Introduction
Colon cancer has one of the highest mortality rates
of any malignant disease globally. According to the annual
age-adjusted cancer incidence and mortality rate in the USA from
1975 to 2002, colon and rectal cancer were among the three most
frequently diagnosed types of cancer (1). Patients with localized colon cancer and
rectal cancer exhibit a high 5-year relative survival rate
(>80%); however, cases exhibiting distant metastases have a
5-year relative survival rate of only ~10% (1). Previous studies have demonstrated that
palliative chemotherapy is able to extend the median survival time
of patients with metastatic colon cancer (2–4). However,
a number of distinct effects of chemotherapies were observed among
different patients with colon cancer. An increased response rate to
treatment and longer continuous effects are required (3,4).
Tumor necrosis factor-α (TNF-α) is an important
cytokine produced primarily by activated macrophages and numerous
other cells including fibroblasts, natural killer cells and cluster
of differentiation (CD)4+ lymphocytes (5,6). TNF-α
exhibits multiple functions in mediating systemic inflammation,
regulating the immune response, cell metabolism, proliferation and
apoptosis (7,8). There are two types of TNF-α receptor
(TNFRs) which mediate these complex functions: TNFR1 and TNFR2
(9,10). Previous studies have demonstrated that
TNF-α serves roles in promoting the development of a number of
malignant tumors, including breast, renal and pancreatic cancer
(10–13). Tumor cells themselves are able to
produce TNF-α (14). Anti-TNF-α
treatment has been studied in a number of these tumors, and
favorable effects have been observed (15).
Inflammation is associated with the initiation of
colon cancer (16).
Colitis-associated cancer, a subtype of colorectal cancer, is
associated with inflammatory bowel disease (16). Currently anti-TNF-α treatment is used
against a number of inflammatory diseases, including rheumatoid
arthritis and Crohn's disease. As TNF-α is a key pro-inflammatory
cytokine, it was hypothesized that anti-TNF-α may promote the
effects of chemotherapy on patients with colon cancer. In the
present study, the effects of combining anti-TNF-α treatment
(infliximab) and oxaliplatin (OXA) on colon cancer were explored in
an animal model.
Materials and methods
Cell culture and cell line
establishment
Human colon cancer cell line SW480 and murine colon
cancer cell line CT-26 (American Type Culture Collection, Manassas,
VA, USA) were cultured with RPMI-1640 medium (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.) and Leibovitz's
L-15 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
supplemented with 10% FBS, respectively. Additionally, 100 µg/ml
streptomycin (Thermo Fisher Scientific, Inc.) and 100 U/ml
penicillin (Thermo Fisher Scientific, Inc.) was added to the medium
throughout the cell culture. All cells were cultured using a
humidified incubator at 37°C with 5% CO2. Cells were
subcultured when the cells were 90% confluent. To induce
OXA-resistant cell lines, SW480 and CT-26 cell lines were treated
with 0.1 µM OXA (Sigma-Aldrich; Merck KGaA) for 1 month to induce
drug resistance (culture medium was changed with fresh medium
containing OXA every three days). The drug resistance was confirmed
using a cell viability assay following treatment with increasing
doses of OXA (10 and 1,000 µM). The drug-resistant cells (SW480-R
and CT-26-R) were used for further study.
Patient samples
Formalin-fixed paraffin-embedded 4-µm thick colon
cancer tissue samples were collected from 60 patients to evaluate
their TNF-α expression levels. All patients were diagnosed with
colon cancer at the First Affiliated Hospital of Hebei North
University between June 2011 and April 2014. Tissue samples were
collected during resective surgery. A total of 27 (45.0%) of these
patients were female and 33 (55.0%) were male. The mean age of
these patients was 52.5 years (range, 46–74 years). The present
study was approved by the Local Committee of Medical Research
Ethics of the First Affiliated Hospital of Hebei North University
(Zhangjiakou, China). Written informed consent was obtained from
the patients or their representatives. All tissue samples were
collected via surgery prior to the patients receiving chemotherapy.
Following surgery, these patients received OXA as adjuvant
chemotherapy. The response to OXA treatment was monitored and
evaluated by computed tomography scans and X-ray examinations
according to the revised RECIST guidelines (version 1.1) (16).
Immunohistochemical staining
The expression levels of TNF-α in the FFPE tissues
from the patients with colon cancer were assessed using
immunohistochemistry (IHC). Standard IHC procedures were followed
(17). Briefly, sample sections were
deparaffinized using xylene and blocked by incubating with 3%
H2O2 in methanol, followed by rehydration
with decreasing concentrations of ethanol. Antigen retrieval was
performed by heating the sections with Reveal Decloaker (Biocare
Medical, LLC, Paheco, CA, USA) in a microwave for 10 min at 100°C.
