
used to analyze the expression of ARK5. The HCC Huh7 cell line,
with an epithelial phenotype, exhibited low expression of ARK5,
whereas the mesenchymal phenotype HCC SNU387 cell line exhibited
high expression of ARK5 (Fig. 1),
indicating that the level of ARK5 may be associated with invasion
and migration in HCC cells (P<0.05 vs. Huh7).
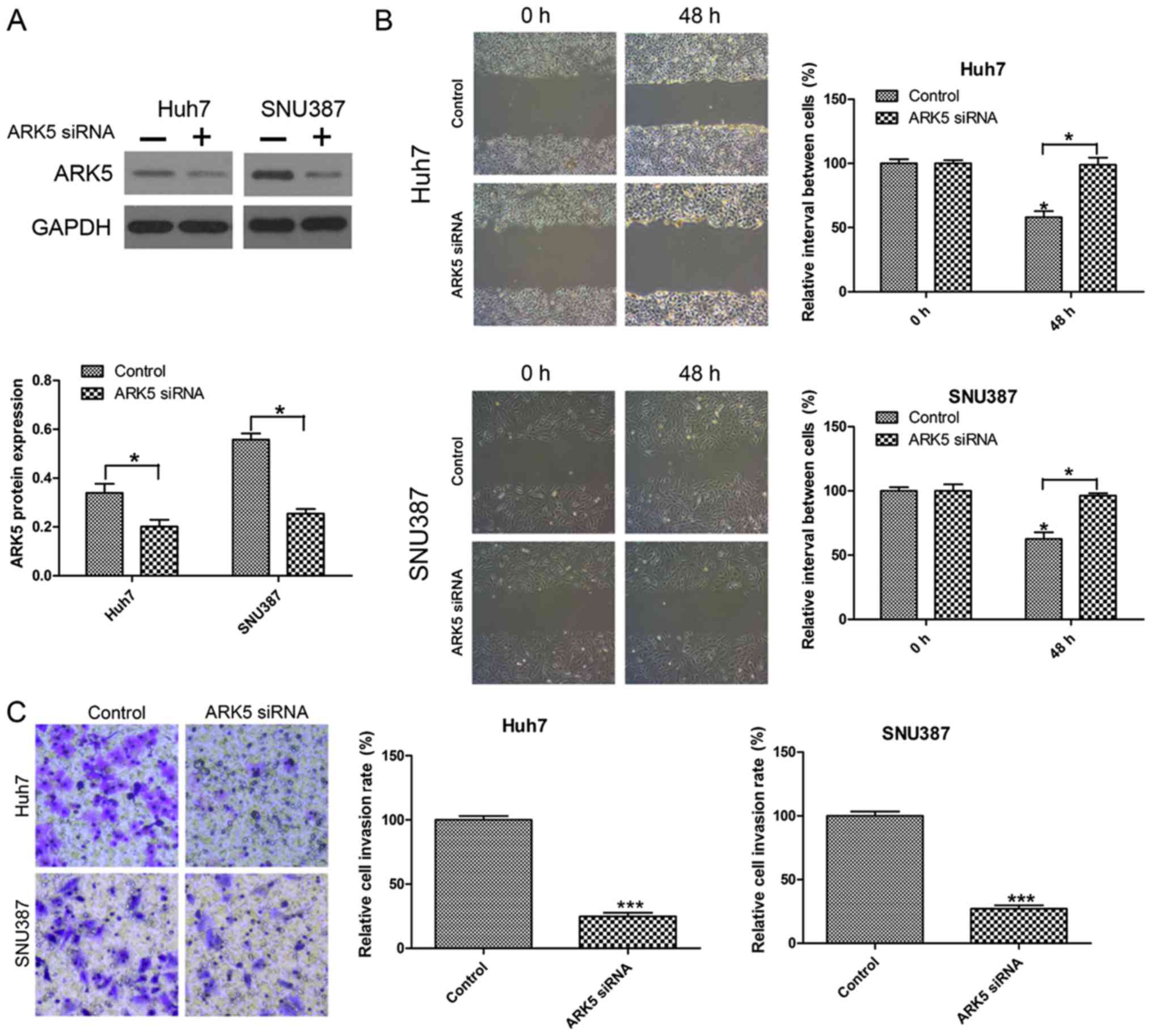
Knockdown of ARK5 inhibits invasion
and migration in Huh7 and SNU387 cells
To investigate the function of ARK5 in invasion and
migration in HCC cells, the effect of ARK5 knockdown on metastasis
was investigated by Transwell invasion and wound healing migration
assays. The knockdown efficiency was examined by western blot
analysis (Fig. 2A). The results
indicated that inhibiting the expression of ARK5 was able to
significantly reduce cell migration compared with the control under
normal conditions after 24 h in wound healing migration assays
(Fig. 2B). In the Transwell invasion
assay, it was observed that the number of HCC cells transfected
with ARK5 siRNA passed through the Transwell membranes were
decreased compared with the control group (Fig. 2C).
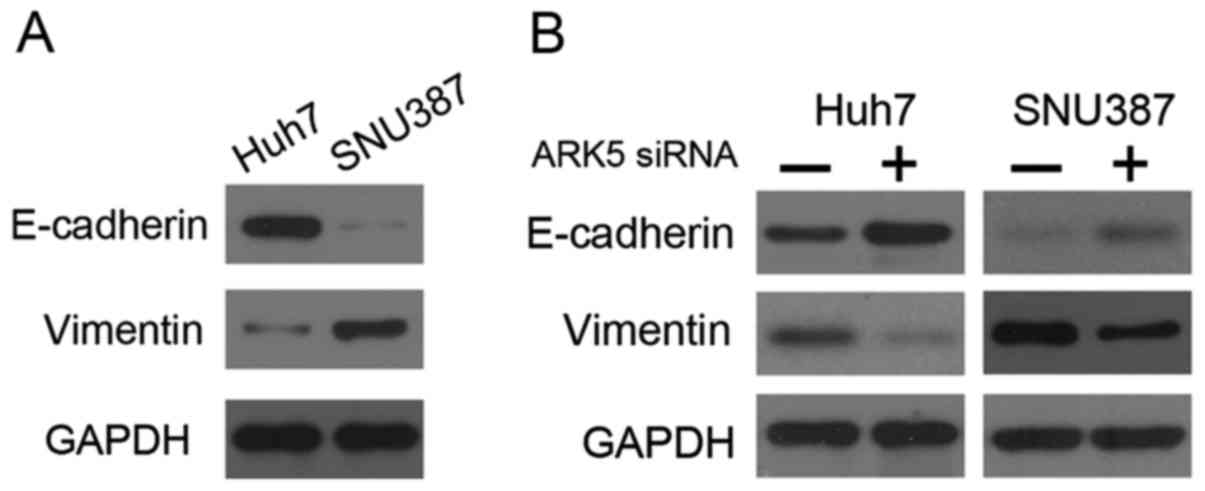
Knockdown of ARK5 may upregulate the
expression of E-cadherin and downregulate vimentin expression in
HCC cells
It has been reported that EMT serves a notable role
in the development of drug resistance in a number of different
tumor types, including cancer of the breast, lung, colon, and
pancreas, with tumor cells that have gained drug resistance
displaying a higher degree of malignant, and migratory and invasive
ability (17–20). Studies have demonstrated that the
inhibition of ARK5 results in the upregulation of E-cadherin
expression and downregulation of vimentin expression in HCC,
ovarian cancer and non-small lung cancer cells (21–23). In
the present study, western blot analysis revealed that Huh7 cells
exhibited high E-cadherin expression and low vimentin expression,
whereas SNU387 cells exhibited the opposite pattern of expression
(Fig. 3A). The results of the present
study also demonstrated that siRNA-mediated silencing of ARK5
resulted in upregulation of E-cadherin and downregulation of
vimentin in Huh7 and SNU387 cells (Fig.