Primary antibody (anti-TNF-α antibody; 1:100 dilution; cat. no.
ab9635; Abcam, Cambridge, MA, USA) was added prior to incubation at
4°C overnight. Secondary antibody (horseradish
peroxidase-conjugated; 1:100 dilution; cat. no. ab6721; Abcam) was
added prior to incubation for 1 h at room temperature. Then
3,3-diaminobenzidine was added and washed away immediately once the
stain had developed. The slides were mounted and observed using
light microscopy. Each slide was evaluated by two researchers
independently without knowledge of the background information of
the slides. TNF-α expression was divided into five levels based on
the proportion of positive cells: 5, >80%; 4, >60%; 3,
>40%; 2, >20%; 1, <20%.
Cell viability
Cell viability was determined using a Cell Counting
Kit-8 (CCK-8) kit (Merck KGaA, Darmstadt, Germany), according to
the manufacturer's protocol. Equal numbers of cells
(5×104 cells/well) were seeded on 96-well plates
containing 100 µl culture medium. Various concentrations of OXA
were added to the cells prior to incubation for 48 h. CCK-8
solution (10 µl/well) was added prior to incubation for 30 min at
37°C. Finally, the absorbance at 450 nm was assessed using an MRX
microplate reader (Dynex Technologies, Chantilly, VA, USA). The
absorbance value of each treated group relative to that of the
control group was used to represent the cell viability.
Antibody-dependent cellular
cytotoxicity (ADCC) assay and complement-dependent cytotoxicity
(CDC) assay
ADCC and CDC assays were performed to evaluate the
cytotoxic effects of infliximab on OXA-resistant colon cancer cell
lines SW480-R and CT-26-R. Equal numbers of these two cell lines
were seeded on 96-well plates (1×105 cells/well) with
100 µl RPMI-1640 medium (Sigma-Aldrich; Merck KGaA). Infliximab
(Thermo Fisher Scientific, Inc.) was added prior to incubation for
1 h at 37°C with 5% CO2. Effector cells [macrophages
were isolated from Balb/c mice as previously described (18)] were added prior to incubation for
another 48 h under identical conditions. For the CDC assay, the
effector cells were replaced with guinea pig serum containing mixed
active complements (Sigma-Aldrich; Merck KGaA), which were added to
the target cells and incubated for 5 h at 37°C with 5%
CO2. The complement was replaced with phosphate-buffered
saline in the control group. The cell viability was assessed
according to the aforementioned protocol.
Animal model
A colon cancer xenograft mouse model was established
using the CT-26-R cell line and Balb/c mice (30 females; 20–22 g; 6
weeks of age; Shanghai Experimental Animal Center, Chinese Academy
of Science, Shanghai, China). Equal numbers of CT-26-R cells
(1×106) were inoculated into the flanks of these mice
subcutaneously. At 1 week after the inoculation, the mice were
randomly divided into three groups (10 per group) to receive
treatments once per week: OXA (4 mg/kg) alone; OXA (4 mg/kg) +
infliximab (6 mg/kg); or saline (control). The agents were injected
into the tail vein of these mice once a week. The tumor volume
(width2 × length × π/6) and survival date was observed
and recorded. All mice were raised in a specific pathogen-free
environment (at room temperature, with fresh air and a humidity of
40–50%) with a 12-h light/12-h dark cycle and free access to water
and food. This animal study was approved by the Experimental Animal
Use Committee of the First Affiliated Hospital of Hebei North
University.
Fluorescence-activated cell sorter
(FACS) analysis
FACS analysis was performed to determine the levels
of TNF-α, cleaved poly(ADP-ribose) polymerase (PARP) and
bromodeoxyuridine (BrdU) in colon cancer cell lines SW480, SW480-R,
CT-26 and CT-26-R or xenograft mouse model-derived tumor cells.
Cells were incubated with the following primary antibodies:
anti-TNF-α (1:100 dilution; cat. no. ab1793; Abcam) and
anti-cleaved PARP (1:100 dilution; cat. no. ab110315; Abcam) for 30
min at room temperature followed by washing with PBS three times.
Next, a fluorescence-conjugated secondary antibody (1:1,000
dilution; cat. no. ab6789; Abcam) was added prior to incubation for
20 min at room temperature followed by washing with PBS three
times. The cells were then analyzed using a BD FACSCanto™ II
machine (BD Biosciences, Franklin Lakes, NJ, USA) and FlowJo
software 9.7.1 (FlowJo LLC, Ashland, OR, USA).