3B). These results demonstrated that the inhibition of ARK5 may
reverse EMT.
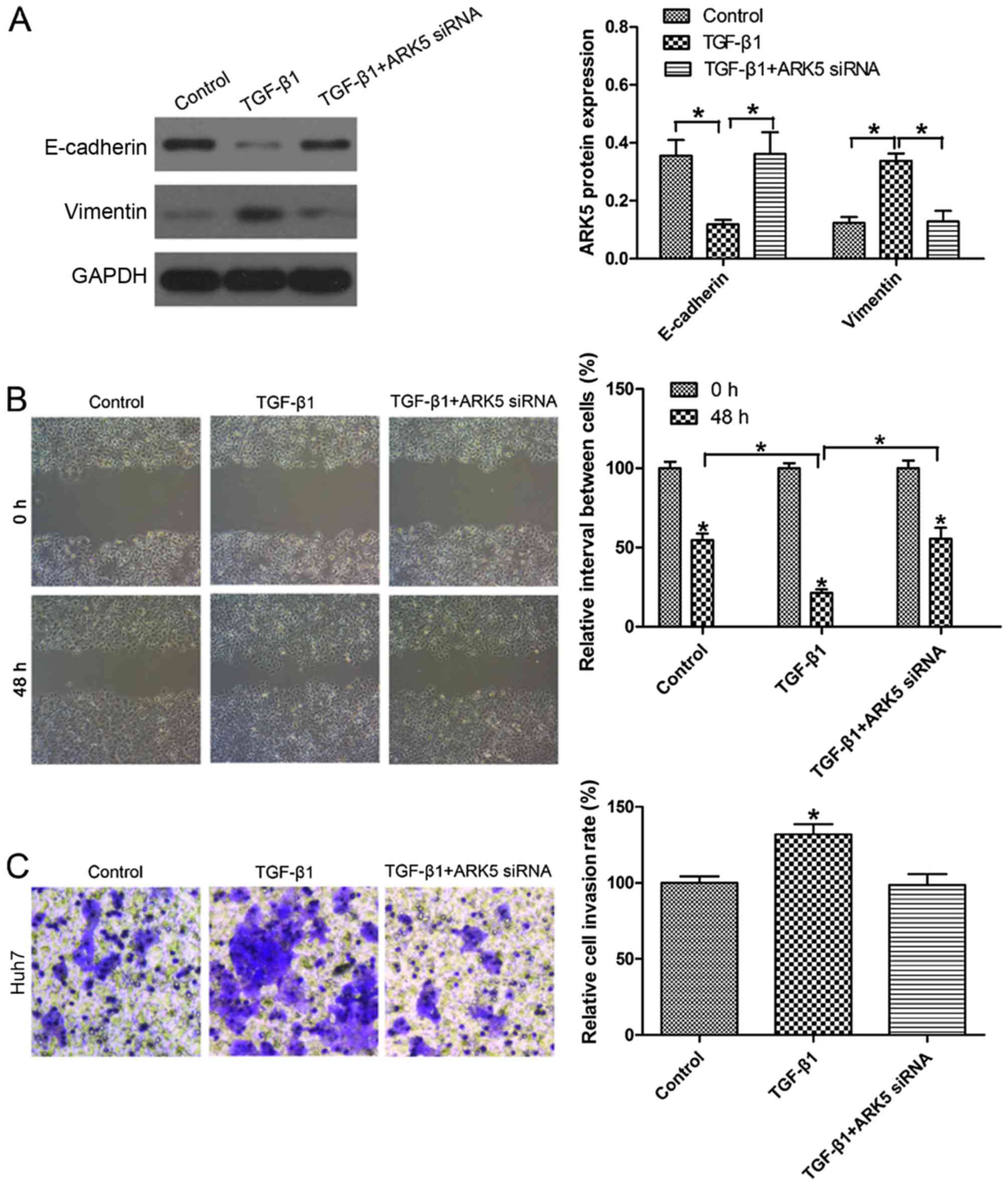
Inhibition of ARK5 reverses EMT under
TGF-β1 treatment in Hep3B cells
Previously, TGF-β1 has been identified as the key
driver in inducing EMT (24).