Statistics
All statistical analysis and data visualization was
performed using GraphPad 7 (GraphPad Software, Inc., La Jolla, CA,
USA) or SPSS software 17.0 (SPSS, Inc., Chicago, IL, USA). The
difference between means of various experimental groups were
analyzed using either Student's t-test or a one-way analysis of
variance with Bonferroni's pairwise comparison. Survival analysis
was performed using the Kaplan-Meier estimator method. The
differences between the survival curves were analyzed using a
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
TNF-α is increased in the tumor tissue
of patients with OXA-resistant colon cancer and OXA-resistant colon
cancer cell lines
A total of 60 tumor tissue samples were collected
from patients with colon cancer who accepted OXA treatment
following surgery, half of whom exhibited sensitivity to the OXA
treatment, with the other half exhibiting resistance to the OXA
treatment. These samples were divided into two groups according to
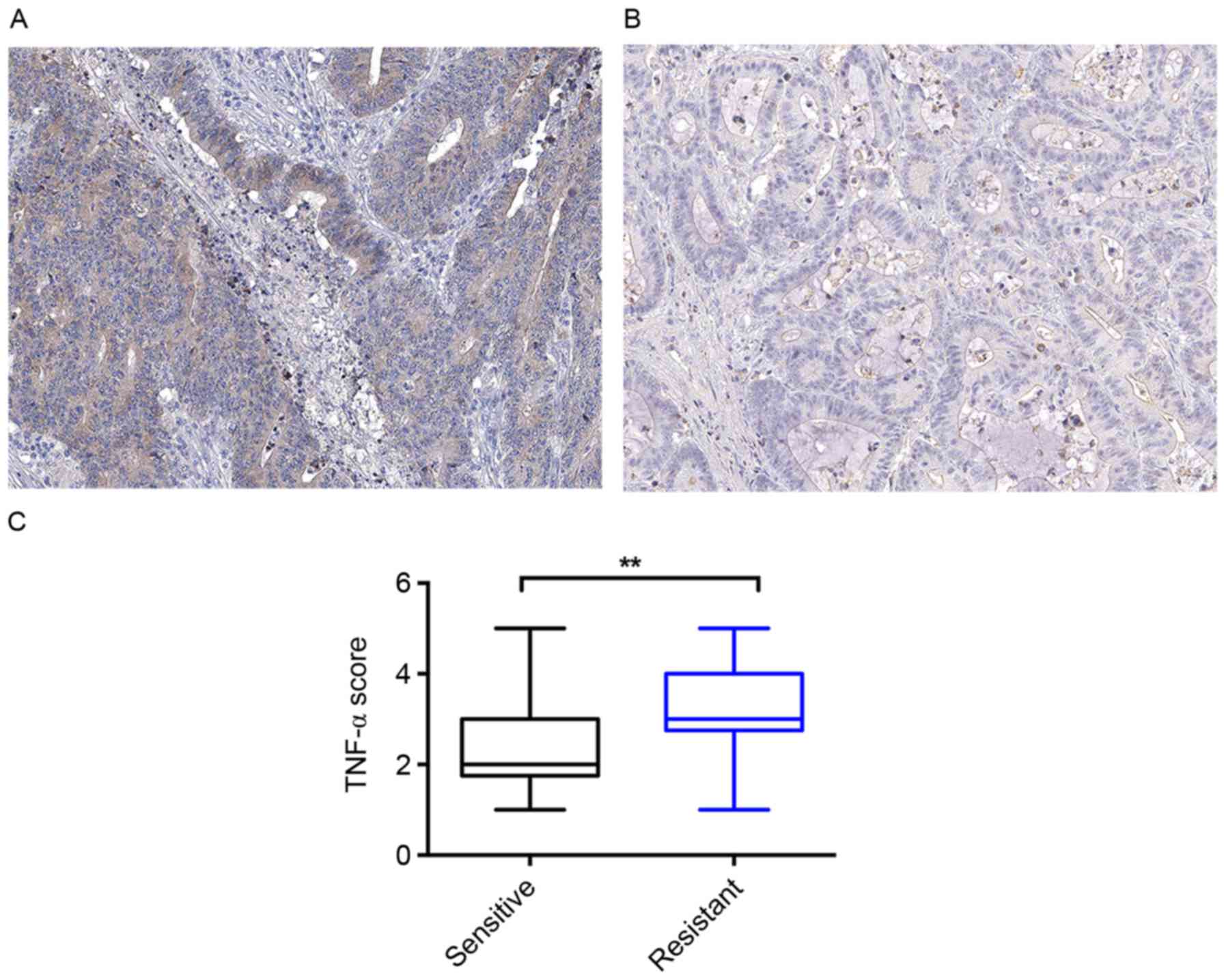
their response to the OXA treatment. IHC was performed to evaluate
the TNF-α level of these tissue samples. As presented in Fig. 1A and B, these samples expressed
distinct levels of TNF-α. Quantitative analysis identified that the
patients with colon cancer who were sensitive to the OXA treatment
had a lower mean TNF-α level compared with that of the patients who
were resistant to the OXA treatment (Fig.