Following exposure to 10 ng/ml TGF-β1 for 48 h, E-cadherin
expression in Huh7 cells was markedly decreased. Notably, the
expression of E-cadherin in Huh7 cells was upregulated upon
transfection with ARK5 siRNA following TGF-β1 treatment compared
with TGF-β1-treated cells (Fig. 4A).
Wound healing assays revealed that the knockdown of ARK5 was able
to reverse TGF-β1-induced cell migratory ability (Fig. 4B). Transwell invasion assays also
revealed that the knockdown of ARK5 was able to reverse
TGF-β1-induced invasion (Fig. 4C).
There was no significant difference between the group transfected
with AKR5 siRNA combined with TGF-β1 and the control in Huh7 cells.
These findings indicated that the knockdown of ARK5 may reverse
TGF-β1-induced EMT and reduce the invasive and metastatic
capabilities of Huh7 cells, which have an epithelial phenotype.
Discussion
There is a high mortality associated with HCC, the
fifth most common tumor worldwide, due to a lack of effective
treatments (25). HCC is frequently
diagnosed at an advanced stage and thus is associated with poor
prognosis (26). It is known that
cell invasion is essential for tumor metastasis, and Akt has been
identified to serve a key role in invasion and metastasis of tumor
cells (27–29). Therefore, it is important to
understand the molecular basis of the involvement of ARK5 in the
invasion and metastasis of HCC cell lines.
Evidence indicates that EMT serves a key role in
carcinogenicity, metastasis, progression and acquired
chemoresistance in many types of cancer, including in HCC (30,31).
During EMT, expression of the epithelial marker E-cadherin
decreases, whereas that of the mesenchymal marker vimentin
increases (32). ARK5 is a tumor
invasion-associated factor that is downstream of Akt signaling.
Cancer cells with high ARK5 expression exhibit high invasive
activity (11). It has been
demonstrated that the inhibition of ARK5 is able to enhance drug
sensitivity in hepatocellular carcinoma through EMT (21). However, prior to the present study,
there were a limited number of reports concerning the role of ARK5
in the metastasis of other cancer types (14,33),
including HCC.
The results of the present study indicated that the
knockdown of ARK5 markedly decreased migration and invasion of HCC
cells. Furthermore, the inhibition of ARK5 was also able to
increase the expression of the epithelial marker E-cadherin and
reduce expression of the mesenchymal marker vimentin in Huh7 and
SNU387 cells, compared with the control.
TGF-β1 is a pleiotropic cytokine that regulates cell
proliferation and differentiation (34). TGF-β1 induces a mesenchymal phenotype
and functions as a tumor suppressor that restricts cell growth,
resulting in the inhibition of cancer progression during the early
stages of carcinogenesis (35).
However, as cancer progresses, TGF-β1 becomes a tumor promoter,
performing dual roles in the progression and metastasis of HCC
(36–38). The data reported in the present study
indicated that TGF-β treatment of HCC cells was able to induce
invasion and migration. When the epithelial Huh7 cells were treated
with TGF-β1, the invasive capability and migratory rate of these
cells was increased compared with the control. Notably, this
increased invasive capability and migratory rate was reversed upon
ARK5 knockdown and treatment with TGF-β1, suggesting that ARK5 may
reduce invasion and migration in HCC cells. Furthermore, these data
also indicated that the treatment of Huh7 cells with TGF-β1
resulted in decreased E-cadherin expression and increased
expression of vimentin compared with the control. However, the
downregulation of ARK5 was able to reverse TGF-β1-induced EMT in
Huh7 cells. These results indicated that the expression of ARK5 may
serve an important role in cellular invasion and metastasis and EMT
phenotype changes of HCC cells.
In summary, the present study demonstrated that
inhibiting the expression of ARK5 may reduce the ability of the HCC
cells to invade and metastasize via EMT. Therefore, ARK5 has the
potential to be a molecular target for the development of novel
therapy of HCC, which focuses on cell invasion.
References
|
1
|
Waly Raphael S, Yangde Z and Yuxiang C:
Hepatocellular carcinoma: Focus on different aspects of management.
ISRN Oncol. 2012:4216732012.PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistic, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaufhold S and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee TK, Poon RT, Yuen AP, Ling MT, Kwok
WK, Wang XH, Wong YC, Guan XY, Man K, Chau KL and Fan ST: Twist
overexpression correlates with hepatocellular carcinoma metastasis
through induction of epithelialmesenchymal transition. Clin Cancer
Res. 12:5369–5376. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suzuki A, Kusakai G, Kishimoto A, Lu J,
Ogura T, Lavin MF and Esumi H: IdentiWcation of a novel protein
kinase mediating Akt survival signaling to the ATM protein. J Biol
Chem. 278:48–53. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kusakai G, Suzuki A, Ogura T, Miyamoto S,
Ochiai A, Kaminishi M and Esumi H: ARK5 expression in colorectal
cancer and its implications for tumor progression. Am J Pathol.
164:987–995. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ekstrand AI, Jonsson M, Lindblom A, Borg A
and Nilbert M: Frequent alterations of the PI3K/AKT/mTOR pathways
in hereditary nonpolyposis colorectal cancer. Fam Cancer.
9:125–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li B, Tsao SW, Li YY, Wang X, Ling MT,
Wong YC, He QY and Cheung AL: Id-1 promotes tumorigenicity and
metastasis of human esophageal cancer cells through activation of
PI3K/AKT signaling pathway. Int J Cancer. 125:2576–2585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohta T, Isobe M, Takahashi T,
Saitoh-Sekiguchi M, Motoyama T and Kurachi H: The Akt and ERK
activation by platinum-based chemotherapy in ovarian cancer is
associated with favorable patient outcome. Anticancer Res.
29:4639–4647. 2009.PubMed/NCBI
|
|
13
|
Simon PO Jr, McDunn JE, Kashiwagi H, Chang
K, Goedegebuure PS, Hotchkiss RS and Hawkins WG: Targeting AKT and
with the proapoptotic peptide, TAT-CTMP: A novel strategy for the
treatment of human pancreatic adenocarcinoma. Int J Cancer.
125:942–951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang XZ, Yu J, Liu HY, Dong RH and Cao
XC: ARK5 is associated with the invasive and metastatic potential
of human breast cancercells. J Cancer Res Clin Oncol. 138:247–254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu S, Niu N, Guo H, Tang J, Guo W, Liu Z,
Shi L, Sun T, Zhou F, Li H, Zhang J and Zhang B: ARK5 promotes
glioma cell invasion, andits elevated expression is correlated with
poorclinical outcome. Eur J Cancer. 49:752–763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bustin SA: Absolute quantification of mRNA
using real-time reverse transcription polymerase chain reaction
assays. J Mol Endocrinol. 25:169–193. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelialto-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, DU F, Zhao Q, Jin J, Ma X and Li H:
Acquisition of 5-fluorouracil resistanceinduces
epithelial-mesenchymal transitions through the Hedgehog signaling
pathway in HCT-8 colon cancer cells. Oncol Lett. 9:2675–2679.
2015.PubMed/NCBI
|
|
19
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes tochemoresistance. Natur. 527:472–476.
2015. View Article : Google Scholar
|
|
20
|
Mallini P, Lennard T, Kirby J and Meeson
A: Epithelial-to-mesenchymal transition: What is the impact on
breast cancer stem cells and drug resistance. Cancer Treat Rev.
40:341–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu T, Zhang J, Chen W, Pan S, Zhi X, Wen
L, Zhou Y, Chen BW, Qiu J, Zhang Y, et al: ARK5 promotes
doxorubicin resistance in hepatocellular carcinoma via
epithelial-mesenchymal transition. Cancer Lett. 377:140–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang HY, Li JH, Li G and Wang SR:
Activation of ARK5/miR-1181/HOXA10 axis promotes
epithelial-mesenchymal transition in ovarian cancer. Oncol Rep.