1C).
TNF-α is increased in OXA-resistant
colon cancer cell lines
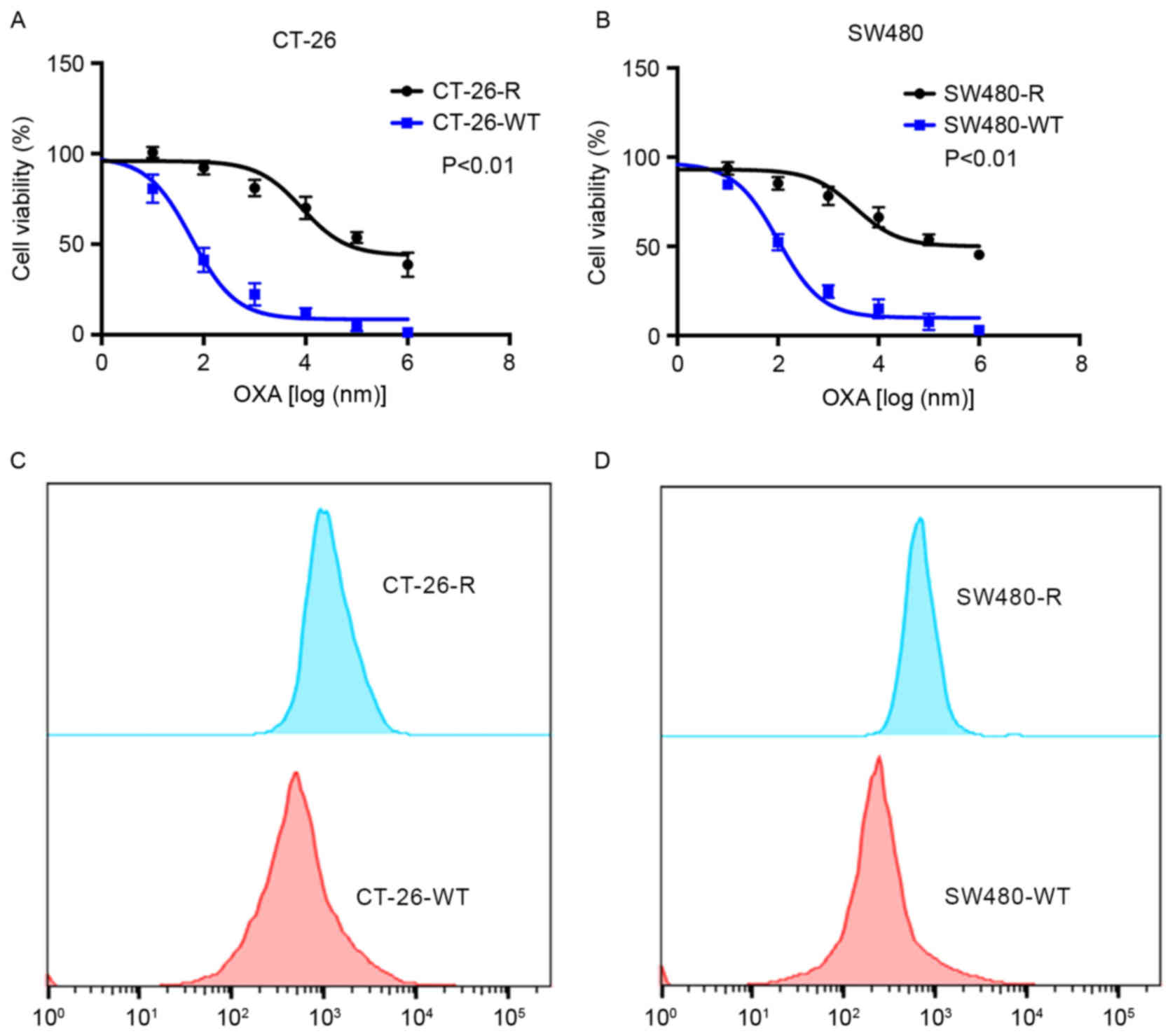
OXA-resistant colon cancer cell lines, CT-26-R and
SW480-R, were created using low-dose OXA treatment. As presented in
Fig. 2A and B, these cell lines
exhibited resistance to increasing concentrations of OXA. The TNF-α
expression was measured using FACS analysis (Fig. 2C and D). These cell lines exhibited
increased levels of TNF-α compared with the wild-type CT-26 and
SW480 cell lines. In summary, the association of TNF-α expression
with OXA sensitivity suggests that TNF-α may alter the effect of
chemotherapy on patients with colon cancer.
Infliximab inhibits the survival of
OXA-resistant colon cancer cell lines by inducing ADCC and CDC
effects
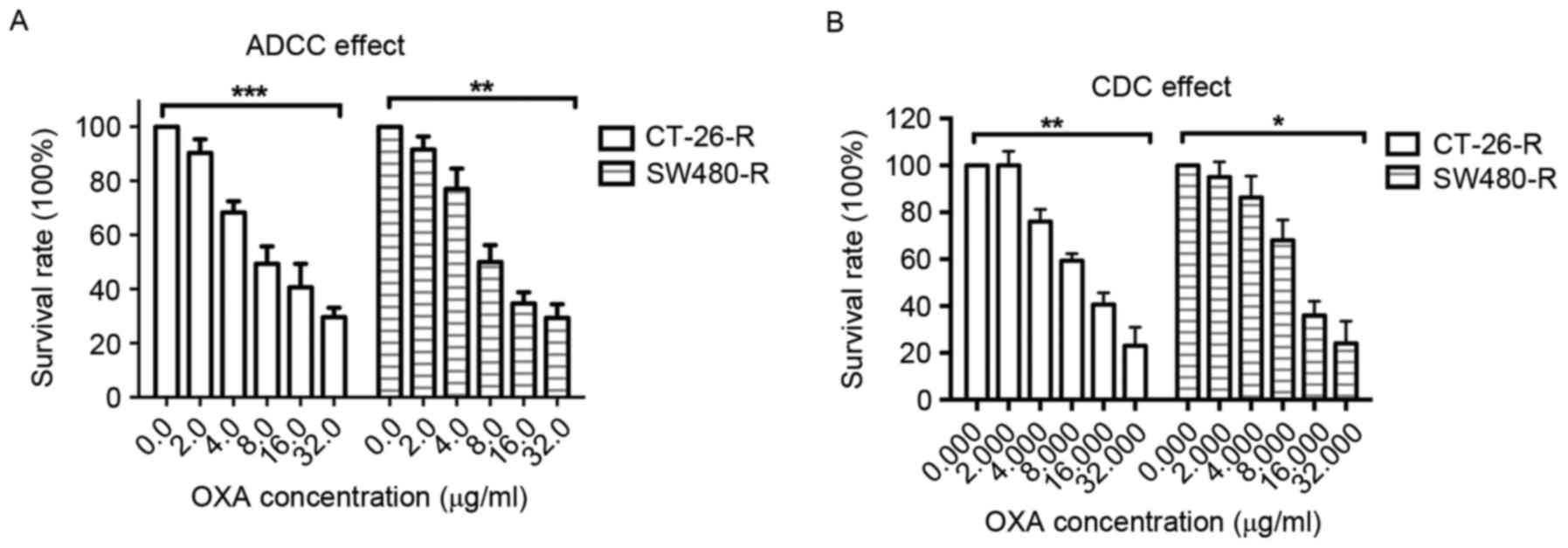
Considering that OXA-resistant colon cancer cell
lines express increased levels of TNF-α and that TNF-α is an
important cytokine which regulates the immune response, the ability
of infliximab to induce ADCC and CDC effects on the OXA-resistant
colon cancer cell lines CT-26-R and SW480-R was investigated.
Notably, it was revealed that infliximab decreased the survival
rate of CT-26-R and SW480-R cells in the presence of macrophages or
active complement (Fig. 3A and B).
These results suggest that TNF-α may promote the resistance of
colon cancer cells to OXA treatment by inhibiting any immune
response to tumor cells.