34:1193–1202. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li M, Zheng C, Xu H, He W, Ruan Y, Ma J,
Zheng J, Ye C and Li W: Inhibition of AMPK-related kinase 5 (ARK5)
enhances cisplatin cytotoxicity in non-small cell lung cancer cells
through regulation of epithelial-mesenchymal transition. Am J
Transl Res. 9:1708–1719. 2017.PubMed/NCBI
|
|
24
|
Park NR, Cha JH, Jang JW, Bae SH, Jang B,
Kim JH, Hur W, Choi JY and Yoon SK: Synergistic effects of CD44 and
TGF-β1 through AKT/GSK-3β/β-catenin signaling during
epithelial-mesenchymal transition in liver cancer cells. Biochem
Biophys Res Commun. 477:568–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
El-Serag HB: Hepatocellular carcinoma. N
Engl J Med. 365:1118–1127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kanda M, Sugimoto H and Kodera Y: Genetic
and epigenetic aspects of initiation and progression of
hepatocellular carcinoma. World J Gastroenterol. 21:10584–10597.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kawasaki K, Smith RS Jr, Hsieh CM, Sun J,
Chao J and Liao JK: Activation of and the phosphatidylinositol
3-kinase/protein kinase Akt pathway mediates nitric oxide-induced
endothelial cell migration and angiogenesis. Mol Cell Biol.
23:5726–5737. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li G, Satyamoorthy K and Herlyn M:
N-cadherin-mediated intercellular interactions promote survival and
migration of melanoma cells. CancerRes. 61:3819–3825. 2001.
|
|
29
|
Liu W, Liu Y and Lowe WL Jr: The role of
phosphatidylinositol 3-kinaseand the mitogen-activated protein
kinases in insulin-like growth factor-I-mediated effects in
vascular endothelial cells. Endocrinology. 142:1710–1719. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi SS and Diehl AM:
Epithelial-to-mesenchymal transitions in the liver. Hepatology.
50:2007–2013. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia H, Ooi LL and Hui KM:
MicroRNA-216a/217-induced epithelial-mesenchymal transition targets
PTEN and SMAD7 to promote drug resistance and recurrenceof liver
cancer. Hepatology. 58:629–641. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thomson S, Petti F, Sujka-Kwok I, Mercado
P, Bean J, Monaghan M, Seymour SL, Argast GM, Epstein DM and Haley
JD: A systems view of epithelial-mesenchymal transition signaling
states. Clin Exp Metastasis. 28:137–155. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen D, Liu G, Xu N, You X, Zhou H, Zhao X
and Liu Q: Knockdown of ARK5 expression suppresses invasion and
metastasis of gastric cancer. Cell Physiol Biochem. 42:1025–1036.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen XF, Zhang HJ, Wang HB, Zhu J, Zhou
WY, Zhang H, Zhao MC, Su JM, Gao W, Zhang L, et al: Transforming
growth factor-β1 induces epithelial-to mesenchymal transition in
human lung cancer cells via PI3K/Akt and MEK/Erk1/2 signaling
pathways. Mol Biol Rep. 39:3549–3556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu Z, Shen MX, Ma DZ, Wang LY and Zha XL:
TGF-beta1-promoted epithelial-to-mesenchymal transformation and
cell adhesion contribute to TGF-beta1-enhanced cell migration in
SMMC-7721 cells. Cell Res. 13:343–350. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dang H, Ding W, Emerson D and Rountree CB:
Snail1 induces epithelial-tomesenchymal transition and tumor
initiating stem cell characteristics. BMC Cancer. 11:3962011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji J and Wang XW: Clinical implications of
cancer stem cell biology in hepatocellular carcinoma. Semin Oncol.
39:461–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang JD, Nakamura I and Roberts LR: The
tumor microenvironment in hepatocellular carcinoma: Current status
and therapeutic targets. Semin Cancer Biol. 21:35–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|