Infliximab decreases the resistance to
OXA in a colon cancer mouse model
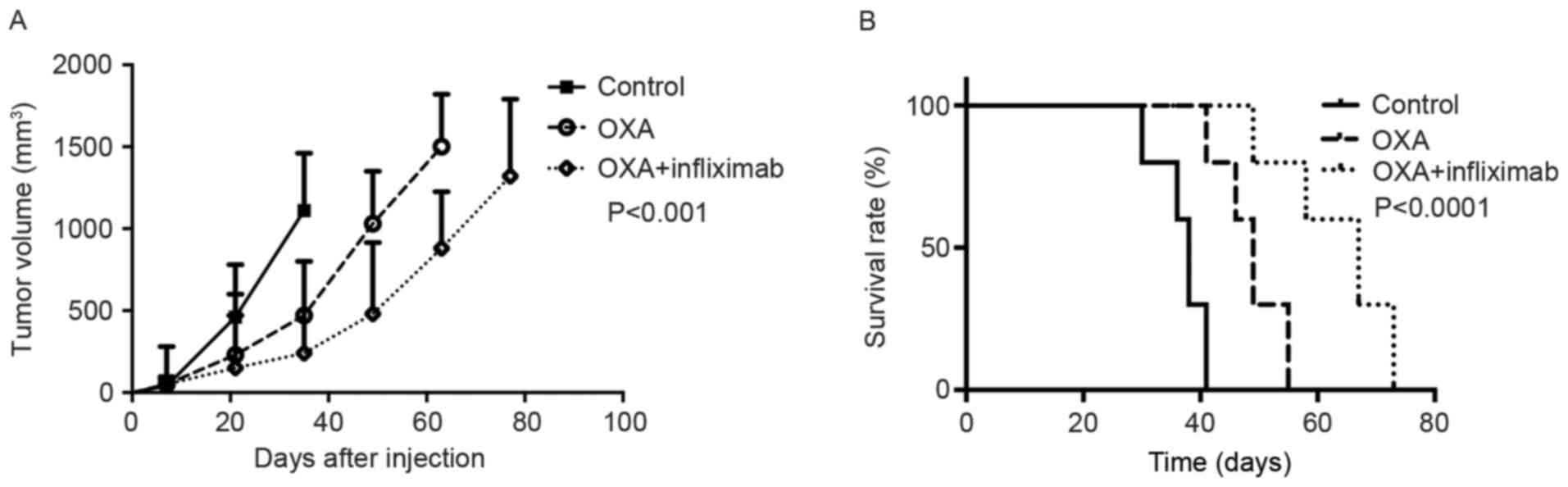
Mice bearing CT-26-R xenograft colon tumors were
assigned to three treatment groups: OXA, OXA + infliximab or
saline. These mice exhibited different survival rates. The group
receiving OXA + infliximab exhibited the longest overall survival
time and most marked tumor growth compared with the group receiving
OXA alone and the control group (Fig.
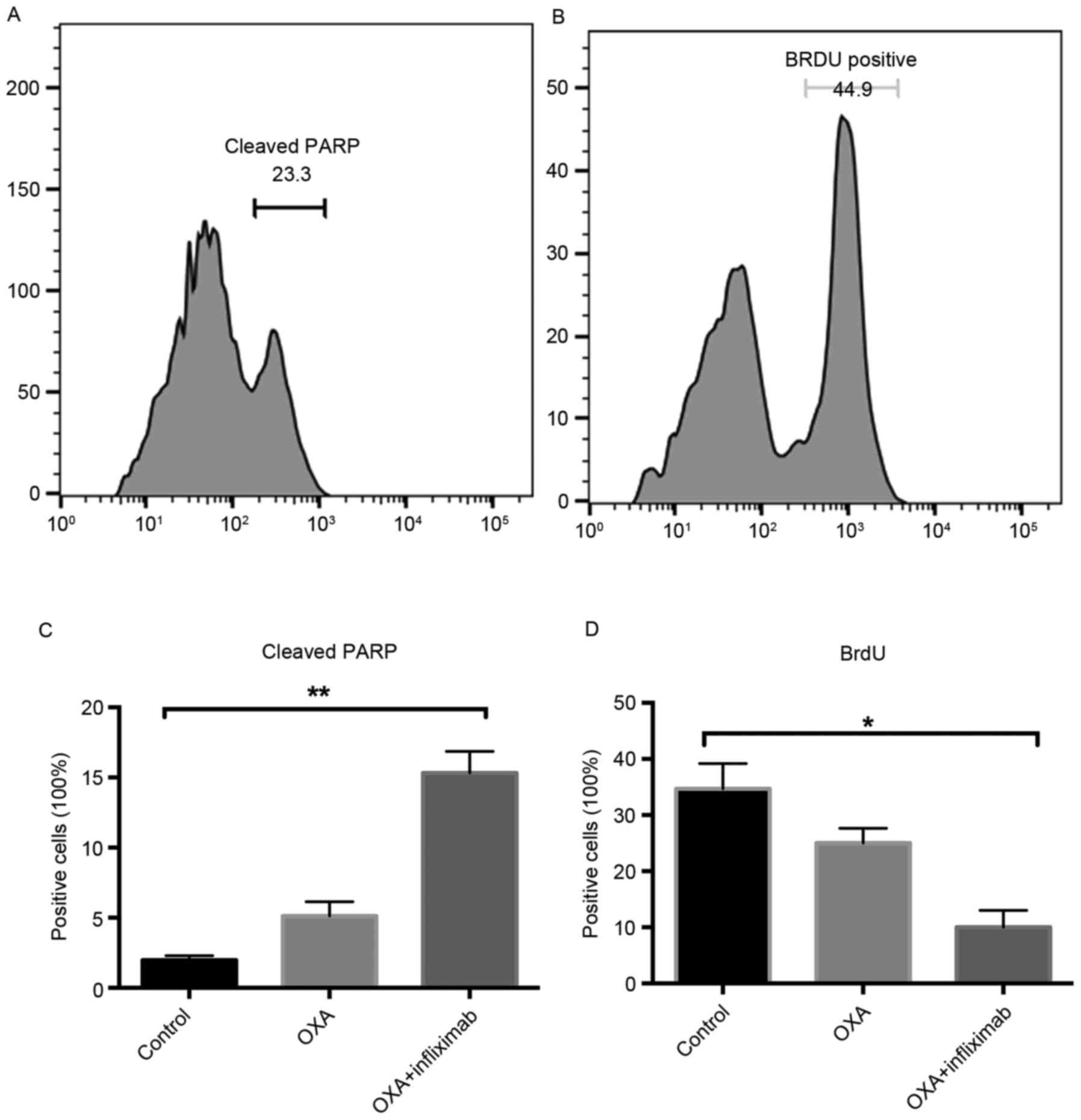
4). Notably, an increased level of cleaved PARP was detected in
the tumor cells of the mice treated with OXA + nfliximab compared
with the OXA group (Fig. 5A and B).
Additionally, the BrdU incorporation rate of these tumor cells was
decreased compared with that of the tumor cells from the mice
receiving OXA (Fig. 5C and D).
Discussion
Although there have been previous studies
demonstrating that local administration of TNF-α may inhibit the
development of advanced solid tumors, including metastatic liver
cancer and soft tissue sarcomas, only a minor effect was observed,
dependent on the combination with other antiblastic agents,
including doxorubicin and melphalan (6,19,20). Conversely, previous studies have
demonstrated that TNF-α may promote the metastasis of tumors
(6,21). A previous study has demonstrated that
TNF-α-deficient mice were resistant to chemical carcinogenesis
(22). These tumor-promoting effects
of TNF-α suggest that anti-TNF-α treatment may be beneficial to
patients with cancer.
Anti-TNF-α treatment has been utilized previously to
treat patients with myelogenous leukemia, multiple myeloma and
myelofibrosis (23). There are
ongoing clinical trials of anti-TNF-α treatment for other
malignances, including ovarian cancer and non-small cell lung
cancer (6,7). Additionally, TNF-α-induced protein 3 was
demonstrated to inhibit the antitumor activity of CD8+ T
cells, which may allow the tumor cells to evade immune surveillance
(24). In the present study, it was
identified that TNF-α expression was increased in the colon cell
lines with induced resistance to OXA treatment. The patients with
colon cancer who were resistant to OXA treatment also tended to
exhibit increased TNF-α expression levels compared with the
patients who were sensitive to OXA treatment. Results of the in
vitro study revealed that infliximab induced ADCC and CDC
effects, inhibiting the survival of OXA-resistant colon cancer cell
lines. In the CT-26-R xenograft mouse model, the combination
treatment of infliximab and OXA resulted in improved effects when
compared with the OXA treatment alone.
In previous studies, to the best of our knowledge,
there was no evidence demonstrating the direct cytotoxicity caused
by anti-TNF-α treatment. The present study provided a possible
explanation of the tumor-inhibiting effects of anti-TNF-α
treatment: Anti-TNF-α treatment may reject tumor cells through
immune response-dependent methods, i.e., ADCC and CDC effects. The
combination of infliximab and OXA achieved apparent favorable
effects in the OXA-resistant colon cancer xenograft mouse model.
This suggests that the anti-TNF-α treatment may have increased the
sensitivity of colon cancer cells to chemotherapy. Furthermore,
increased levels of cleaved PARP and the decreased BrdU
incorporation level were also observed in the infliximab- and
OXA-treated mice, which suggested that the combining treatment
effectively induced apoptosis and inhibited the proliferation of
colon cancer cells.
In conclusion, we hypothesize that anti-TNF-α
treatment may sensitize colon cancer cells to chemotherapy and thus
enhance the efficacy of chemotherapy for the treatment of colon
cancer.
Acknowledgements
The present study sponsored by the Excellent
Clinical Medicine Talent Program of Finance Department of Hebei
Province, 2015: Fundamental and Clinical Research of Gene
Detection, Mechanism, Resistance and Accurate Treatment of
Colorectal Cancer Cases in Regions of Hebei, Shanxi and Inner
Mongolia.
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J,
Smigal C and Thun MJ: Cancer statistics, 2006. CA Cancer J Clin.
56:106–130. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jonker DJ, Maroun JA and Kocha W: Survival
benefit of chemotherapy in metastatic colorectal cancer: A
meta-analysis of randomized controlled trials. Br J Cancer.
82:1789–1794. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohne CH, Cunningham D, Di Costanzo F,
Glimelius B, Blijham G, Aranda E, Scheithauer W, Rougier P, Palmer
M, Wils J, et al: Clinical determinants of survival in patients
with 5-fluorouracil-based treatment for metastatic colorectal
cancer: Results of a multivariate analysis of 3825 patients. Ann
Oncol. 13:308–317. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
André T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eisenman ST, Gibbons SJ, Verhulst PJ,
Cipriani G, Saur D and Farrugia G: Tumor necrosis factor alpha
derived from classically activated ‘M1’ macrophages reduces
interstitial cell of Cajal numbers. Neurogastroenterol Motil.
29:2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mocellin S, Rossi CR, Pilati P and Nitti
D: Tumor necrosis factor, cancer and anticancer therapy. Cytokine
Growth Factor Rev. 16:35–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van Horssen R, Ten Hagen TL and Eggermont
AM: TNF-alpha in cancer treatment: Molecular insights, antitumor
effects, and clinical utility. Oncologist. 11:397–408. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu Y and Zhou BP:
TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and
invasion. Br J Cancer. 102:639–644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen G and Goeddel DV: TNF-R1 signaling: A
beautiful pathway. Science. 296:1634–1635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sedger LM and McDermott MF: TNF and
TNF-receptors: From mediators of cell death and inflammation to
therapeutic giants-past, present and future. Cytokine Growth Factor
Rev. 25:453–472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stuelten CH, DaCosta Byfield S, Arany PR,
Karpova TS, Stetler-Stevenson WG and Roberts AB: Breast cancer
cells induce stromal fibroblasts to express MMP-9 via secretion of
TNF-alpha and TGF-beta. J Cell Sci. 118:2143–2153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harrison ML, Obermueller E, Maisey NR,
Hoare S, Edmonds K, Li NF, Chao D, Hall K, Lee C, Timotheadou E, et
al: Tumor necrosis factor alpha as a new target for renal cell
carcinoma: Two sequential phase II trials of infliximab at standard
and high dose. J Clin Oncol. 25:4542–4549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ohri CM, Shikotra A, Green RH, Waller DA
and Bradding P: Tumour necrosis factor-alpha expression in tumour
islets confers a survival advantage in non-small cell lung cancer.
BMC Cancer. 10:3232010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sethi G, Sung B and Aggarwal BB: TNF: A
master switch for inflammation to cancer. Front Biosci.
13:5094–5107. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Terzic J, Grivennikov S, Karin E and Karin
M: Inflammation and colon cancer. Gastroenterology.
138:2101–2114.e5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamanishi J, Mandai M, Iwasaki M, Okazaki
T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N,
et al: Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+
T lymphocytes are prognostic factors of human ovarian cancer. Proc
Natl Acad Sci USA. 104:pp. 3360–3365. 2007; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ray A and Dittel BN: Isolation of mouse
peritoneal cavity cells. J Vis Exp. pii:14882010.
|
|
19
|
Rossi CR, Foletto M, Pilati P, Mocellin S
and Lise M: Isolated limb perfusion in locally advanced cutaneous
melanoma. Semin Oncol. 29:400–409. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Christoforidis D, Martinet O, Lejeune FJ
and Mosimann F: Isolated liver perfusion for non-resectable liver
tumours: A review. Eur J Surg Oncol. 28:875–890. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Orosz P, Echtenacher B, Falk W, Rüschoff
J, Weber D and Männel DN: Enhancement of experimental metastasis by
tumor necrosis factor. J Exp Med. 177:1391–1398. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suganuma M, Okabe S, Marino MW, Sakai A,
Sueoka E and Fujiki H: Essential role of tumor necrosis factor
alpha (TNF-alpha) in tumor promotion as revealed by
TNF-alpha-deficient mice. Cancer Res. 59:4516–4518. 1999.PubMed/NCBI
|
|
23
|
Tsimberidou AM, Waddelow T, Kantarjian HM,
Albitar M and Giles FJ: Pilot study of recombinant human soluble
tumor necrosis factor (TNF) receptor (p75) fusion protein (TNFR:
Fc; Enbrel) in patients with refractory multiple myeloma: Increase
in plasma TNF alpha levels during treatment. Leuk Res. 27:375–380.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giordano M, Roncagalli R, Bourdely P,
Chasson L, Buferne M, Yamasaki S, Beyaert R, van Loo G,
Auphan-Anezin N, Schmitt-Verhulst AM and Verdeil G: The tumor
necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a
brake on antitumor activity of CD8 T cells. Proc Natl Acad Sci USA.
111:pp. 11115–11120. 2014; View Article : Google Scholar : PubMed/NCBI
|